Abstract
Sex – a marker of biological and social individual differences – matters for drug use, particularly for cigarette smoking, which is the leading cause of preventable death in the United States. More men than women smoke, but women are less likely than men to quit. Resting state brain function, or intrinsic brain activity that occurs in the absence of a goal-directed task, is important for understanding cigarette smoking, as it has been shown to differentiate between smokers and non-smokers. But, it is unclear whether and how sex influences the link between resting state brain function and smoking behavior. In this study, we demonstrate that sex is indeed associated with resting state connectivity in cigarette smokers, and that sex moderates the link between resting state connectivity and self-reported nicotine dependence. Using functional magnetic resonance imaging and behavioral data from 50 adult daily smokers (23 women), we found that women had greater connectivity than men within the default mode network, and that increased connectivity within the reward network was related to increased nicotine tolerance in women but to decreased nicotine tolerance in men. Findings highlight the importance of sex-related individual differences reflected in resting state connectivity for understanding the etiology and treatment of substance use problems.
Keywords: brain function, cigarette smoking, fMRI, resting state, sex differences
Sex matters for drug use (Wetherington, 2007). Sex differences in cigarette smoking are of particular concern (SAMHSA, 2012; USDHHS, 2012). Risk of smoking-related death increased for women across the last 50 years, while remaining stable for men across the last 30 years (Thun et al., 2013). Furthermore, there are notable sex differences in smoking motivation and cessation: Compared to men, women are more likely to use cigarettes in response to non-pharmacological factors (Perkins et al., 2001), and less likely to use cigarettes for the pharmacological effects of nicotine (Perkins, Jacobs, Sanders, & Caggiula, 2002), to initiate cessation, and to succeed when they do try to quit (USDHHS, 2012). Treatment may be more successful in men than in women because it is easier to attenuate pharmacological effects of smoking (e.g., through nicotine reduction therapy) than it is to avoid exposure to non-pharmacological smoking cues (see Perkins & Scott, 2008).
An opportunity to understand individual, including sex, differences in substance use is provided by examinations of brain function (reviewed in Andersen, Sawyer, & Howell, 2012; Beltz, Blakemore, & Berenbaum, 2013), particularly resting state brain function (Sutherland, McHugh, Pariyadath, & Stein, 2012). This “endogenous” brain activity that occurs in the absence of a goal-directed task is thought to explain the brain’s large metabolic demand, and thus, to reflect the brain’s physiological baseline and an individual’s psychological baseline (Gusnard & Raichle, 2001). Resting state brain function has been shown to mark neuropsychiatric disease; for example, it is atypical in individuals with attention deficit hyperactivity disorder and depression (reviewed in Fox & Greicius, 2010), and in substance users (e.g., Gu et al., 2010; Weiland, Sabbineni, Calhoun, Welsh, & Hutchison, 2015).
Resting state brain function shows sex differences and has been associated with cigarette smoking (Biswal et al., 2010; Sutherland et al., 2012). Female smokers appear to have greater resting state connectivity than male smokers between the hippocampus and other brain regions, according to exploratory analyses (Wetherill, Jagannathan, Shin, & Franklin, 2014), but such differences in brain connectivity have not been examined in relation to smoking behavior. Resting state connectivity has also been seen to be reduced in dependent smokers who are deprived of nicotine (Cole, Beckmann, et al., 2010; Hong et al., 2009). This suggests that resting state connectivity is affected by transient nicotine states (i.e., fluctuating with smoking-related behavior), but leaves unanswered questions about the link between connectivity and smoking-related traits (i.e., relatively stable smoking characteristics).
Two resting state networks (spatially distinct brain regions with synchronous endogenous activity) particularly important for smoking are the default mode network (DMN) and the reward network. The DMN includes several regions of interest (ROIs), such as the posterior cingulate cortex, medial prefrontal cortex, and lateral parietal regions; it is a task-negative network (more active during rest than tasks) and supports unconstrained and evaluative processing (Raichle et al., 2001). Women (both smokers and non-smokers) have greater connectivity than men in this network (Biswal et al., 2010; Wetherill et al., 2014). The reward network, including the striatum and orbitofrontal cortex, is a task-positive network (more active during tasks than rest) and supports the processing of appetitive stimuli (Cole, Beckmann, et al., 2010; Janes, Nickerson, Frederick, & Kaufman, 2012). Sex differences in this network have not been studied, but are likely, given evidence from other neuroimaging studies. In a blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) study examining neural responses to images of cigarettes, female smokers were seen to have greater activity than male smokers in the striatum (part of the reward circuitry that facilitates conditioning; McClernon, Kozink, & Rose, 2008). (A failure to replicate this finding is difficult to interpret because of methodological variations; Wetherill et al., 2013). In a study using positron emission tomography (PET) examining dopaminergic responses to smoking, men showed a faster response in the striatum than did women, consistent with sex differences in pharmacological versus non-pharmacological smoking motivations (Cosgrove et al., 2014).
Smoking-related sex differences in brain activity are likely to relate to nicotine dependence, since highly-dependent smokers have the most difficulty quitting (Hymowitz et al., 1997). Some important dimensions of nicotine dependence represent understudied stable, smoking-related traits. One is tolerance, or reduced sensitivity to the effects of nicotine. Although changes in nicotine sensitivity can be acute, tolerance is often conceptualized and assessed as a chronic decrease in the response to nicotine that results from repeated cigarette use (Shiffman, Waters, & Hickcox, 2004). Two others concern a narrowing of the smoking repertoire, in which a user’s smoking rate (continuity) and style (stereotypy) are not influenced by circumstances or surroundings (Shiffman et al., 2004).
We studied sex differences in resting state brain function of cigarette smokers measured with fMRI and links to nicotine dependence, a key aspect of smoking behavior. Our work follows from evidence of sex differences in resting state brain function and links between resting state brain function and cigarette use, and fills a gap by associating sex-related differences in the brain and smoking behavior. We hypothesized (a) that women would have greater connectivity than men in the DMN (consistent with other work; Biswal et al., 2010; Wetherill et al., 2014) and in the reward network (consistent with task-based BOLD fMRI findings; McClernon et al., 2008), and (b) that sex would moderate links between resting state connectivity and self-reported nicotine dependence, focusing on tolerance, continuity, and stereotypy traits.
Methods
Participants
Participants were 51 cigarette smokers (28 men, 23 women), aged 18 to 45 years. One male participant was excluded because he fell asleep during resting state data collection, so analyses included 50 participants with complete, usable data. All participants were recruited through community radio and newspaper advertisements, right-handed, and native English speakers who provided informed consent. Inclusion criteria were smoking at least 10 cigarettes per day for the past 12 months, no plans to quit smoking, no cardiovascular or respiratory disease during the previous year, no use of psychiatric medications, no current dependence on a substance other than nicotine based upon a brief structured interview (substance-related sections of the Mini-International Neuropsychiatric Interview), and no current depression (defined as a score > 16 on the Center for Epidemiologic Studies Depression Scale). There were no significant sex differences in demographic or smoking characteristics, with means and standard deviations shown by sex in Table 1. Men and women did not differ on age, t(48) = −1.29, p > .05, race, χ2(3, N=50) = 1.24, p > .05, number of years of education, t(48) = −.06, p > .05, number of years smoked, t(48) = −.24, p > .05, number of cigarettes smoked per day, t(48) = 1.14, p > .05, or carbon monoxide (CO) expired at the baseline or experimental session, t(48) = −.83, p > .05, and t(48) = .36, p > .05, respectively. Sample data on the neural correlates of smoking expectancy have been previously reported (Wilson et al., 2014).
Table 1.
Sample demographics and smoking characteristics by sex
| Men | Women | Total | |
|---|---|---|---|
| N | 27 | 23 | 50 |
| Age M (SD) | 24.4 (5.9) | 26.9 (7.6) | 25.6 (6.8) |
|
Race % White |
93 | 87 | 90 |
| Total years of education M (SD) | 13.8 (3.2) | 13.9 (2.9) | 13.9 (3.0) |
| Number of years smoked M (SD) | 5.2 (4.4) | 5.5 (6.3) | 5.3 (5.3) |
| Number of cigarettes smoked per day M (SD) | 15.7 (3.7) | 14.6 (3.3) | 15.2 (3.5) |
| Baseline CO (in ppm) during behavioral data collection M (SD) | 19.6 (7.2) | 22.1 (9.1) | 20.7 (8.1) |
| Experimental CO (in ppm) during imaging data collection M (SD) | 6.5 (2.5) | 6.2 (3.3) | 6.4 (2.9) |
Note. M: mean; SD: standard deviation; CO: carbon monoxide; ppm: parts per million. No significant sex differences, examined with a chi-square test of independence for race and two-tailed t-tests for all other variables with a Type I error of .05; see text for test results.
Procedures
Participants visited the lab for two sessions (details provided in Wilson et al., 2014). In the baseline session, participants provided an expired-air CO sample to verify smoking status (≥10 parts per million; BreathCo, Vitalograph, Lenexa, Kansas), completed self-report measures on smoking behavior, and were scheduled for a separate, two-hour experimental session. They were instructed to abstain from smoking and using nicotine-containing products for the 12 hours preceding the experimental session.
In the experimental session, participants reported the last time they smoked a cigarette and provided a CO sample to verify smoking abstinence, and thus, compliance with the deprivation instructions; they were required to have a CO level that was half of their baseline session sample or less in parts per million (rounded to the nearest integer), a cutoff used in similar studies (e.g., Wilson, Sayette, & Fiez, 2012). All participants met this requirement (sample CO reduction: M = 68%, SD = 12%). Participants then provided MRI data, including resting state data in a 5 min. 20 s. functional scan; this is a sufficient amount of time to acquire reliable resting state brain function measurements for group difference tests in network connectivity (Cole, Smith, & Beckmann, 2010; Van Dijk et al., 2010). During this scan, participants were instructed to relax, keep their eyes closed, not think of anything in particular, and stay awake.
Measures
Nicotine dependence
Nicotine dependence was assessed with the 19-item self-report Nicotine Dependence Syndrome Scale (NDSS; Shiffman et al., 2004). Participants indicated how true smoking-related statements were of them on a Likert-type scale from 1 (not true at all) to 5 (extremely true). The NDSS provides a multidimensional operationalization of nicotine dependence, with the three subscales that can be conceptualized as smoking-related traits considered here: tolerance (reduced sensitivity to the effects of smoking), continuity (regularity of smoking or smoking without interruption), and stereotypy (fixed smoking pattern, impervious to context). The NDSS is reliable, with the three subscales of interest having internal consistencies ranging from .55 to .70 and test-retest reliabilities ranging from .71 to .77 (Shiffman et al., 2004). It is also valid, concurrently related to other measures of nicotine dependence and predictive of smoking cessation (Shiffman et al., 2004). Composite scores were created using procedures standard for the measure: For each subscale, participants’ raw data were combined with regression-based intercept and orthogonal factor scores generated during measure validation (Shiffman et al., 2004); high values reflect high dependence.
Resting state brain function
MRI data were collected using a 3-Tesla Siemens Trio scanner. For resting state scans, a series of 160 functional volumes was acquired using an echo-planar imaging pulse sequence (TR=2,000ms; TE=25ms; flip angle=80°; 64x64 matrix; 34 slices; 3mm3 voxels). For registering functional data to standard space, high resolution three-dimensional structural volumes were acquired using a T1-weighted magnetization-prepared rapid gradient echo sequence.
Preprocessing
Preprocessing was conducted using the fMRI Expert Analysis Tool Version 6.00 in FSL (www.fmrib.ox.ac.uk/fsl; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). The following steps were applied to the data: removal of the first four volumes to ensure magnetic field stabilization; motion correction using linear registration; slice-timing correction; non-brain removal; spatial smoothing using a 6mm full-width at half-maximum Gaussian kernel; grand-mean intensity normalization of the four-dimensional dataset by a single multiplicative factor; highpass temporal filtering (sigma=100.0 s) to remove BOLD signal occurring at less than .01 Hz. Motion correction included covarying six movement vectors from the data (X, Y, Z, pitch, yaw, roll). Registration to Montreal Neurological Institute stereotaxic space was carried out using linear registration, further refined with nonlinear registration. Whole-brain and physiological noise (e.g., cardiac and respiratory signal) reflected in white matter (central coordinate: x=26, y=−12, z=35) and cerebral spinal fluid (central coordinate: x=19, y=−33, z=18) ROIs was covaried from the data; ROI size depended upon brain size, as described below. This is a meaningful way to increase the signal to noise ratio in resting state networks that are derived from BOLD signal in gray matter ROIs (Chang & Glover, 2009).
ROI selection and time series extraction
Four a priori ROIs defined each network. They are listed in Table 2 along with their central coordinates, selected from past resting state research. ROI size was scaled according to participant brain volume to account for the sex difference in brain size (for a discussion of correcting for sex differences in brain size, see Beltz et al., 2013). In this sample, brain size (sum of gray matter and white matter volumes) was significantly greater in men (M = 1266 cm3, SD = 99 cm3) than in women (M = 1098 cm3, SD = 91 cm3), t(48) = 6.20, p < .001, d = 1.76. ROIs with 6.5 mm radii were used for the median brain size and linearly scaled for other sizes, spheres were created around the central coordinates of ROIs, and mean BOLD signal across voxels was extracted for each volume. Figure 1 shows the median brain DMN ROIs (red in sagittal and axial slices) and reward network ROIs (blue in coronal slice) overlaid on a standard brain template (in radiological orientation).
Table 2.
Network ROIs and their central coordinates
| Resting state network | ROIs | ROI central coordinates (in MNI space: x, y, z) | References |
|---|---|---|---|
| DMN | PCC | −5, −49, 40 | (Biswal et al. 2010; Van Dijk et al. 2010) |
| MPFC | −1, 47, −4 | ||
| RLP | 46, −62, 32 | ||
| LLP | −45, −67, 36 | ||
| Reward | RS* | 12, 7, 13 | (Cole, Beckmann et al. 2010; Janes et al. 2012) |
| LS* | −12, 7, 13 | ||
| ROFC | 36, 16, −26 | ||
| LOFC | −28, 12, −20 |
Note. ROI: region of interest; MNI: Montreal Neurological Institute; DMN: default mode network; PCC: posterior cingulate cortex; MPFC: medial prefrontal cortex; RLP: right lateral parietal; LLP: left lateral parietal; RS: right striatum; LS: left striatum; ROFC: right orbitofrontal cortex; LOFC: left orbitofrontal cortex.
MNI coordinates converted from Talairach space (using algorithm by Lacadie, Fulbright, Rajeevan, Constable, & Papademetris, 2008).
Figure 1.
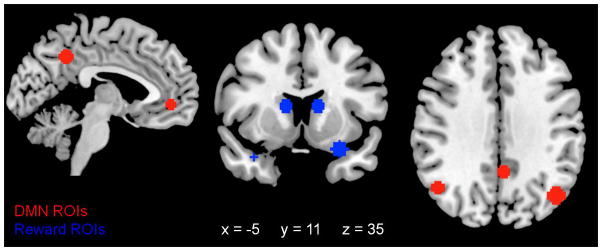
Resting state network ROIs for the median brain volume (i.e., ROIs with a 6.5mm radius) overlaid on a Montreal Neurological Institute stereotaxic space brain in radiological orientation at x = −5, y = 11, and z = 35. DMN ROIs (posterior cingulate cortex, medial prefrontal cortex, right and left lateral parietal lobules) are in red and shown in the sagittal and axial slices. Reward network ROIs (right and left striatum, right and left orbitofrontal cortices) are in blue and shown in the coronal slice. DMN: default mode network.
Functional connectivity
Functional connectivity was computed in several steps (van den Heuvel & Hulshoff Pol, 2010; Zhou et al., 2009). First, Pearson product moment correlations were used to quantify the functional connectivity among the six pairs of ROIs within each network; the time series of each ROI was correlated with the time series of every other ROI in the same network. Second, correlations were transformed to a normal distribution using Fisher’s r-to-z` transformation because Pearson r is not normally distributed; this created z(r) scores and facilitated the combination of correlations across networks and their comparison across participants (see e.g., Van Dijk et al., 2010). Third, principal component analyses (PCAs) were used to create network composite scores. PCAs were conducted on the covariance matrices of the six centered z(r) scores constituting each network, extracting one component. No rotation was conducted, so the single components reflect the maximum network variance explained by the contributing z(r) scores and can be interpreted as the dominant functional process among a set of ROI pairs, independent of other processes. The first component explained 55% of the variance in the DMN, and connectivity between the MPFC and RLP contributed the most with a component loading of .88. The first component explained 45% of the variance in the reward network, and connectivity between the RS and LOFC contributed the most with a component loading of .85. Fourth, component loadings were used to compute regression-based composite scores for each participant.
Data Analysis Plan
Type I error was set at .05 and age was a covariate in all analyses. There was a large age range in this sample, and age is related to resting state connectivity (e.g., Dosenbach et al., 2010) and smoking behavior (e.g., number of years smoked, r(48) = .64, p < .001). Baseline CO was a covariate in analyses concerning nicotine dependence, in order to control for any state-like effects of nicotine satiety participants experienced while completing the NDSS. Some links between nicotine dependence and resting state connectivity in smokers are affected by satiety and related to CO (e.g., Hong et al., 2009).
Analyses of covariance (ANCOVAs) were conducted to examine sex differences in the resting state connectivity of smokers. The independent variable was sex, dependent variables were the composite scores from PCAs characterizing connectivity within the DMN and reward network, and the covariate was age. Effect size (η2p) was reported for significant results.
Hierarchical multiple regressions were conducted to identify sex-moderated links between resting state connectivity and nicotine dependence. Age and baseline CO were entered in step 1, sex and DMN or reward network connectivity were entered in step 2, and the interaction of sex and network connectivity was entered in step 3. Dependent variables were the NDSS tolerance, continuity, and stereotypy subscales. Effect size (ΔR2) was reported for all steps, and confidence intervals were provided for variables within significant steps.
Results
Results of ANCOVAs examining sex differences in resting state connectivity revealed the expected significant sex difference in connectivity within the DMN, F(1,46) = 4.83, p < .05, η2p = .09, with connectivity greater in women (M = .28, SD = 1.08) than in men (M = −.24, SD = .87). There were no significant sex differences in connectivity within the reward network, F(1,46) = .90, p > .05, but means were in the expected direction (women: M = .14, SD = 1.07; men: M = −.12, SD = .94). Age was not a significant covariate in either case, F(1,46) = 2.86, p > .05 and F(1,46) = .20, p > .05, and analyses without age provided the same pattern of results.
Hierarchical regressions examining sex moderation of links between resting state connectivity and nicotine dependence assessed with the NDSS revealed significant effects for the reward network, but not for the DMN. Table 3 shows results for sex moderation of links between connectivity within the reward network and nicotine dependence. Only models (steps) significant at Type I error of .05 are interpreted in the text, but all models are shown in the table for completeness and to inform future work. Sex moderated the link between reward connectivity and nicotine tolerance (step 3), with age a significant covariate (step 1). The nature of the interaction is plotted in Figure 2, with simple correlations for each sex: Increased connectivity was associated with increased tolerance for women, but with decreased tolerance for men.
Table 3.
Sex moderation of links between reward connectivity and self-reported nicotine dependence
| NDSS scale | Model and variables | β | F | df for F | t | R2 (ΔR2) | 95% CI for β |
|---|---|---|---|---|---|---|---|
| Tolerance | Step 1 | 5.20** | 2,47 | .18** | |||
| Age | −.37 | −2.68* | −.64, −.09 | ||||
| Baseline CO | −.13 | −.95 | −.41, .15 | ||||
| Step 2 | 2.59* | 4,45 | .19 (.01) | ||||
| Age | −.37 | −2.58* | −.65, −.08 | ||||
| Baseline CO | −.15 | −1.04 | −.44, .14 | ||||
| Sex | .04 | .28 | −.24, .32 | ||||
| Connectivity | .06 | .45 | −.22, .35 | ||||
| Step 3 | 3.11* | 5,44 | .26 (.07)* | ||||
| Age | −.35 | −2.58* | −.63, −.08 | ||||
| Baseline CO | −.17 | −1.24 | −.46, .11 | ||||
| Sex | .04 | .29 | −.23, .31 | ||||
| Connectivity | .03 | .21 | −.25, .30 | ||||
| Sex x Connectivity | .28 | 2.10* | .01, .55 | ||||
| Continuity | Step 1 | .20 | 2,47 | .01 | |||
| Step 2 | .30 | 4,45 | .03 (.02) | ||||
| Step 3 | .36 | 5,44 | .04 (.01) | ||||
| Stereotypy | Step 1 | 2.54† | 2,47 | .10† | |||
| Age | .21 | 1.42 | −.09, .50 | ||||
| Baseline CO | .18 | 1.27 | −.11, .48 | ||||
| Step 2 | 1.55 | 4,45 | .12 (.02) | ||||
| Step 3 | 1.46 | 5,44 | .14 (.02) |
Note. NDSS: Nicotine Dependence Syndrome Scale; df: degrees of freedom; CI: confidence interval; CO: carbon monoxide. Step 1 included covariates (age and baseline CO). Step 2 included sex and network connectivity. Step 3 included the interaction of sex and network connectivity. Models, variables, and ΔR2 significant at
p < .10;
p < .05;
p < .01.
Figure 2.
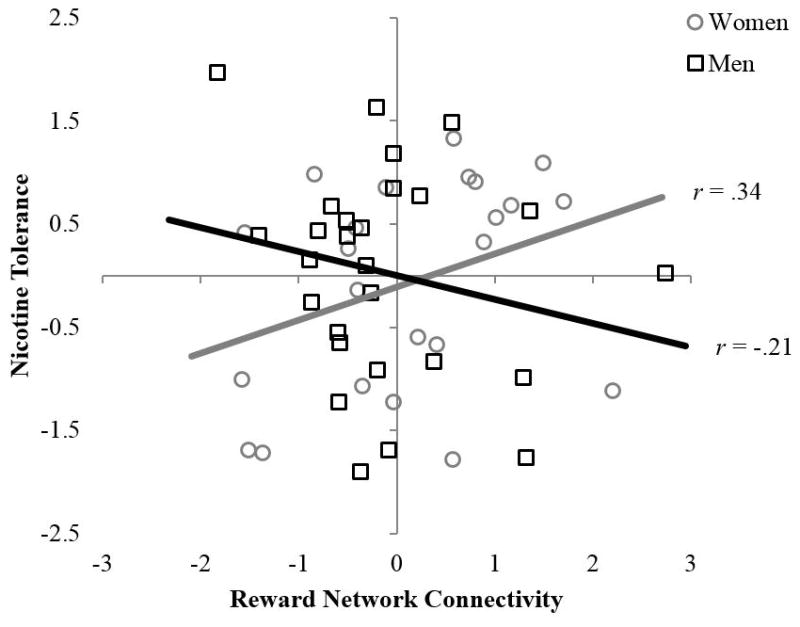
Relation between reward connectivity and self-reported nicotine tolerance by sex. Sex-moderation significant by hierarchical multiple regression at p < .05, with effects of age and baseline carbon monoxide controlled in step 1, main effects of sex and connectivity estimated in step 2, and interaction of sex and connectivity estimated in step 3. Unadjusted data points and zero-order correlations (ps > .05) are shown for ease of interpretation. Data points for men are shown as black squares, with a black linear trend line. Data points for women are shown as gray circles, with a gray linear trend line.
There were no significant effects of DMN, sex, or their interaction on nicotine dependence assessed with the NDSS. Step 1 model results are the same as those presented in Table 2 for the reward network. Step 2 models revealed no main effects, F(4,45) = 2.53, p > .05, ΔR2 = .00, F(4,45) = .64, p > .05, ΔR2 = .04, and F(4,45) = 1.52, p > .05, ΔR2 = .02 for tolerance, continuity, and stereotypy, respectively. Step 3 models also revealed no interactions, F(5,44) = 2.23, p > .05, ΔR2 = .02, F(5,44) = 1.04, p > .05, ΔR2 = .07, and F(5,44) = 1.22, p > .05, ΔR2 = .00, respectively. Excluding covariates (i.e., step 1) did not alter the pattern of results revealed in the regression analyses.
Discussion
The goal of this study was to identify how sex differences in resting state connectivity are linked to self-reported smoking behavior, and thus, to illustrate how resting state brain function reflects individual differences important for addiction. Using fMRI and behavioral data from 27 male and 23 female adult regular smokers, we examined sex differences in resting state connectivity in the DMN and reward networks, and sex-moderated links between connectivity and self-reported trait-like dimensions of nicotine dependence, extending past work on state-like dimensions of dependence (Cole, Beckmann, et al., 2010; Hong et al., 2009).
Results concerning DMN connectivity partially confirmed expectations. As predicted, connectivity within the DMN was greater in women than in men, with a small-to-moderate effect size, consistent with evidence from smokers and non-smokers (Biswal et al., 2010; Wetherill et al., 2014). But, this sex difference did not relate to nicotine dependence. This might suggest that smokers have DMN connectivity that is typical for their sex (i.e., women have greater DMN connectivity than men regardless of smoking status), or that DMN connectivity is only related to smoking states (e.g., modulated by nicotine withdrawal and replacement; Cole, Beckmann, et al., 2010), and not to smoking-related traits.
Results concerning reward connectivity also partially confirmed expectations. There was no sex difference in reward connectivity (consistent with the task-based fMRI study of Wetherill et al., 2013), but sex did moderate the link between reward connectivity and self-reported nicotine tolerance, with a small effect size. Increased connectivity within the reward network was associated with increased tolerance in women but with decreased tolerance in men. This finding is consistent with those from fMRI and PET studies showing that reward-related brain regions (particularly the striatum) facilitate women’s (more than men’s) smoking through non-pharmacological factors (Cosgrove et al., 2014; McClernon et al., 2008) and with data showing that tolerance is the only NDSS subscale associated with non-pharmacological reasons for smoking (Shiffman et al., 2004). To the extent that non-pharmacological factors influence women’s (more than men’s) difficulty in quitting smoking, these findings have implications for aiding cessation in women. For example, personalized interventions that use resting state connectivity as a marker of treatment efficacy could help women extinguish learned associations between environmental cues and smoking rewards (Fox & Greicius, 2010) and are likely to be more beneficial than currently available treatments, such as nicotine replacement patches, which are most effective for men (Perkins & Scott, 2008).
Findings concerning the DMN and reward network provide an interesting contrast: There was a sex difference in DMN connectivity that was not related to self-reported smoking behavior, whereas there was not a significant sex difference in reward connectivity despite reward connectivity being related to behavior in a sex-dependent fashion. This pattern highlights an important, but oft forgotten, characteristic of individual differences that is relevant to neuroimaging research: The interpretation of individual differences in one domain or level of analysis may also depend on individual differences in another domain or level of analysis. For example, not every sex difference in the brain is linked to behavior because some brain-based sex differences compensate for others (De Vries, 2004). Some sex differences in brain function actually offset the sex difference in brain size; small and large brains simply operate differently in order to perform the same task. Other sex differences in brain size, however, are not equalized by brain function and relate to behavioral sex differences (see examples in Beltz et al., 2013).
Sex differences in smoking-related brain and behavioral processes reflect how the prototypical male smoker differs from the prototypical female smoker, but there is variability in the extent to which individuals are typical for their sex. It is important to specify the sources of the sex-related individual differences that underlie sex-typed DMN connectivity and the reward network-tolerance link. In other words, what exactly is it about being male or female that matters for the neural substrates of smoking? Possibilities include sex hormones, neurotransmitters, genes, hypothalamic-pituitary adrenal axis function, and experience (Andoh et al., 2008; Cosgrove et al., 2012; Ray et al., 2006; Richards et al., 2011). Among the possibilities, sex hormones are the most promising for future investigations. For example, menstrual cycle phase has been linked to subjective smoking experiences, particularly during cessation attempts (reviewed in Carpenter, Upadhyaya, LaRowe, Saladin, & Brady, 2006), and to differences in brain responses to smoking cues (Mendrek, Dinh-Williams, Bourque, & Potvin, 2014).
Our results should be interpreted with respect to features of the study design. First, menstrual cycle phase was not considered, but this actually decreased our ability to detect sex differences. Second, we did not find sex differences in nicotine dependence assessed with the NDSS; this may reflect varying sex differences in self-report versus experimental manipulations (e.g., Perkins et al., 2001). Third, we found that resting state connectivity was only related to one of three stable dimensions of self-reported nicotine dependence. This may suggest that our finding is not robust (i.e., it is a chance result from analyses that were not corrected for multiple comparisons) even though it is consistent with behavioral work showing that the theoretically and statistically orthogonal dependence dimensions have differential links with addiction-related behavior (Shiffman et al., 2004; Wilson & MacLean, 2013).
Fourth, our confirmatory analysis approach complements exploratory work on sex differences in the resting state connectivity of smokers (Wetherill et al., 2014), but required several assumptions. Resting state networks were defined a priori, and functional connectivity was characterized by correlations and PCAs, a powerful data reduction technique that streamlines interpretation (see e.g., Zhou et al., 2009). PCAs create a data-driven network composite, allowing some ROI relations to contribute to the network representation more than others and permitting a single inferential test. Although ROI-based PCA analyses have some disadvantages (e.g., ROI selection can limit inferences about systems-level brain function), alternative approaches, such as seed-based correlations and independent component analysis, are exploratory and require multiple comparisons and post hoc interpretation of results, making them suboptimal for the hypothesis-driven analyses conducted here (see van den Heuvel & Hulshoff Pol, 2010).
Conclusions
Resting state brain function reflects biological and social processes underlying sex differences in multiple domains, including substance use and addiction. Utilizing fMRI data from male and female regular smokers, we found that a sex difference in one resting state network (the DMN) was not linked to self-reported trait-like dimensions of nicotine dependence, but that another network (reward network) was related to nicotine dependence in a sex-dependent fashion, such that increased connectivity was related to increased tolerance in women, but to decreased tolerance in men. These findings are important for public health, by suggesting that resting state brain function is a mechanism underlying sex differences in smoking that might be leveraged to help women quit (e.g., through monitoring the efficacy of personalized interventions that tap non-pharmacological rewards associated with cigarette use). Moreover, our approach illustrates how studying individual differences reflected in resting state brain function and substance use can be examined – and understood – in tandem.
References
- Andersen ML, Sawyer EK, Howell LL. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Experimental and Clinical Psychopharmacology. 2012;20(1):2–15. doi: 10.1037/a0025219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh J, Verhulst S, Ganesh M, Hopkins-Price P, Edson B, Sood A. Sex- and race-related differences among smokers using a national helpline are not explained by socioeconomic status. Journal of the National Medical Association. 2008;100(2):200–207. doi: 10.1089/ped.2013.0257. [DOI] [PubMed] [Google Scholar]
- Beltz AM, Blakemore JEO, Berenbaum SA. Sex differences in brain and behavioral development. In: Rakic P, Rubenstein J, editors. Comprehensive developmental neuroscience. Vol. 3. Oxford: Elsevier; 2013. pp. 467–499. [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine & Tobacco Research. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47(4):1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52(2):590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience. 2010;4:article 8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, Bois F, Seibyl JP, Mazure CM, O'Malley SS. Sex differences in availability of β(2)*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Archives of General Psychiatry. 2012;69(4):418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, Morris ED. Sex differences in the brain's dopaminergic signature of cigarette smoking. The Journal of Neuroscience. 2014;34(50):16851–16855. doi: 10.1523/JNEUROSCI.3661-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience. 2010;4:article 19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Stein ES. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Archives of General Psychiatry. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco Control. 1997;6(suppl 2):S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick BD, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and Alcohol Dependence. 2012;125(3):252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42(2):717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Dinh-Williams L, Bourque J, Potvin S. Sex differences and menstrual cycle phase-dependent modulation of craving for cigarette: An fMRI pilot study. Psychiatry Journal. 2014:article 723632. doi: 10.1155/2014/723632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchinson S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research. 2001;3(2):141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology. 2002;163(2):194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research. 2008;10(7):1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, O'Malley S. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology. 2006;188(3):355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez CW. Biological mechanisms underlying the relationship between stress and smoking: State of the science and directions for future work. Biological Psychology. 2011;88(1):1–12. doi: 10.1016/j.biopsycho.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2011 National Survey on Drug Use and Health: Summary of national findings, NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6(2):327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62(4):2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Gapstur SM. 50-year trends in smoking-related mortality in the United States. The New England Journal of Medicine. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. Tobacco Addiction, NIDA Research Report Series, NIH Publication Number 12-4342. Washington, D.C: National Institute of Drug Abuse; 2012. [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE. Reduced executive and default network functional connectivity in cigarette smokers. Human Brain Mapping. 2015;36(3):872–882. doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addictive Behaviors. 2014;39(4):789–792. doi: 10.1016/j.addbeh.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Young KA, Jagannathan K, Shin J, O'Brien CP, Childress AR, Franklin TR. The impact of sex on brain responses to smoking cues: A perfusion fMRI study. Biology of Sex Differences. 2013;4(1):article 9. doi: 10.1186/2042-6410-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: A shift in the burden of proof? Experimental and Clinical Psychopharmacology. 2007;15(5):411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(4):1196–1207. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, MacLean RR. Associations between self-control and dimensions of nicotine dependence: A preliminary report. Addictive Behaviors. 2013;38(3):1812–1815. doi: 10.1016/j.addbeh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Quitting-unmotivated and quitting-motivated cigarette smokers exhibit different patterns of cue-elicited brain activation when anticipating an opportunity to smoke. Journal of Abnormal Psychology. 2012;121(1):198–211. doi: 10.1037/a0025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Chen Y, Ding M, Wright P, Lu Z, Liu Y. Analyzing brain networks with PCA and conditional Granger causality. Human Brain Mapping. 2009;30(7):2197–2206. doi: 10.1002/hbm.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]


