Abstract
Background
Despite strong evidence supporting a genetic and gene-environment interaction in the irritable bowel syndrome (IBS), the role of genes and early life in another common functional bowel disorder, chronic constipation (CC) has been little studied.
Aim
To determine if familial aggregation occurs in CC, and whether risk factors differed in those with family members.
Methods
A randomly selected population-based cohort from Olmsted County, MN, was surveyed (n=8006); 3831 completed questionnaires (response rate 48%). Cases were identified based upon their responses to a validated questionnaire and meeting Rome criteria for CC. Controls were matched one-to-one by age and gender. IBS (by Rome II criteria) was excluded. Recruitment of case and control probands occurred in 2010–2011 by mailing a family information form; then first degree relatives (FDR) were mailed a questionnaire. All potential proband participants were not informed of CC status.
Results
Overall 1185 cases who met criteria for CC without symptoms of IBS and 1185 controls were surveyed; 309 case and 336 control probands provided data. The proportion of family members having CC was not associated with case status, and the constipation status of FDR was not significantly associated with case-controls status of the respective probands. Symptom burden in FDR was associated with gender and SSC score, but not age or proband status.
Conclusions
No evidence of familial aggregation was observed in adults from the community with CC (excluding IBS). Our data suggest environmental factors in later life more likely account for adult CC.
Keywords: chronic constipation, irritable bowel syndrome, functional gastrointestinal disorder, familial aggregation
INTRODUCTION
In the United States, chronic constipation (CC) is a common gastrointestinal (GI) disorder estimated to affect from 12% to 19% of the general population [1,2]. The high prevalence of this functional gastrointestinal disorder (FGID) in the community results in a significant burden on both patients and health care resources. CC was the primary complaint for over six million patient visits in the United States in 2004, accounting for a total cost of $1.7 billion, and has a mean annual cost of treatment of $7552 per patient [3–5]. In addition to the financial burden of CC, there is growing evidence of significant associated morbidity and mortality; CC has been observed to negatively impact survival and is associated with an increased risk of cardiovascular disease [6,7].
Irritable bowel syndrome (IBS) is another prevalent FGID that overlaps with CC, leading some to suggest CC and IBS with predominant constipation should be reclassified as one and the same disorder [8,9]. There is no doubt that IBS consistently aggregates in families [10–14], and a strong and growing body of evidence has identified genetic and environmental risk factors in IBS. On the other hand, only very limited information exists on whether CC aggregates in families like IBS, and no data in adults from the West has been published. A recent observation from Asia reported familial aggregation of constipation in adult patients evaluated at a single tertiary center and suggested that those with constipation and a positive family history of CC may be clinically different than constipated patients without a family history [15,16]. Familial aggregation of CC was also reported to occur in pediatric patients in a tertiary care setting [17]. Whether familial aggregation truly occurs in CC in the community remains to be documented, and if clustering occurs whether this is explained by genetic factors, environmental factors, or an interplay of both is currently unknown. Population-based studies for CC are critical given how common the disorder is in the community and to avoid potential biases in the tertiary setting. Therefore, this study sought to determine whether chronic constipation aggregates in families from a population-based perspective.
METHODS
This is a population-based cohort study of randomly selected subjects from Olmsted County, Minnesota, and their identified first degree relatives. This research was approved by the Mayo Clinic Institutional Review Board.
Subjects
The Olmsted County population is comprised of approximately 120,000 residents of whom 89% are Caucasian. Importantly, sociodemographically the Olmsted County population is very similar to that of the general U.S. white population, except for a slightly higher education level as well as a greater proportion employed in the health-related services industry, and it is generally accepted that random samples are likely to be reasonably representative of U.S. whites [18,19] The medical records linkage system of the Rochester Epidemiology Project (REP) allows access to details of the medical care provided to county residents for research who have not revoked research authorization (per Minnesota law) and provides a nearly complete enumeration of the population from which random samples can be drawn.
Population sampling, inclusion, and exclusion criteria
Utilizing the data resources of the REP, a series of age- and gender-stratified random samples of Olmsted County residents were drawn beginning in 1988, and most recently, from 2008 to 2009. Subjects in these samples were mailed validated GI symptom questionnaires (discussed below). The most recent survey (2008–2009) identified all subjects previously mailed to and a new random sample of additional county residents was also generated. For these surveys, a study questionnaire and an explanatory letter were mailed to all eligible subjects (e.g. thought to still be alive and residing in the county). Reminder letters were mailed at subsequent intervals thereafter. Subjects who indicated that they did not wish to participate at any point were not contacted further. Subjects were assumed to no longer reside within Olmsted County if the survey was undeliverable and no new address was known. The subjects that returned questionnaires (which contained criteria for defining chronic constipation as well as other FGIDs) were considered for inclusion in the current study. At approximately ten weeks from the initial survey, any non-respondent was then contacted by telephone to request their participation and verify their residency within the county. Subjects who no longer resided in the county or were deceased were excluded.
Questionnaire
The original Talley Bowel Disease Questionnaire (BDQ) was designed as a self-report instrument to measure GI symptoms experienced over the prior year and to collect information on past medical history [20]. The current and previous versions of the Talley BDQ have been shown to have adequate reliability and validity [20–22]. The current Talley BDQ is comprised of 27 gastrointestinal symptoms including constipation-related questions and the Somatic Symptom Checklist (SSC), which is a valid measure of somatic complaints [23]. The SSC is a self-report scale that contains somatic complaints that are rated based on frequency and intensity [24]. Responses for the SSC items for frequency, and separately, intensity, were first averaged, and then the mean of these two average (frequency and intensity) scores computed to yield a composite score.
Definition of chronic constipation
For this study, subjects were classified a priori with CC based on their responses to the current Talley BDQ. Importantly, respondents meeting symptom criteria for IBS were not classified as CC. The definition for chronic constipation was based on the Rome classification and defined as having two or more of the following: 1) less than three bowel movements per week; 2) straining 25% or more of the time with bowel movements (BM); 3) having hard stools 25% or more of the time with BM; and 4) sensation of incomplete evacuation 25% or more of the time with BM [25].
Case and control (proband) recruitment
The cases for this study were identified based on their responses to the BDQ which indicated they met the criteria used to define CC. Controls were selected from among the respondents not meeting criteria for CC, and matched (in a one-to-one fashion) on age and gender. For both cases and controls, subjects meeting symptom criteria for IBS were excluded. Recruitment of case and control probands occurred from 2010 to 2011 through mailings of a study packet that contained an explanatory letter about the study, consent form, and a family information form (FIF). The FIF was designed to ascertain contact information for all first degree relatives (FDR) of the probands and the original version had been used in a prior study [14]. Probands were asked to provide permission to contact their relatives by providing their mailing addresses. Specifically, the FIF utilized in this study asked for the names, mailing addresses, vital status (alive versus deceased), approximate age or birth year, and exact relationship type for all FDR. Probands were given the opportunity to decline permission to contact their relatives and were asked to complete all aspects of the FIF except for the mailing addresses of the relatives they did not want contacted. All potential proband participants were not informed of their CC status, were assured that their relatives would not be informed of their responses, and were offered remuneration for participation with a copy of the Mayo Clinic Diet Book. The Mayo Clinic Survey Research Center was utilized for the proband recruitment process and for development of a database that linked probands with their respective FDR and the FIF information about the FDR. The opportunity to decline participation was contained in the study packet and any proband declining participation was not further contacted. Non-respondents were mailed reminder packets at three and six weeks from the time of the initial mailing. If there was no response after the two subsequent mailings, no further attempt at contact was made.
Relative Recruitment
Relatives that the proband provided permission to contact were mailed recruitment packets which contained an explanatory letter for the study; the Talley BDQ, and a consent form. The proband relatives were never informed of the CC status of their respective relative and were asked to complete the study packet regardless if they had bowel symptoms or not. FDR were excluded if they were younger than the age of 18 or if they resided outside of the United States. All potential FDR were offered remuneration for participation with a copy of the Mayo Clinic Diet Book. The opportunity to decline participation was contained in the study packet and any FDR declining participation was not further contacted. Non-respondents were mailed reminder packets at three and six weeks from the time of the initial mailing. If there was no response after the two subsequent mailings, no further attempt at contact was made. The Mayo Clinic Survey Research Center was again utilized to send out, receive and track the study packets mailed to FDR. Data collected for the study was entered in an electronic database using a double entry verification keypunch system. The same definition for CC used to identify case and control probands was applied to the FDR respondents.
Statistical Analysis
Several logistic regression models were used to assess potential response bias. First, the association of age and gender with response (no vs. yes) to the primary survey (2008–2009) was assessed. Then in the set of potential probands (cases and controls) identified, the association of age, gender, SSC score with proband status (case vs. control), and separately, with returning an FIF (no vs. yes) was assessed. In addition, among the probands that returned an FIF, the association of age, gender and SSC score with proband status (case vs. control) was checked. Finally, the association of survey response (no vs. yes) with age, gender, and corresponding proband status (case vs. control) in all FDR mailed a study packet was examined; this latter model accounting for potential within family correlations by identifying the family as a “cluster” (exchangeable correlation structure used).
The data obtained from FDR was assessed using logistic regression models ignoring the matching of case FDR and corresponding matched control FDR due to the limited number of “matched families” among the FDR respondents. The FDR questionnaire responses were used to identify those FDR meeting CC criteria. Two types of models were used, one in which the individual FDR status (CC vs. no CC) was the binary response and the status (case vs. control) of their respective proband was considered the primary predictor variable, with the FDR’s age, gender, BMI, smoking status, and alcohol use (none, <7, and ≥ 7 drinks per week with <7 as the reference level) included as covariates. This model accounted for potential within family correlations by identifying the family as a “cluster” (exchangeable correlation structure). A second model examined the ratio of the number of FDR family members meeting the CC criteria divided by the total number of FDR family members responding. In this model, only the respective proband status (case vs. control) of the family was included. The estimated coefficients from these models were used to calculate odds ratios (OR) (95% CI) for CC in FDR. The association of individual CC symptoms with the FDR corresponding proband status (case vs. control) was also assessed using a similar logistic model as the initial FDR model described above, but only using data from FDR meeting CC criteria. Finally, the “symptom burden” (number of constipation symptoms) in FDR meeting criteria for functional constipation were assessed using a model containing age, gender, SSC score, and FDR proband status to predict the number of symptoms each individual reported. This model accounted for within family correlations assuming an exchangeable correlation structure.
RESULTS
Participation and subject characteristics
A total of 8006 randomly selected Olmsted County residents were mailed a survey in 2008–2009 which included questions necessary to define CC as well as other FGIDs. Of these, a total of 3831 returned a questionnaire (response rate 48%). The odds for response were slightly greater in females and increased with age (p<0.05). Based on the responses to specific questions in the questionnaire, 1185 met the criteria for chronic constipation and did not meet criteria for IBS (case probands). A total of 1185 matched (age and gender) control probands were identified that did not meet criteria for CC or IBS. The SSC scores were associated with proband status with decreasing odds of being a case with increasing SSC score (OR [95% CI] per SSC score unit = 0.74 [0.64,0.85], p<0.001).
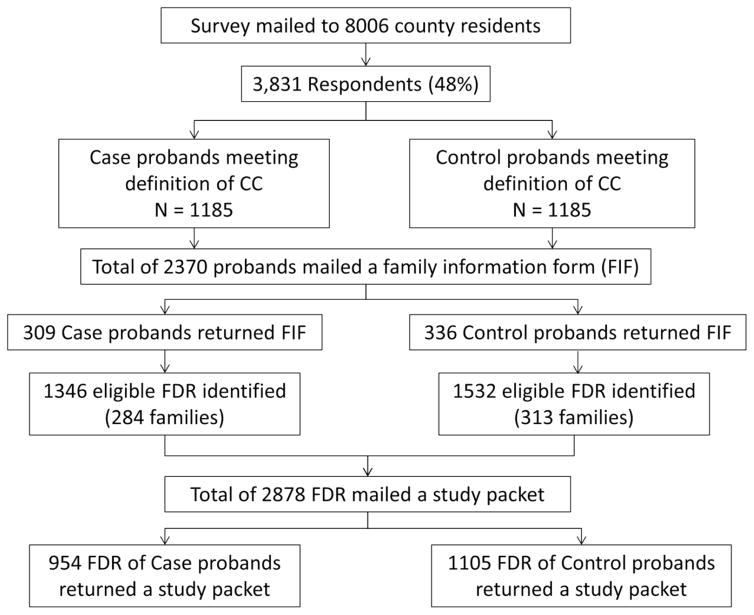
Figure 1 summarizes the recruitment of cases, controls, case-FDR, and control-FDR. Of the 1185 subjects with CC, 309 (26%) returned an FIF with a list of FDR available for contact while among the 1185 matched controls, 336 (28%) returned an FIF with a list of FDR available for contact. No association of response (returned FIF vs. not) and proband status (case vs. control) or SSC score was observed (p>0.1). However the odds for returning an FIF were increased in female probands (OR [95% CI] = 1.3 [1.1,1.6], p<0.001) and also increased with age (OR [95% CI] per 10 years = 1.14 [1.07,1.21], p<0.001). Sociodemographically, the case and control groups were similar to one another in regards to age, marital status, and race [Table 1].
Figure 1.
Flow diagram of subject recruitment
Table 1a.
Sociodemographic and symptom status of all probands from Olmsted County, MN.
| Cases (n=1185) | Controls (n=1185) | |
|---|---|---|
| Age (Median [range]) | 64 [25, 93] | 64 [25, 93] |
| Female | 584 (49%) | 584 (49%) |
| <3 BM/week | 269 (23%) | 189 (17%) |
| Hard or lumpy stools | 1042 (88%) | 647 (58%) |
| Strain | 1068 (90%) | 619 (54%) |
| Incomplete evacuation | 714 (60%) | 536 (47%) |
| Feel stool could not pass | 406 (34%) | 351 (31%) |
| Digitation | 263 (22%) | 196 (17%) |
| Laxatives | 334 (28%) | 304 (26%) |
| No loose or mushy stools | 472 (40%) | 411 (36%) |
Values in the table are N (%) unless otherwise noted.
The 309 case probands identified a total of 1392 FDR on their FIF of which 4 (0.3%) were underage, 37 (2.7%) resided outside of the United States and 5 (0.3%) were found to be deceased. This resulted in 1346 (96.7%) eligible case-FDR available for recruitment. The 336 control probands identified a total of 1565 FDR on their FIF. Of the identified control-FDR, 4 (0.3%) were underage, 25 (1.6%) resided outside of the United States and 4 (0.3%) were found to be deceased. This resulted in 1532 (97.8%) eligible control-FDR available for recruitment.
Out of the 1346 case-FDR mailed a recruitment packet, 954 (71%) agreed to participate and completed a questionnaire while a total of 1105 (72%) control-FDR agreed to participate and completed a questionnaire. Although recruitment rates were similar between case-FDR and control-FDR, the odds for participating were increased in females (OR=1.7 [1.5,2.0], p<0.001) in both FDR groups. The age, gender, and relationship with respective probands for the case-FDR and control-FDR that responded are summarized in Table 2.
Table 2.
Familial aggregation in First Degree Relatives (FDR) who returned a Family Information Form (FIF).
| % FDR with CC | OR | p-value | |
|---|---|---|---|
|
| |||
| Proband Status: | |||
| Case | 279 (29%) | 1.17 (0.95,1.43) † | 0.14 |
|
| |||
| Control | 291 (27%) | 1.0 (ref) | |
|
| |||
| Model with age per 10 years, female (vs. male) and proband by gender status | |||
|
| |||
| Proband by gender Status: | |||
| Case male | 125 (28%) | 1.17 (0.88,1.55) | 0.28 |
|
| |||
| Case female | 154 (31%) | 1.42 (1.07,1.89) | 0.01 |
|
| |||
| Control male | 137 (29%) | 1.0 ref for case male | |
|
| |||
| Control female | 154 (25%) | 1.0 ref for case female | |
|
| |||
| Age < 60 | 251 (31%) | 1.11 (1.04,1.17) * | 0.001 |
|
| |||
| Age >= 60 | 319 (26%) | ||
|
| |||
| Male | 298 (33%) | 1.0 (ref) | |
|
| |||
| Female | 272 (24%) | 0.63 (0.52,0.76) | <0.001 |
|
| |||
| Each variable below is adjusted for age per 10 years, female (vs. male) and proband by gender status (case male, case female, control male, control female) | |||
|
| |||
| BMI < 25 | 187 (25%) | 0.99 (0.98,1.02) + | 0.94 |
|
| |||
| BMI >= 25 | 368 (29%) | ||
|
| |||
| Smoking status: | |||
| Never smoked | 348 (28%) | 1.0 (ref) | |
|
| |||
| Current smoker | 37 (23%) | 0.82 (0.57,1.20) | 0.31 |
|
| |||
| Past smoker | 181 (29%) | 0.98 (0.78,1.23) | 0.90 |
|
| |||
| Alcohol: | |||
| No alcohol | 284 (29%) | 1.08 (0.86,1.34) | 0.51 |
|
| |||
| 1–6 drinks/week | 201 (27%) | 1.0 (ref) | |
|
| |||
| >= 7 drinks/week | 82 (28%) | 0.92 (0.69,1.23) | 0.58 |
|
| |||
| Education: | |||
| Less than HS | 25 (37%) | 1.28 (0.78,2.10) | 0.33 |
|
| |||
| HS or some college | 277 (29%) | 1.0 (ref) | |
|
| |||
| College or pro training | 265 (26%) | 0.88 (0.71,1.08) | 0.21 |
|
| |||
| Married | 409 (28%) | 0.93 (0.74,1.16) | 0.51 |
|
| |||
| Not married | 157 (28%) | 1.0 (ref) | |
|
| |||
| Employment: | |||
| Employed | 332 (26%) | 1.0 (ref) | |
|
| |||
| Not employed | 27 (20%) | 0.74 (0.48,1.15) | 0.18 |
|
| |||
| Retired | 209 (33%) | 1.25 (0.94,1.67) | 0.13 |
per 10 years of age
per 1 unit of BMI
model adjusted for age per 10 years, and female (vs. male)
Familial aggregation of chronic constipation
Overall, 279 (29%) of the 954 case-FDR met symptom criteria for chronic constipation, compared to 291 (26%) of the 1105 control-FDR who met the criteria for chronic constipation based on their questionnaire responses. The corresponding proband status of FDR was not associated with the FDR reporting symptoms consistent with CC in the individual FDR logistic model (OR=1.2 [0.9,1.4], p=0.14), or in the family unit based logistic model (OR=1.1 [0.9,1.4], p=0.20). The odds for CC did depend on gender of the proband, i.e. an “interaction” was detected. Specifically, the odds for CC in FDR of case proband females were increased relative to FDR of control proband females (OR=1.4 [1.1,1.9], p=0.01), but not for FDR of case proband males (relative to control proband males).
Influence of case-control status on symptoms of constipation for relatives
Table 3 summarizes the comparison of individual symptoms of constipation between case-FDR with CC and control-FDR with CC. Symptoms of constipation were similar and no significant differences were detected for any individual symptom. Symptom burden was associated with gender and SSC score but not age or FDR proband status.
Table 3.
Symptom burden in first-degree-relatives (FDR) with chronic constipation (CC)
| Number of chronic constipation symptoms | |||
|---|---|---|---|
|
| |||
| 2 symptoms n (%) |
3 symptoms n (%) |
4 symptoms n (%) |
|
|
| |||
| Male (n=298) | 161 (54.0%) | 115 (38.6%) | 22 (7.4%) |
| Female# (n=272) | 103 (37.9%) | 119 (43.8%) | 50 (18.4%) |
|
| |||
| Age ≤ 57§ (n=293) | 133 (45.4%) | 124 (42.3%) | 36 (12.3%) |
| Age > 57 (n=277) | 131 (47.3%) | 110 (39.7%) | 36 (13.0%) |
|
| |||
| Case (n=279) | 129 (46.2%) | 115 (41.2%) | 35 (12.5%) |
| Control (n=291) | 135 (46.4%) | 119 (40.9%) | 37 (12.7%) |
|
| |||
| SSC* ≤ 0.67§ (n=291) | 156 (53.6%) | 107 (36.8%) | 28 (9.6%) |
| SSC > 0.67 (n=279) | 108 (38.7%) | 127 (45.5%) | 44 (15.8%) |
Overall median value
p<0.001(Spearman correlation)
p<0.001(chi-square test)
SSC, somatic symptom checklist
DISCUSSION
CC is a common GI disorder in the community that poses a significant burden on both patients as well as healthcare resources. The etiopathogenesis of CC remains unclear and various risk factors have been described including physical activity, lower socioeconomic status, history of abuse, non-narcotic analgesic use, female gender, and age [3,8,26]. However, the potential roles of intrafamilial learning and genetics in functional GI disorders have been of growing interest given the consistent demonstration of familial aggregation in disorders such as IBS [10–14]. A population-based investigation to determine familial clustering in CC is crucial given that although CC is common in the community, many do not actively seek health care for their symptoms. The aim of this study was to determine whether CC clusters in families in subjects from Olmsted County, Minnesota.
Over 600 case and control probands participated in this study. The proportion of family members having CC was not associated with case status, and the constipation status of FDR was not significantly associated with case-controls status of the respective proband. Furthermore, the symptoms as well as symptom burden of chronic constipation did not appear to differ between those with a positive family history of constipation. Therefore, this novel population-based study did not detect familial aggregation occurring in subjects from the community with chronic constipation.
Familial clustering of functional GI disorders may be explained by shared disease susceptibility genes among family members; shared disease susceptibility genes for another disease entity that may increase the risk of the GI disorder of interest; shared household environment, shared behaviors, or shared household experiences; or a combination of these various factors [14]. Our negative findings are important given the positive findings in IBS [10–14], because the data suggest chronic constipation and constipation-predominant IBS are separate entities in the community. Our results differ from limited studies in Asia but this likely reflects the differing “populations” that are being investigated. The study by Chan et al. was in a specialty clinic in a tertiary referral center [15]. Patients with CC who present for care at a referral center have characteristics that differ significantly from those with CC in the community who do not seek referral based care. For example, women who are evaluated at a referral-based gastroenterology practice have an increased risk for a history of physical and sexual abuse [27]. This may highlight the possibility that differing intrafamilial environment may be significant in those with CC who seek referral based care and those that do not. These differing environments may explain our negative findings from a population-based perspective but the apparently positive findings from referral based settings [15–17].
There are a few potential limitations and weaknesses of our study. First, the response rate of our case and control probands was not as high as we had hoped. Our lower response rate may reflect a few factors. First, Olmsted County residents who typically respond to a particular survey, have previously responded to an average of 4.5 different surveys in the previous year and approximately a quarter of these respondents find these surveys to be burdensome [28]. Furthermore, the likelihood of future participation in health-related surveys in this community has been found to be negatively related to good health status, a busy schedule, and perceptions that a survey is too long [28]. Therefore, our lower participation rate may reflect “survey burnout” in this community. We did attempt to improve our potential response rate with the inclusion of remuneration in the form of a copy of the Mayo Clinic Diet Book. However, this was provided following the completion of a survey. Prepaid incentive by providing the book with the initial study packet may have improved our response rate as prepaid monetary incentive has been previously demonstrated to improve survey response rates in the community [29]. Another potential limitation is the high prevalence of respondents who met the criteria for chronic constipation, which raises the possibility of misclassification bias. In addition, our data may not be applicable to the entire U.S. population given that the racial composition of this community is predominantly white. However, major response bias was not apparent when responders were compared with non-responders. Further, we have shown that response bias is highly unlikely even with lower response rates in this population to GI surveys [30]. We acknowledge the study was conducted in one localized cohort of one country comprising mostly middle class Caucasians, and thus the results of this study cannot be generalized universally. Our results however should apply to the general Caucasian population in the United States. Further studies are needed to investigate the potential role of genetics and familial risk factors which may lead to more targeted therapies in different patient populations.
In conclusion, this population-based study did not demonstrate familial aggregation of chronic constipation in this community. Our results may highlight the differences between CC patients seen in the referral-based setting and those in the general community who do not seek care for their symptoms. The findings further suggest that IBS with constipation and chronic constipation have different familial risk factors. Additional population-based studies are needed to validate our findings.
Table 1b.
Sociodemographic and symptom status of all Probands from Olmsted County, MN Probands who returned the Family Information Form
| Cases (n=309) | Controls (n=336) | |
|---|---|---|
| Age (Median [range]) | 66 [27, 91] | 66 [25, 91] |
| n (%) | n (%) | |
| Female | 154 (45%) | 187 (55%) |
| Male | 155 (51%) | 149 (49%) |
| < 3 BM/week§ | 60 (56%) | 48 (44%) |
| Not < 3 BM/week | 246 (47%) | 273 (53%) |
| Hard or lumpy stools§ | 270 (60%) | 183 (40%) |
| No hard or lumpy stools | 37 (21%) | 137 (79%) |
| Straining§ | 279 (60%) | 185 (40%) |
| No straining | 30 (18%) | 134 (82%) |
| Incomplete evacuation§ | 186 (55%) | 153 (45%) |
| No incomplete evacuation | 121 (42%) | 166 (58%) |
| Anal blockage§ | 102 (52%) | 95 (48%) |
| No blockage | 205 (48%) | 225 (52%) |
| Digitation§ | 79 (59%) | 54 (41%) |
| No digitation | 230 (46%) | 265 (54%) |
| Laxatives | 88 (48%) | 96 (52%) |
| No laxatives | 221 (48%) | 250 (52%) |
| No loose or mushy stools | 119 (48%) | 109 (52%) |
| Loose or mushy stools§ | 190 (47%) | 212 (53%) |
variables defined as never vs. (sometimes or more)
Values in the table are N (%) unless otherwise noted.
Acknowledgments
Grant Support: Study data were obtained from the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Dr. N. J. Talley and Mayo Clinic have licensed the Talley Bowel Disease Questionnaire.
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in north america: A systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Locke GR, 3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Go VL, Johannes RS, et al. A longitudinal survey of self-reported bowel habits in the united states. Dig Dis Sci. 1989;34:1153–1162. doi: 10.1007/BF01537261. [DOI] [PubMed] [Google Scholar]
- 4.Nyrop KA, Palsson OS, Levy RL, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 5.Rantis PC, Jr, Vernava AM, 3rd, Daniel GL, Longo WE. Chronic constipation--is the work-up worth the cost? Dis Colon Rectum. 1997;40:280–286. doi: 10.1007/BF02050416. [DOI] [PubMed] [Google Scholar]
- 6.Chang JY, Locke GR, 3rd, McNally MA, et al. Impact of functional gastrointestinal disorders on survival in the community. Am J Gastroenterol. 2010;105:822–832. doi: 10.1038/ajg.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med. 2011;124:714–723. doi: 10.1016/j.amjmed.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. doi: 10.1038/ajg.2011.164. quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 9.Wong RK, Palsson OS, Turner MJ, et al. Inability of the rome iii criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228–2234. doi: 10.1038/ajg.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalantar JS, Locke GR, 3rd, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: A prospective study. Gut. 2003;52:1703–1707. doi: 10.1136/gut.52.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy RL, Jones KR, Whitehead WE, et al. Irritable bowel syndrome in twins: Heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., 3rd Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 13.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 14.Saito YA, Petersen GM, Larson JJ, et al. Familial aggregation of irritable bowel syndrome: A family case-control study. Am J Gastroenterol. 2010;105:833–841. doi: 10.1038/ajg.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AO, Hui WM, Lam KF, et al. Familial aggregation in constipated subjects in a tertiary referral center. Am J Gastroenterol. 2007;102:149–152. doi: 10.1111/j.1572-0241.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 16.Chan AO, Lam KF, Hui WM, et al. Influence of positive family history on clinical characteristics of functional constipation. Clin Gastroenterol Hepatol. 2007;5:197–200. doi: 10.1016/j.cgh.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Ostwani W, Dolan J, Elitsur Y. Familial clustering of habitual constipation: A prospective study in children from west virginia. J Pediatr Gastroenterol Nutr. 2010;50:287–289. doi: 10.1097/MPG.0b013e3181a0a595. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ., 3rd History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the rochester epidemiology project: Half a century of medical records linkage in a us population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: The bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 21.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 22.Reilly WT, Talley NJ, Pemberton JH, Zinsmeister AR. Validation of a questionnaire to assess fecal incontinence and associated risk factors: Fecal incontinence questionnaire. Dis Colon Rectum. 2000;43:146–153. doi: 10.1007/BF02236971. discussion 153–144. [DOI] [PubMed] [Google Scholar]
- 23.Choung RS, Locke GR, 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: A psychological component is the rule. Am J Gastroenterol. 2009;104:1772–1779. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the sunya revision of the psychosomatic symptom checklist. J Behav Med. 1984;7:247–257. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 25.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Chang JY, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Risk factors for chronic constipation and a possible role of analgesics. Neurogastroenterol Motil. 2007;19:905–911. doi: 10.1111/j.1365-2982.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 28.Beebe TJ, Jenkins SM, Anderson KJ, Davern ME. Survey-related experiential and attitudinal correlates of future health survey participation: Results of a statewide survey. Mayo Clin Proc. 2008;83:1358–1363. doi: 10.1016/S0025-6196(11)60784-2. [DOI] [PubMed] [Google Scholar]
- 29.Beebe TJ, Davern ME, McAlpine DD, Call KT, Rockwood TH. Increasing response rates in a survey of medicaid enrollees: The effect of a prepaid monetary incentive and mixed modes (mail and telephone) Med Care. 2005;43:411–414. doi: 10.1097/01.mlr.0000156858.81146.0e. [DOI] [PubMed] [Google Scholar]
- 30.Choung R, Locke GR, III, Schleck C, et al. A low response rate does not necessarily indicate non-response bias in gastroenterology survey research: A population-based study. J Public Health. 2013;21:87–95. [Google Scholar]