Abstract
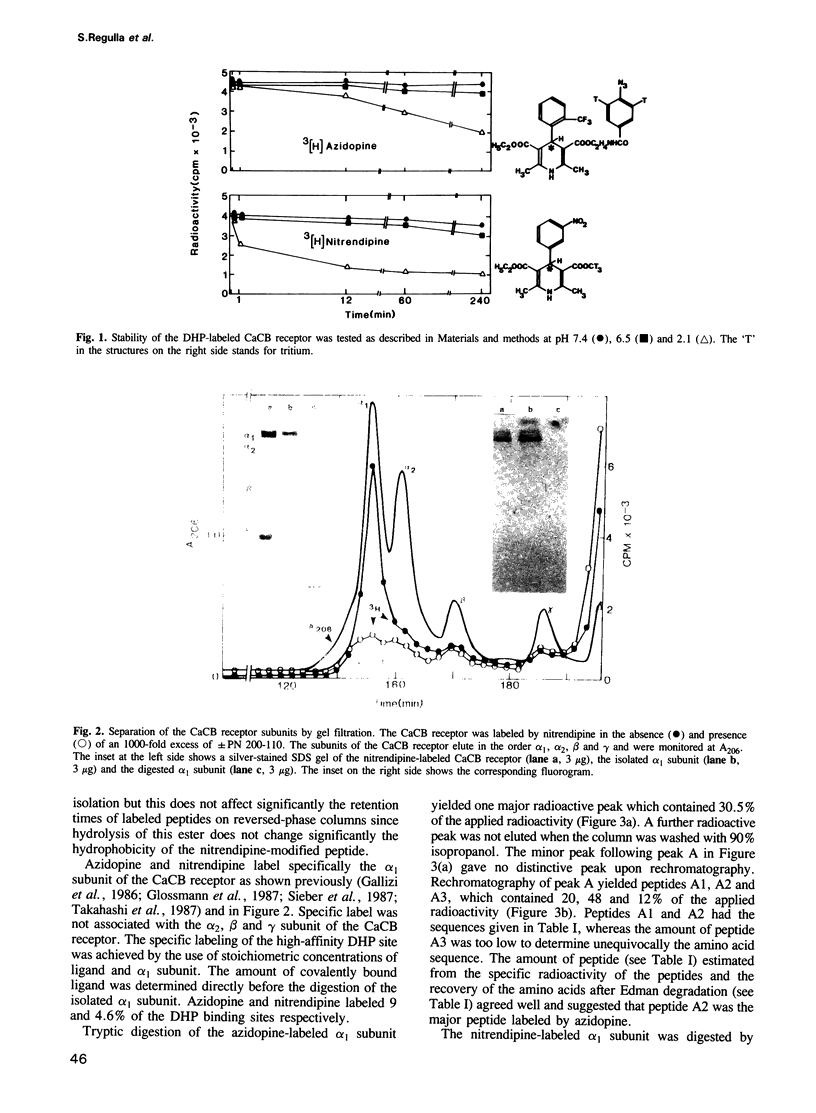
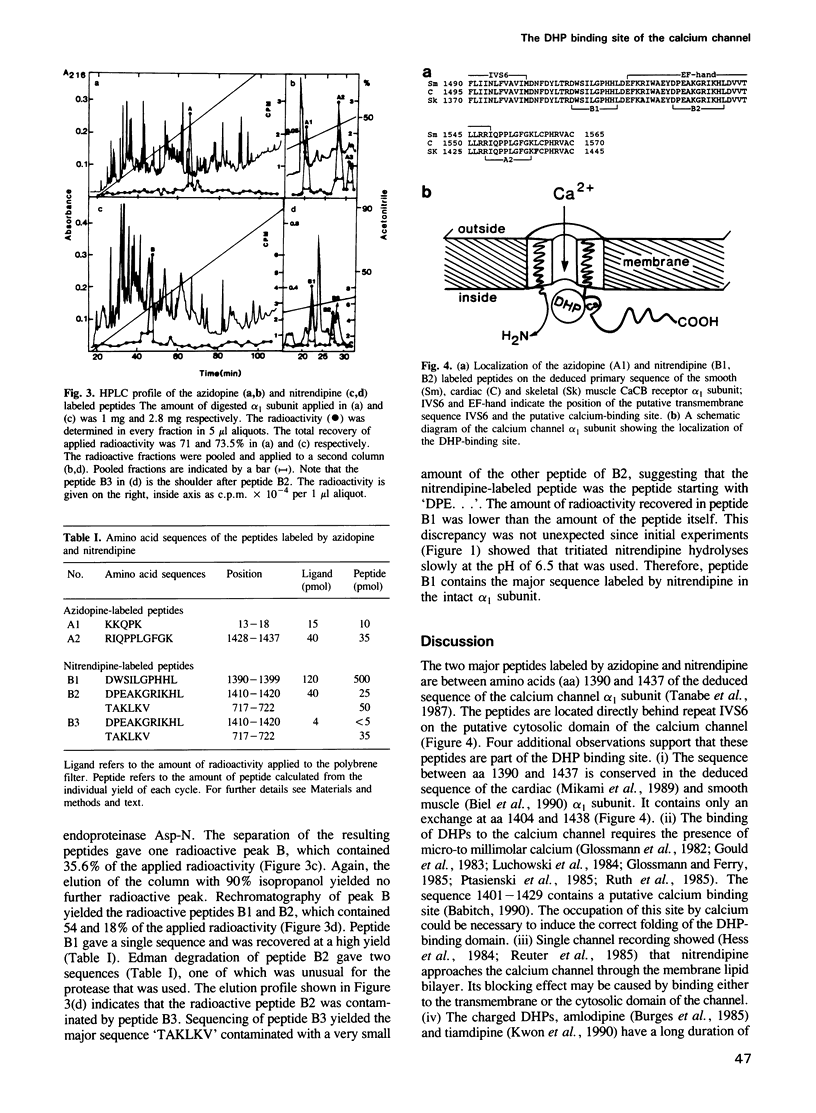
The dihydropyridine binding site of the rabbit skeletal muscle calcium channel alpha 1 subunit was identified using tritiated azidopine and nitrendipine as ligands. The purified receptor complex was incubated either with azidopine or nitrenidpine at an alpha 1 subunit to ligand ratio of 1:1. The samples were then irradiated by a 200 W UV lamp. The ligands were only incorporated into the alpha 1 subunit, which was isolated by size exclusion chromatography and digested either by trypsin (azidopine) or endoproteinase Asp-N (nitrendipine). Each digest contained two radioactive peptides, which were isolated and sequenced. The azidopine peptides were identical with amino acids 13-18 (minor peak) and 1428-1437 (major peak) of the primary sequence of the skeletal muscle alpha 1 subunit. The nitrendipine peptides were identical with amino acids 1390-1399 (major peak) and 1410-1420 (minor peak). The sequence from amino acids 1390 to 1437 is identical in the alpha 1 subunits of skeletal, cardiac and smooth muscle and follows directly repeat IVS6. These results indicate that dihydropyridines bind to an area that is located at the putative cytosolic domain of the calcium channel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babitch J. Channel hands. Nature. 1990 Jul 26;346(6282):321–322. doi: 10.1038/346321b0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Fosset M., Lazdunski M. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca2+ channel. Purification and subunit composition. J Biol Chem. 1985 Nov 15;260(26):14255–14263. [PubMed] [Google Scholar]
- Campbell K. P., Lipshutz G. M., Denney G. H. Direct photoaffinity labeling of the high affinity nitrendipine-binding site in subcellular membrane fractions isolated from canine myocardium. J Biol Chem. 1984 May 10;259(9):5384–5387. [PubMed] [Google Scholar]
- Dalbon P., Brandolin G., Boulay F., Hoppe J., Vignais P. V. Mapping of the nucleotide-binding sites in the ADP/ATP carrier of beef heart mitochondria by photolabeling with 2-azido[alpha-32P]adenosine diphosphate. Biochemistry. 1988 Jul 12;27(14):5141–5149. doi: 10.1021/bi00414a029. [DOI] [PubMed] [Google Scholar]
- Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. Amino acids of the Torpedo marmorata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 1988 Apr 5;27(7):2346–2357. doi: 10.1021/bi00407a016. [DOI] [PubMed] [Google Scholar]
- Ebel S., Schütz H., Hornitschek A. Untersuchungen zur Analytik von Nifedipin unter besonderer Berücksichtigung der bei Lichtexposition entstehenden Umwandlungsprodukte. Arzneimittelforschung. 1978;28(12):2188–2193. [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Rombush M., Goll A., Glossmann H. Photoaffinity labelling of Ca2+ channels with [3H]azidopine. FEBS Lett. 1984 Apr 9;169(1):112–118. doi: 10.1016/0014-5793(84)80299-9. [DOI] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F. Purification of a functional receptor for calcium-channel blockers from rabbit skeletal-muscle microsomes. Eur J Biochem. 1986 Nov 17;161(1):217–224. doi: 10.1111/j.1432-1033.1986.tb10145.x. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R. Assay for calcium channels. Methods Enzymol. 1985;109:513–550. doi: 10.1016/0076-6879(85)09112-1. [DOI] [PubMed] [Google Scholar]
- Gould R. J., Murphy K. M., Reynolds I. J., Snyder S. H. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5122–5125. doi: 10.1073/pnas.80.16.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Flockerzi V., Nastainczyk W., Ruth P., Schneider T. The molecular structure and regulation of muscular calcium channels. Curr Top Cell Regul. 1990;31:223–239. doi: 10.1016/b978-0-12-152831-7.50008-1. [DOI] [PubMed] [Google Scholar]
- Ichida S., Masada A., Fujisue T., Yoshioka T., Matsuda N. Photoaffinity labeling with dihydropyridine derivatives of crude membranes from rat skeletal, cardiac, ileal, and uterine muscles and whole brain. J Biochem. 1989 May;105(5):767–774. doi: 10.1093/oxfordjournals.jbchem.a122742. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y. W., Zhong Q., Wei X. Y., Zheng W., Triggle D. J. The interactions of 1,4-dihydropyridines bearing a 2-(2-aminoethylthio)methyl substituent at voltage-dependent Ca2+ channels of smooth muscle, cardiac muscle and neuronal tissues. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):128–136. doi: 10.1007/BF00195069. [DOI] [PubMed] [Google Scholar]
- Leung A. T., Imagawa T., Block B., Franzini-Armstrong C., Campbell K. P. Biochemical and ultrastructural characterization of the 1,4-dihydropyridine receptor from rabbit skeletal muscle. Evidence for a 52,000 Da subunit. J Biol Chem. 1988 Jan 15;263(2):994–1001. [PubMed] [Google Scholar]
- Luchowski E. M., Yousif F., Triggle D. J., Maurer S. C., Sarmiento J. G., Janis R. A. Effects of metal cations and calmodulin antagonists on [3H] nitrendipine binding in smooth and cardiac muscle. J Pharmacol Exp Ther. 1984 Sep;230(3):607–613. [PubMed] [Google Scholar]
- Mason R. P., Campbell S. F., Wang S. D., Herbette L. G. Comparison of location and binding for the positively charged 1,4-dihydropyridine calcium channel antagonist amlodipine with uncharged drugs of this class in cardiac membranes. Mol Pharmacol. 1989 Oct;36(4):634–640. [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Oberthür W., Muhn P., Baumann H., Lottspeich F., Wittmann-Liebold B., Hucho F. The reaction site of a non-competitive antagonist in the delta-subunit of the nicotinic acetylcholine receptor. EMBO J. 1986 Aug;5(8):1815–1819. doi: 10.1002/j.1460-2075.1986.tb04431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptasienski J., McMahon K. K., Hosey M. M. High and low affinity states of the dihydropyridine and phenylalkylamine receptors on the cardiac calcium channel and their interconversion by divalent cations. Biochem Biophys Res Commun. 1985 Jun 28;129(3):910–917. doi: 10.1016/0006-291x(85)91978-3. [DOI] [PubMed] [Google Scholar]
- Ruth P., Flockerzi V., von Nettelbladt E., Oeken J., Hofmann F. Characterization of the binding sites for nimodipine and (-)-desmethoxyverapamil in bovine cardiac sarcolemma. Eur J Biochem. 1985 Jul 15;150(2):313–322. doi: 10.1111/j.1432-1033.1985.tb09023.x. [DOI] [PubMed] [Google Scholar]
- Sieber M., Nastainczyk W., Zubor V., Wernet W., Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987 Aug 17;167(1):117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- Swiderek K., Jaquet K., Meyer H. E., Heilmeyer L. M., Jr Cardiac troponin I, isolated from bovine heart, contains two adjacent phosphoserines. A first example of phosphoserine determination by derivatization to S-ethylcysteine. Eur J Biochem. 1988 Sep 15;176(2):335–342. doi: 10.1111/j.1432-1033.1988.tb14286.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tejedor F. J., Catterall W. A. Site of covalent attachment of alpha-scorpion toxin derivatives in domain I of the sodium channel alpha subunit. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8742–8746. doi: 10.1073/pnas.85.22.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Langs D. A., Janis R. A. Ca2+ channel ligands: structure-function relationships of the 1,4-dihydropyridines. Med Res Rev. 1989 Apr-Jun;9(2):123–180. doi: 10.1002/med.2610090203. [DOI] [PubMed] [Google Scholar]
- Vaghy P. L., Striessnig J., Miwa K., Knaus H. G., Itagaki K., McKenna E., Glossmann H., Schwartz A. Identification of a novel 1,4-dihydropyridine- and phenylalkylamine-binding polypeptide in calcium channel preparations. J Biol Chem. 1987 Oct 15;262(29):14337–14342. [PubMed] [Google Scholar]