Abstract
The present study aimed to investigate the effects of endogenous hydrogen sulfide (H2S) on the expression levels of angiotensin II type 1 receptor (AGTR1) in a rat model of carbon tetrachloride (CCl4)-induced hepatic fibrosis. A total of 56 Wistar rats were randomly divided into four groups: Normal control group, model group, sodium hydrosulfide (NaHS) group, and DL-propargylglycine (PAG) group. Hepatic fibrosis was induced by CCl4. The rats in the PAG group were intraperitoneally injected with PAG, an inhibitor of cystathionine-γ-lyase (CSE). The rats in the NaHS group were intraperitoneally injected with NaHS. An equal volume of saline solution was intraperitoneally injected into both the control and model groups. All rats were sacrificed at week three or four following treatment. The serum levels of hyaluronidase (HA), laminin protein (LN), procollagen III (PcIII), and collagen IV (cIV) were detected using ELISA. The serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and albumin (ALB) were detected using an automatic biochemical analyzer. The liver mRNA expression levels of CSE were detected by reverse transcription-quantitative polymerase chain reaction. The liver expression levels of AGTR1 and the plasma expression levels of H2S were detected using western blot analyses. The results indicated that the severity of hepatic fibrosis, the serum expression levels of HA, LN, PcIII, cIV, ALT, and AST, the liver expression levels of CSE and AGTR1, and the plasma expression levels of H2S were significantly higher in the PAG group, as compared with the model group (P<0.05). Conversely, the expression levels of ALB were significantly lower in the PAG group, as compared with the model group. In addition, the severity of hepatic fibrosis, the serum expression levels of HA, LN, PcIII, cIV, ALT, and AST, the liver expression levels of CSE and AGTR1, and the plasma expression levels of H2S were significantly lower in the NaHS group, as compared with the model group (P<0.05). These results suggest that endogenous H2S is associated with CCl4-induced hepatic fibrosis in rats, and may exhibit anti-fibrotic effects. Furthermore, H2S reduced the liver expression levels of AGTR1, which may be associated with the delayed progression of hepatic fibrosis.
Keywords: DL-propargylglycine, sodium hydrosulfide, carbon tetrachloride, hepatic fibrosis, angiotensin II type 1, hydrogen sulfide, cystathionine-β-synthase
Introduction
Hydrogen sulfide (H2S) has previously been identified as a signaling molecule that exhibits numerous physiological and pathological activities (1). Exogenous sodium hydrosulfide (NaHS) releases H2S, in order to induce physiological responses. Previous studies have demonstrated that H2S may relax vascular and ileal smooth muscle in the cardiovascular system (2), increase colonic secretion and reduce gastric injury in the gastrointestinal system (3), attenuate neuronal injury (4), and prevent the development of hypertension (5). The roles of H2S also include the inhibition of oxidative stress (6), the production of lipid peroxidation and inflammatory factors (7), and the activation of ATP-sensitive potassium channels (KATP) (8).
H2S regulates hepatic-related injury, and is produced in the liver by both cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), which use L-cysteine as a substrate to produce H2S. H2S exhibits anti-inflammatory and cytoprotective activities against hepatic ischemia reperfusion (I/R) injury (9). Carbon tetrachloride (CCl4)-induced downregulation of H2S production and CSE expression is associated with the development of increased intrahepatic resistance, portal hypertension, and hepatic fibrosis in a rat model of liver cirrhosis (10). A previous study suggested that H2S and CSE exhibit anti-fibrotic effects in pulmonary fibrosis (11). However, to the best of our knowledge, there have been few attempts to investigate the role of H2S in hepatic fibrosis.
Hepatic fibrosis is a common response to chronic liver injury caused by various diseases. The mechanisms underlying the development of hepatic fibrosis consist predominantly of the activation of hepatic stellate cell (HSCs), and the accumulation of extracellular matrix components within the liver (12). The renin angiotensin system (RAS) is involved in the pathogenesis of fibrosis, both in the heart and various other organs (13,14). Notably, activated human HSCs express RAS components and synthesize angiotensin II. Furthermore, angiotensin II type 1 receptors (AGTR1) are located in HSCs (15). Previous studies have demonstrated that the control of RAS activation by AGTR1 antagonists may have therapeutic potential in the treatment of hepatic fibrosis. H2S and NaHS decrease AGTR1 binding, as well as AGTR1 binding affinity in spontaneously hypertensive rats (16). The present study aimed to investigate whether H2S affects hepatic fibrosis by regulating the expression of AGTR1.
Materials and methods
Materials and reagents
Pathogen-free male Wistar rats (weighing 200–300 g) were purchased from XiPuer-Rubicam Experimental Animals, Ltd. (Shanghai, China). Analytical grade CCl4 and DL-propargylglycine (PAG) were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing, China). Analytical grade zinc cetate, N,N-dimethy-p-phenylenediamine, HCl, trichloroacetic acid, and NaHS were purchased from Sigma-Aldrich (St. Louis, MO, USA). The rat ELISA kit was purchased from Sigma-Aldrich, the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) kit was obtained from Takara Bio, Inc. (Tokyo, Japan), and the Western Blotting kit was from Abcam (Cambridge, UK). The current study was approved by the ethics committee of Shanghai Jiaotong University Affiliated Sixth People's Hospital (Shanghai, China).
Experimental design
The rats were randomly divided into four groups (n=14/group): Normal control group, model hepatic fibrosis group, NaHS group, and PAG group. NaHS is a H2S donor, and PAG is a CBS inhibitor. Each group was then randomly divided into two subgroups each containing seven rats; one of the subgroups received treatment for three weeks, whereas the other one received treatment for four weeks. The rats were maintained in a sterile environment with ad libitum access to drinking water, and underwent a 12 h light/dark cycle. Hepatic fibrosis was induced using 5 ml/kg 40% CCl4 in corn oil tree time weekly for three or four weeks in all groups, except for the normal control group. The rats in the PAG group were intraperitoneally injected with 45 µmol/kg/day PAG, a CBS inhibitor. The rats in the NaHS group were intraperitone-ally injected with 56 µmol/kg/day NaHS, H2S donor. An equal volume of saline was intraperitoneally injected into the control and model group rats. The rats were then sacrificed with 2% pentobarbital (H. Lundbeck Co., Copenhagen, Denmark) at week three or four following modeling, depending on their subgroup.
Serum biochemical measurements
The serum expression levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB) were determined using commercially available kits (Diatech Diagnostics, Hungary) using the Autobiochemical Analyzer (Toshiba, Tokyo, Japan) following centrifugation at 3,000 × g at 4°C of the blood collected at 4,000 rpm for 15 min. The serum expression levels of hyaluronidase (HA), laminin protein (LN), procollagen III (PcIII), and collagen IV (cIV) were measured using an ELISA kit.
Histopathological examination
The livers were removed and washed with saline to remove excess blood. Liver tissue sections (5 µm) were fixed in formalin and embedded in paraffin prior to examination. The liver sections were then stained using hematoxylin and eosin (HE) and Masson's trichrome (Wuhan Biotechnology Ltd., Co., Wuhan, China), in order to determine the stage of hepatic fibrosis. The fibrotic stages were scored according to Brunt (17): S0, no fibrosis; S1, portal fibrous expansion; S2, thin fibrous septa emanating from portal triads; S3, fibrous septa bridging portal triads and central veins; S4, cirrhosis.
RT-qPCR
RT-qPCR was performed in order to analyze the mRNA expression levels of CSE in hepatic tissue, as previously described (18). Total RNA (2 µg) was extracted from the hepatic samples and reverse transcribed using the RevertAid First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's instructions. The PCR primer sequences from Takara Bio, Inc. (Tokyo, Japan) were as follows: CSE, forward 5′-CCACCACAACGATTACCCA-3′, reverse 5′-TCAGCACCCAGAGCCAAAG-3′; and β-actin, forward 5′-TCCTGACCCTGAAGTACCCCATTG-3′, and reverse 5′-GGAACCGCTCATTGCCGATAGT-3′. RT-qPCR was performed using the SYBR Green qPCR Super Mixture (Takara Bio, Inc.) and an ABI Prism 7500 Sequence Detection System (Applied Biosystems Life Technologies, Foster City, CA, USA). Amplification was performed with the following cycles: 95°C for 2 min, followed by 40 cycles of denaturing at 95°C for 15 sec, and annealing at 60°C for 32 sec. All reactions were performed in triplicate. Data analysis was performed using the 2−ΔΔCT method, as described by Livak and Schmittgen (19), with β-actin acting as a reference gene.
Western blot analysis
The protein expression levels of AGTR1 were detected by western blotting. The membrane protein fractions were prepared as previously described (20). Briefly, 100 mg liver tissue samples were first homogenized ultrasonically for 4 min using a Tissue Pulverizer (BioSpec Products, Bartlesville, OK, USA). The samples were then lysed in radioimmunoprecipitation buffer, separated by 10% SDS-PAGE and electro-transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with 5% non-fat dry milk in tris-buffered saline containing Tween® 20 [TBST; 10 mmol/l Tris-hydrochloric acid (HCl) pH 8.0, 150 mmol/l NaCl, and 1% Tween® 20]. The membranes were probed with a rabbit polyclonal AGTR1 antibody (cat. no. sc-1173; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a dilution of 1:1,000 in blocking solution (10 mM Tris-HCl, 5% powdered milk, 2% bovine serum albumin, 0.1% Tween® 20, pH 7.6; Sigma-Aldrich). Following extensive washing with TBST, the membranes were incubated for 1 h with a secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (cat. no. A6154; Sigma-Aldrich) at a dilution of 1:2,000 in phosphate-buffered saline (PBS). After a final washing step with TBST, the blots were visualized using an enhanced chemiluminescence kit (GE Healthcare Life Sciences, Chalfont, UK). In order to detect β-actin, the membranes were stripped with 0.2% SDS, 0.1% mercaptoethanol, and 1 M Tris pH 6.8 for 30 min at 70°C, prior to being incubated with a mouse monoclonal β-actin antibody (cat. no. A5441, Sigma-Aldrich) at a dilution of 1:4,000 in blocking solution for 1 h. The membranes were then incubated with the HRP-conjugated goat anti-rabbit antibody (cat. no. A6154, Sigma-Aldrich) at a dilution of 1:2,000 in PBS for 1 h. The results of the enhanced chemiluminescence analysis were digitized by conventional scanning, and quantified using computerized image analysis software Alpha Imager 2000, according to the manufacturer's instructions. The densitometric results were expressed in relation to the ratio of AGTR1 and β-actin.
Measurement of H2S serum levels
The serum levels of H2S were measured as described previously (13). Briefly, 75 µl aliquots of sera were mixed with 100 µl distilled water, and 300 µl 10% trichloroacetic acid. The reaction was terminated by the addition of 150 µl 1% zinc acetate. A total of 20 µM N,N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl, and FeCl3 (30 µM; 133 µl) in 1.2 M HCl was then added to the solution. Following a 15 min incubation at room temperature, the absorbance of the resulting solution was measured using a UV-2550 spectrophotometer at 670 nm (Shimadzu Corporation, Tokyo, Japan). All samples were assayed in triplicate, and the levels of H2S were calculated against the NaHS calibration curve (0.122–250 µM).
Statistical analysis
The results are expressed as the mean ± standard deviation. The data were analyzed by a one-way analysis of variance followed by Newman-Keuls comparisons using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of H2S on CCl4-induced hepatic fibrosis
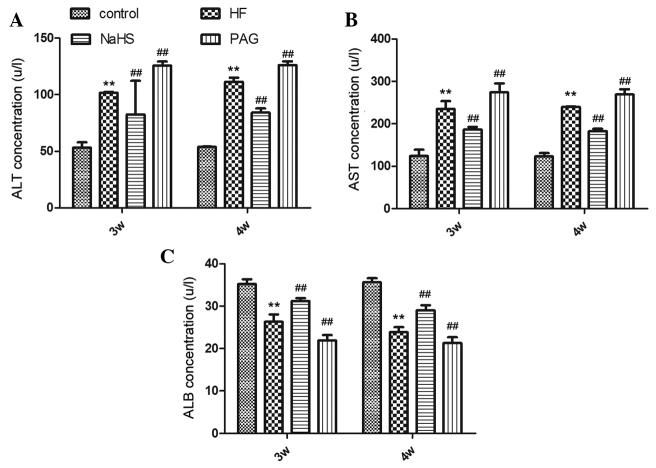
Treatment with NaHS significantly attenuated the CCl4-induced serum expression levels of ALT and AST, whereas PAG increased the serum expression levels of AST and ALT. Conversely, the serum expression levels of ALB were decreased in the PAG group, and increased in the NaHS group (Fig. 1). The observed CCl4-induced impaired liver function and the protective effects of H2S were supported by histological alterations. The liver sections from the normal control group rats exhibited normal histology (Table I), whereas the liver sections from the CCl4-induced model group rats exhibited focal necrosis and degeneration. NaHS delayed both necrosis and vacuolization, whereas PAG aggravated hepatic injury and caused increased inflammatory cell infiltration.
Figure 1.
Serum expression levels of (A) alanine transaminase, (B) aspartate transaminase, and (C) albumin for each group. The error bars indicated the mean ± standard deviation (**P<0.01 vs. normal control group, ##P<0.01 vs. the model group). NaHS, sodium hydrosulfide; PAG, DL-propargylglycine; HF, hepatic fibrosis.
Table I.
Number of rats present in each stage of hepatic fibrosis for each group.
| Groups | S0 | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| Normal control group | 14 | ||||
| Model group (3 w) | 3 | 4 | |||
| NaHS group (3 w) | 2 | 2 | 3 | ||
| PAG group (3 w) | 2 | 5 | |||
| Model group (4 w) | 3 | 4 | |||
| NaHS group (4 w) | 1 | 2 | 3 | 1 | |
| PAG group (4 w) | 2 | 5 |
NaHS, sodium hydrosulfide; PAG, DL-propargylglycine; S, stage; w, weeks.
Effects of NaHS and PAG on CCl4-induced hepatic fibrosis
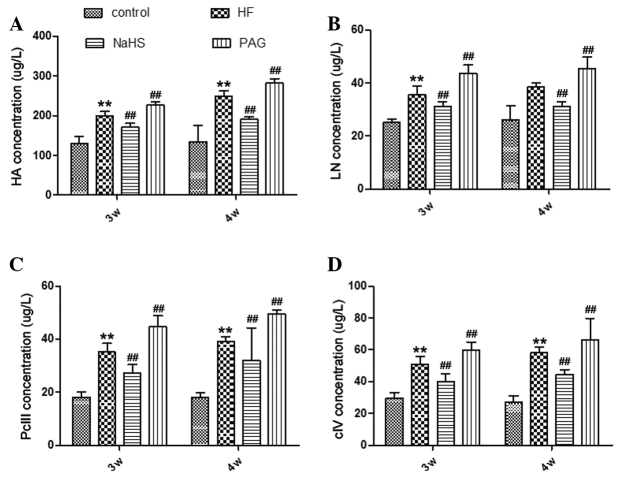
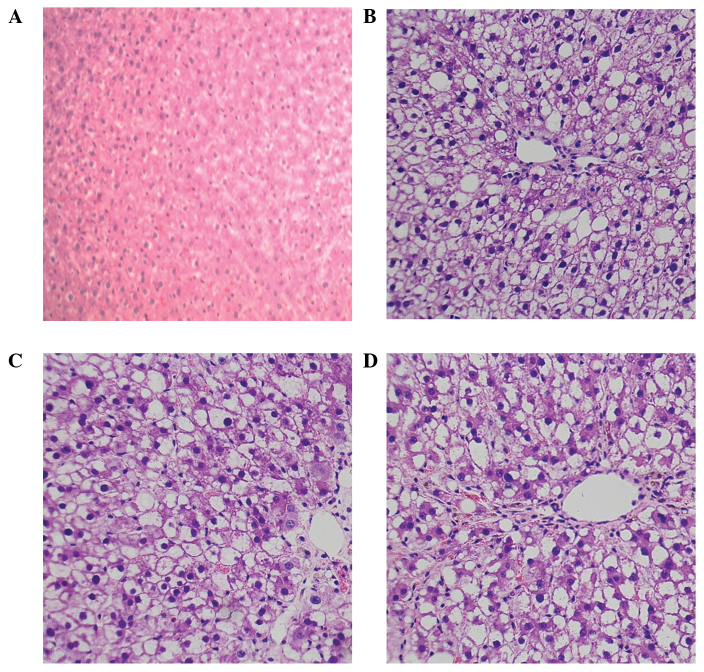
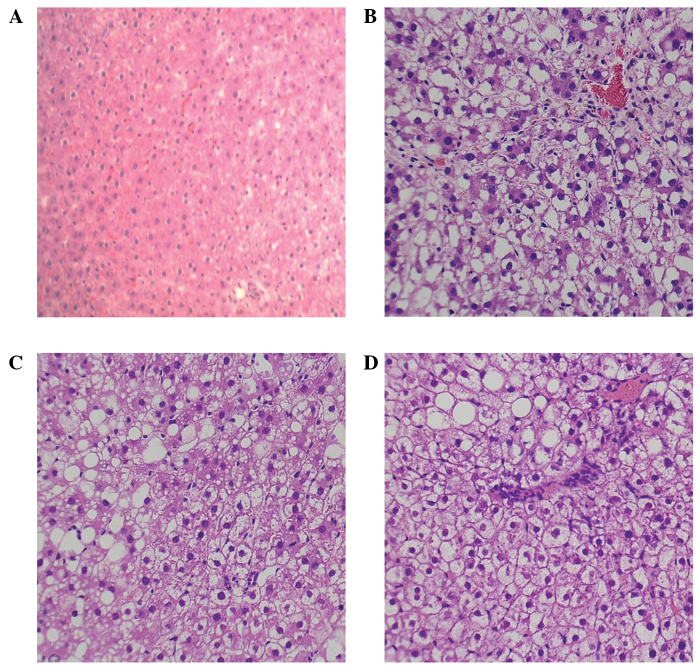
Evidence of liver injury in the model group, which received an intraperitoneal injection of CCl4, was indicated by significantly increased serum expression levels of HA, LN, PcIII, and cIV (Fig. 2), indicative of fibrosis. Pathological damage was evident when compared with the control group. Treatment with PAG resulted in increased serum expression levels of HA, LN, PcIII, and cIV, as compared with the control group, whereas treatment with NaHS resulted in significantly lower serum expression levels of HA, LN, PcIII, and cIV compared with the model group (Fig. 2). The results of HE staining demonstrated that the PAG group exhibited early-onset necrosis and increased inflammatory cell infiltration, as compared with the control group (Figs. 3 and 4). The subgroup treated for four weeks with PAG exhibited severe liver necrosis. The number of blue pixels in the Masson-stained liver sections was measured in order to quantify the amount of collagen fibers. The results of the Masson staining of the control group liver sections suggested a near absence of collagen fibers, whereas treatment with CCl4 significantly increased the number of blue pixels in the liver sections, suggesting a large number of collagen fibers were present. Treatment with NaHS resulted in a significantly lower number of blue pixels, whereas treatment with PAG was associated with a significantly higher number of blue pixels, as compared with the control and model groups (Figs. 5 and 6).
Figure 2.
Serum expression levels of (A) hyaluronidase, (B) laminin protein, (C) procollagen III, and (D) collagen IV for each group. The error bars indicated the mean ± standard deviation (**P<0.01 vs. normal control group, ##P<0.01 vs. the model group). NaHS, sodium hydrosulfide; PAG, DL-propargylglycine; HF, hepatic fibrosis.
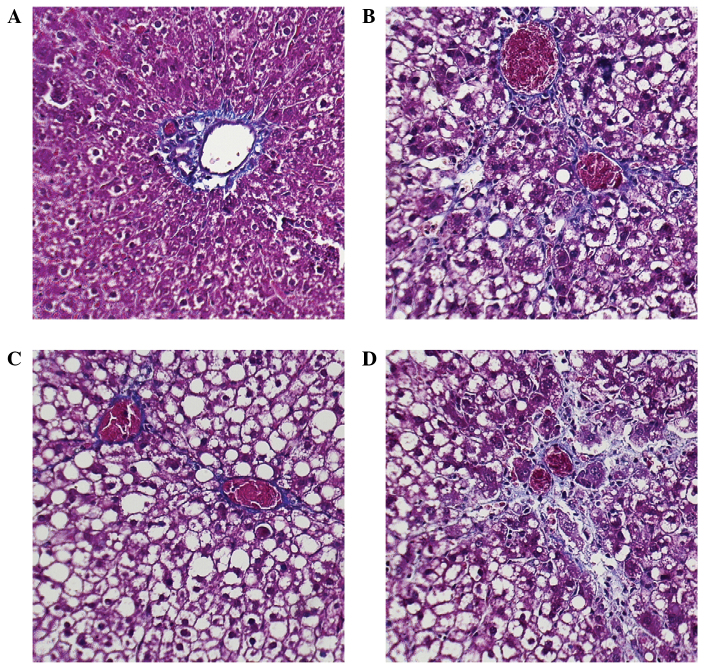
Figure 3.
Pathology of the hepatic tissues for each group at week three. The tissues were stained with hematoxylin and eosin (magnification ×200). (A) Control group, (B) model group, (C) sodium hydrosulfide group, (D) DL-propargylglycine.
Figure 4.
Pathology of the hepatic tissues for each group at week four. The tissues were stained with hematoxylin and eosin (magnification ×200). (A) Control group, (B) model group, (C) sodium hydrosulfide group, (D) DL-propargylglycine.
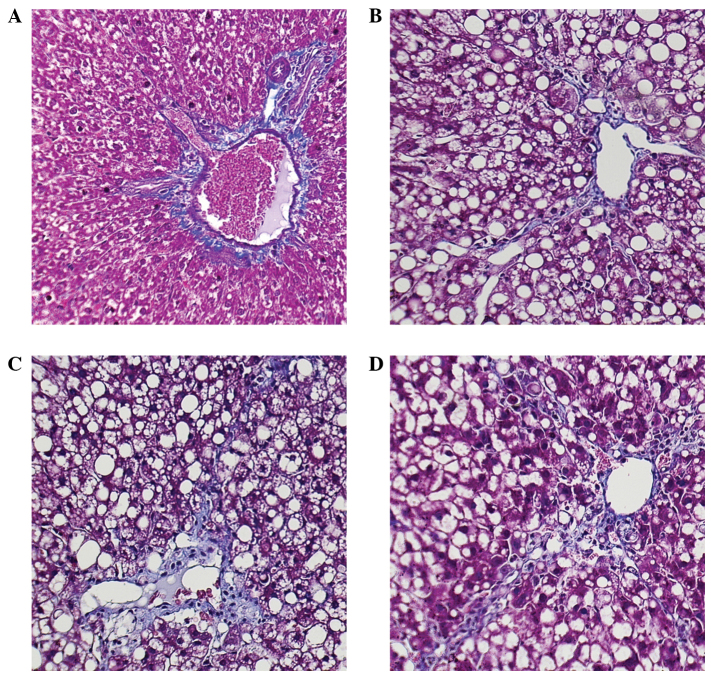
Figure 5.
Pathology of the hepatic tissues for each group at week three. The tissues were stained with Masson's trichrome (magnification ×200). (A) Control group, (B) model group, (C) sodium hydrosulfide group, (D) DL-propargylglycine.
Figure 6.
Pathology of the hepatic tissues for each group at week three. The tissues were stained with Masson's trichrome (magnification ×200). (A) Control group, (B) model group, (C) sodium hydrosulfide group, (D) DL-propargylglycine.
Effects of NaHS and PAG on the mRNA expression levels of CSE and on H2S synthesis in rat liver
The gene encoding the H2S-forming enzyme CSE was detected in the rat liver, by RT-qPCR of liver RNA. The mRNA expression levels of CSE in the model group were markedly lower, as compared with the normal control group, these results were accentuated by PAG treatment. Conversely, in the NaHS group, CSE content was significantly increased compared with the model group (Fig. 7A). The serum expression levels of H2S in the CCl4-treated rats were markedly lower than in the normal control group, suggesting that CCl4 significantly reduced hepatic H2S-producing activity (Fig. 7B).
Figure 7.
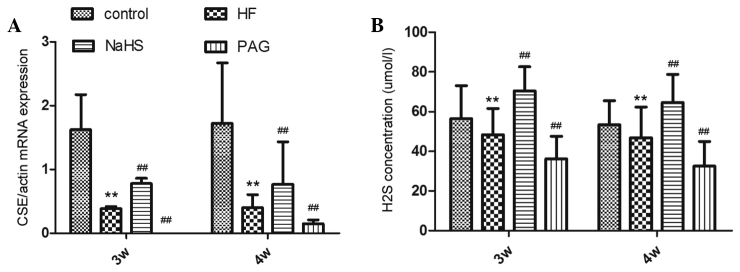
Expression levels of cystathionine-γ-lyase (CSE), hydrogen sulfide (H2S) and angiotensin II type 1 receptor (AGTR1) in rats with hepatic fibrosis. (A) The mRNA expression levels of CSE were measured in each group using reverse transcription-quantitative polymerase chain reaction, which demonstrated similar results between week three and four. (B) H2S levels were decreased in the DL-propargylglycine group, and conversely increased in the sodium hydrosulfide group, as compared with the control. The error bars indicated the mean ± standard deviation (**P<0.01 vs. normal control group, ##P<0.01 vs. the model group). HF, hepatic fibrosis.
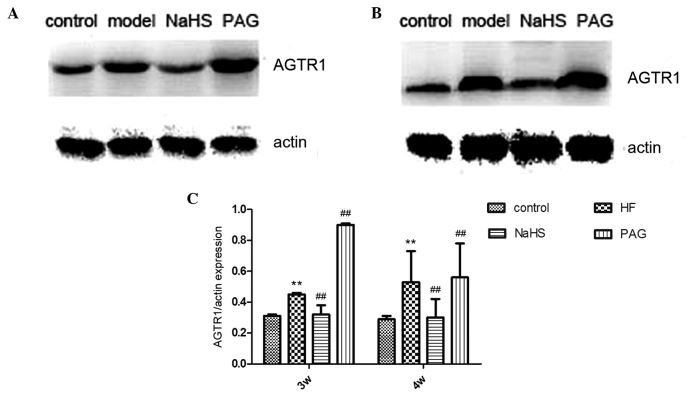
Effects of NaHS and PAG on the expression levels of AGTR1 in CCl4-induced hepatic fibrosis
The hepatic protein expression levels of AGTR1 were evaluated by immunoblot analysis. In the model group, CCl4-induced hepatic fibrosis resulted in the significant upregulation of AGTR1 expression, as compared with the normal control group. Conversely, treatment with NaHS resulted in a significant downregulation of AGTR1 expression, as compared with the control group; however, this finding was not significant. PAG treatment significantly increased the expression levels of AGTR1, as compared with the control group (Fig. 8).
Figure 8.
Protein expresssion levels of angiotensin II type 1 receptors (AGTR1) as determined by western blot analysis in the rat groups at (A) week three and (B) week four. Actin was used as an endogenous control. Actin was used as an endogenous control. (C) Quantification of AGTR1 expression levels. The error bars indicated the mean ± standard deviation (**P<0.01 vs. normal control group, ##P<0.01 vs. the model group). NaHS, sodium hydrosulfide; PAG, DL-propargylglycine.
Discussion
CCl4 is widely used to induce a chemical model of hepatic fibrosis. CCl4 is metabolized to a trichloromethyl radical, leading to increased lipid peroxidation, depletion of glutathione, impaired hepatic anti-oxidant activity, and hepatocyte necrosis (21). Fan et al (22) reported that H2S administration attenuated hepatic fibrosis and collagen I protein expression in rats exhibiting CCl4-induced hepatic fibrosis, inhibited cellular proliferation, and induced cell cycle arrest and apoptosis of activated HSCs. Jha et al (23) demonstrated that H2S significantly attenuated hepatic I/R injury via preservation of the intracellular redox balance and inhibition of apoptosis during I/R injury. These results suggested that H2S may serve as a promising therapeutic agent in the treatment of hepatic I/R injury.
HSCs have a crucial role in the onset of hepatic fibrosis. HSCs express AGTR1 (15), and are activated by the binding of angiotensin II to AGTR1, which in turn leads to the secretion of extracellular matrix components resulting in the development of hepatic fibrosis (24). Activated HSCs also express numerous cytokines, which accelerate hepatic inflammation (24).
Fibrogenesis in chronic liver disease is stimulated by angiotensin II via AGTR1, and may be modulated by angiotensin-converting enzyme inhibitors and AGTR1 antagonists (25,26). In the present study, advanced liver fibrosis was effectively induced by CCl4. The results of the present study demonstrated that the protein expression levels of AGTR1 were negatively correlated with the degree of liver fibrosis. Töx et al (27) showed that angiotensin II may influence transforming growth factor (TGF)-β-mediated processes via AGTR1, by enhancing Smad2 gene expression in the liver.
Tan et al (28) previously investigated the protective role of H2S on CCl4-induced acute hepatotoxicity, as well as the prophylactic and therapeutic effects of H2S on long-term CCl4-induced cirrhosis and portal hypertension, mediated by the multiple functions of H2S, including antioxidation, anti-inflammation, cytoprotection, and anti-fibrosis. The results of the study indicated that the use of H2S may provide potent therapeutic effects against liver cirrhosis and portal hypertension.
The regulation of sinusoidal resistance depends on the aggregation of HSCs around sinusoidal endothelial cells (29). A previous study demonstrated that H2S is an autocrine neurotransmitter that is involved in the regulation of HSC contraction and the maintenance of portal venous pressure via KATP channels (29). H2S counteracts impaired vasodilation and HSC contraction, thus reducing portal hypertension in cirrhotic livers (29).
Angiotensin II has been shown to increase the expression levels of hepatic TGF-β1 during the development of hepatic fibrosis (30). Connective tissue growth factor (CTGF) is a hepatic profibrotic mediator, which is a downstream target of TGF-β1 in HSCs (31,32). Tamaki et al (33) demonstrated that telmisartan (an AGTR1 receptor blocker) inhibited hepatic fibrosis, induced downregulation of tumour necrosis factor-α, TGF-β1, and CTGF mRNA expression, and reduced the number of α-smooth muscle actin-positive cells in the liver.
In conclusion, the results of the present study demonstrated that H2S was able to inhibit liver fibrosis, and hinder the formation of hepatic fibrosis. Downregulation of AGTR1 was closely associated with the progression of liver fibrosis, suggesting that H2S may inhibit the expression of AGTR1. The results of the present study contribute to the understanding of the protective effects of H2S in liver fibrosis, but whether the mechanisms underlying H2S protection, its correlation with angiotensin receptor inhibitors, and its application in human hepatic fibrosis has similar protective effects requires further study.
Acknowledgments
The present study was supported by grants from the National Nature Science Foundation of China (grant nos. 81302093 and 81272752).
References
- 1.Łowicka E, Bełtowski J. Hydrogen sulfide (H S) - the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 2.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorucci S, Antonelli E, Distrutti E, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastro-enterology. 2005;129(1):210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LM, Jiang CX, Liu DW. Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats. Neurochem Res. 2009;34:1984–1992. doi: 10.1007/s11064-009-0006-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhong G, Chen F, Cheng Y, et al. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 7.Lou LX, Geng B, Du JB, Tang CS. Hydrogen sulphide-induced hypothermia attenuates stress-related ulceration in rats. Clin Exp Pharmacol Physiol. 2008;35:223–228. doi: 10.1111/j.1440-1681.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhong GZ, Li YB, Liu XL, et al. Hydrogen sulfide opens the KATP channel on rat atrial and ventricular myocytes. Cardiology. 2010;115:120–126. doi: 10.1159/000260073. [DOI] [PubMed] [Google Scholar]
- 9.Kang K, Zhao M, Jiang H, et al. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S, Antonelli E, Mencarelli A, et al. The third gas: H S regulates perfusion pressure in both the isolated and perfused 2 normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 11.Fang L, Li H, Tang C, et al. Hydrogen sulfide attenuates the pathogenesis of pulmonary fibrosis induced by bleomycin in rats. Can J Physiol Pharmacol. 2009;87:531–538. doi: 10.1139/Y09-039. [DOI] [PubMed] [Google Scholar]
- 12.Tacke F, Weiskirchen R. Update on hepatic stellate cells: Pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol. 2012;6:67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- 13.Stock P, Liefeldt L, Paul M, Ganten M. Local renin-angiotensin systems in cardiovascular tissues: Localization and functional role. Cardiology. 1995;86(Suppl 1):2–8. doi: 10.1159/000176938. [DOI] [PubMed] [Google Scholar]
- 14.Yoshiji H, Kuriyama S, Yoshii J, et al. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745–750. doi: 10.1053/jhep.2001.28231. [DOI] [PubMed] [Google Scholar]
- 15.Bataller R, Sancho-Bru P, Ginès P, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117–125. doi: 10.1016/S0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Zhang LK, Zhang CY, et al. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res. 2008;31:1619–1630. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 17.Brunt EM. Nonalcoholic steatohepatitis: Definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Sun B, Chen H, et al. Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine. 2010;49:15–23. doi: 10.1016/j.cyto.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Nickenig G, Laufs U, Schnabel P, et al. Down-regulation of aortic and cardiac AT1 receptor gene expression in transgenic (mRen-2) 27 rats. Br J Pharmacol. 1997;121:134–140. doi: 10.1038/sj.bjp.0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009;30:29–41. doi: 10.1016/j.mam.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Fan HN, Wang HJ, Yang-Dan CR, et al. Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep. 2013;7:247–53. doi: 10.3892/mmr.2012.1153. [DOI] [PubMed] [Google Scholar]
- 23.Jha S, Calvert JW, Duranski MR. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaida I, Matsumura Y, Kubota M, et al. The prolyl 4-hydroxylase inhibitor HOE 077 prevents activation of Ito cells, reducing procollagen gene expression in rat liver fibrosis induced by choline-deficient L-amino acid-defined diet. Hepatology. 1996;23:755–63. doi: 10.1053/jhep.1996.v23.pm0008666329. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson JR, Clouston AD, Ando Y, et al. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148–155. doi: 10.1053/gast.2001.25480. [DOI] [PubMed] [Google Scholar]
- 26.Tuncer I, Ozbek H, Ugras S, Bayram I. Anti-fibrogenic effects of captopril and candesartan cilexetil on the hepatic fibrosis development in rat. The effect of AT1-R blocker on the hepatic fibrosis. Exp Toxicol Pathol. 2003;55:159–166. doi: 10.1078/0940-2993-00309. [DOI] [PubMed] [Google Scholar]
- 27.Töx U, Scheller I, Kociok N, et al. Expression of angiotensin II receptor type 1 is reduced in advanced rat liver fibrosis. Dig Dis Sci. 2007;52:1995–2005. doi: 10.1007/s10620-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 28.Tan G, Pan S, Li J, et al. Hydrogen sulfide attenuates carbon tetrachloride-induced hepatotoxicity, liver cirrhosis and portal hypertension in rats. Plos One. 2011;6:e25943. doi: 10.1371/journal.pone.0025943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Distrutti E, Mencarelli A, Santucci L, et al. The methionine connection: Homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology. 2008;47:659–667. doi: 10.1002/hep.22037. [DOI] [PubMed] [Google Scholar]
- 30.Bataller R, Gabele E, Parsons CJ, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–1055. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 31.Grotendorst GR. Connective tissue growth factor: A mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/S1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 32.Paradis V, Dargere D, Bonvoust F, et al. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82:767–774. doi: 10.1097/01.LAB.0000017365.18894.D3. [DOI] [PubMed] [Google Scholar]
- 33.Tamaki Y, Nakade Y, Yamauchi T, et al. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J Gastroenterol. 2013;48:491–503. doi: 10.1007/s00535-012-0651-7. [DOI] [PubMed] [Google Scholar]