Abstract
Primary resistant, recurrent and relapsed solid tumors are often non-responsive to conventional anti-neoplastic therapies. Moreover, in responsive tumors, the therapeutic to toxic range of these interventions remains quite narrow, such that side effects of therapy are substantial. Targeted therapies, such as adoptive T cell transfer, not only spare normal tissues but also use alternative killing mechanisms to which the tumor cells are usually not immune. Adoptive T cell transfer for solid tumors faces unique challenges because of the inherent heterogeneity of tumor parenchyma, the complexity of the tumor microenvironment, and tumor occurrence in areas with limited therapeutic accessibility. In this review, we examine the recent evolution of various T cell-based immunotherapeutics, the mechanisms of action behind their antitumor activity, their increasing complexity, and the prospect of building on previous successes in the treatment of solid tumors.
Introduction
Early stage solid cancers, defined as solid malignancies of non-lymphoreticular origins, are fairly well controlled using standard-of-care therapies. Resistant, metastatic or recurrent tumors are often surgically unresectable and are frequently nonresponsive to further radiation or chemotherapies. Recently alternative strategies, including immunotherapies using selected or engineered T cells, have shown promise in the treatment of blood cancers.
Immunotherapies are of particular interest in solid malignancies because of the peculiar interaction between the immune system and the tumor complex (1). The immune system acts in duality by providing anti-tumor activity via CD8+ and CD4+ T cells and their immune activating cytokines while conversely shielding the tumor from death through the activity of T regulatory cells and their immunosuppressive cytokines. There are various modalities of T cell-based therapies that rely on the T cells' ability to recognize and kill aberrant cells (Table 1). T cell therapies for solid tumors, however, face a number of unique challenges. Here, we discuss the evolution of adoptive T cell transfer, from the simplest forms to the more recent and more sophisticated approaches employed to overcome solid tumors' immune-evasion strategies.
Table 1. Representative pre-clinical studies investigating the use of adoptive T cell transfer in solid tumors.
| Target | Pre-clinical model |
|---|---|
| TILs | |
| Putative colon carcinoma antigens | TILs derived in a hybrid mouse model to treat autologous lung metastases of colon carcinoma (51) |
| CTLs | |
| CMV immunodominant antigens pp65 and IE1-72 | Ex vivo studies on primary GBM lines (12). No animal model exists. |
| EBV antigens LMP1/2, BARF1, EBNA1 | No pre-clinical studies reported |
| tgTCR | |
| Survivin-specific HLA-A2 restricted tgTCR T cells | In vitro studies in leukemia and multiple myeloma-derived cell lines, In vivo studies in leukemia cell lines (20) |
| pmel-I / CXCR2 | Melanoma (8) |
| Colon carcinoma (8) | |
| CAR | |
| HER2 | Medulloblastoma (52) |
| GBM (53) | |
| Osteosarcoma (54) | |
| IL13Rα2 | High grade glioma (55) |
| EphA2 | GBM (56) |
| HER2 and IL13Rα2 bispecific CAR T cells (HIL BiCAR T cells) | GBM (32) |
| HER2 CD19 bispecific Tandem CAR T cells Prototype Molecule | HER2+ CD19 inducible cell lines (33) |
| HER2 and IL13Rα2 bispecific Tandem CAR T cells (HIL TanCAR T cells) | GBM (Hegde, unpublished, 33, 57) |
| Engager T Cells | |
| CD3/EphA2 BiTEs T cells | GBM (31) |
| Lung cancer (31) | |
T-cell Transfer: The Promise
For those tumors that traditional therapeutics have failed, alternative strategies are needed. Based on recent insight into tumor biology and immunology, harnessing a patient's own immune system to enhance treatment has become an increasingly attractive option. Adoptive cell therapy (ACT) is the process by which immune cells are transferred to a recipient to induce an antitumor effect (2). T cells are capable of homing to tumor sites throughout the body, providing an advantage for use in ACT over antibodies, which neither effectively cross the blood brain barrier (BBB) nor consistently achieve adequate biodistribution deep inside solid tumors. Theoretically, T cells are capable of inducing a response powerful enough to mediate meaningful anti-tumor regression. T cells can be enriched from tumor-specific precursors and/or modified to possess a predetermined antigenic specificity and can be expanded ex vivo to clinically relevant numbers. Moreover, adoptive T cell transfer could provide a long-lasting therapeutic effect following a small number of treatments, if a memory subset of T cells is successfully attained. The arduous and expensive production process, mostly restricted to autologous T cell products, is an important disadvantage to T cell therapies and an impediment to their commercialization. However, recent efforts led to simplification of the production processes (3) as well as exploration of third-party lines (NCT02108522), enabling T cell therapy to become an “off the shelf” therapy to a greater extent.
Tumor infiltrating lymphocytes (TILs) were the earliest effective form of T cell transfer for solid tumors (Table 2). TILs were isolated from tumor tissue, expanded ex vivo in IL-2 (interleukin 2), and systemically administered to lymphodepleted advanced melanoma patients (4). TILs maintain specificity to tumor antigens and are capable of recognizing intracellular antigenic peptides presented within the context of the MHC-I/T cell receptor (TCR) (5) (Table 3). Objective clinical responses in 50-70% (6), and even complete tumor regression in 22% of patients with metastatic melanoma (7), launched a new era of efficacious T cell therapy for solid tumors. Recently, T cells have been further modified with homing receptors, demonstrating enhanced localization to tumor sites in pre-clinical melanoma studies (8). These promising data have led to the development of a clinical trial using modified TILs for the treatment of metastatic melanoma (NCT01740557).
Table 2. Examples of clinical studies employing various T cell-based therapies for the treatment of solid tumors.
| Target | Solid tumor therapy | Reported responses |
|---|---|---|
| TILs | ||
| Putative melanoma antigens | Autologous TILs in patients with metastatic melanoma | Objective response in 50-70% (6)22% complete response (7) |
| T cells transduced with CXCR2 to enhance homing or NGFR for metastatic melanoma | NCT01740557 | |
| CTLs | ||
| CMV immunodominant antigen pp65 | CMV-specific CTLs in patients with GBM | NCT01205334 |
| CMV-specific CTLs and CMV-specific DCs in patients with GBM | Ongoing, NCT00693095 | |
| EBV antigens LMP1/2, EBNA1/2/3 | EBV-specific CTLs in patients with nasopharyngeal carcinoma | 2 complete response, 1 partial response, 1 stable disease (11) |
| tgTCR | ||
| NY-ESO-1 | Autologous TCR-transduced T cells in patients with synovial cell carcinoma and melanoma | 9 partial response, 2 complete response (18) |
| CEA | Autologous TCR-transduced T cells for patients with colorectal cancer | 1 partial response (58) |
| CAR | ||
| HER2 | CMV-specific CTLs expressing HER2 CAR in patients with GBM | NCT01109095 |
| HER2 CAR and TGF-β resistant CTLs in patients with HER2+ malignancy (NCT00889954) | NCT00889954 | |
| Autologous HER2 CAR T cells in patients with osteosarcoma (HEROS) | 4 stable disease, median overall survival 10.3 months (59) | |
| Autologous HER2 CAR T cells in patients with sarcoma after fludarabine/cyclophosphamide lymphodepletion (HEROS 2.0) | NCT00902044 | |
| IL13Rα2 | IL13Rα2 CAR CD8+ CTLs in patients with high grade glioma | Results unpublished, NCT00730613 |
| GD2 | GD2 CAR expressed in activated T cells or EBV-CTLs in patients with neuroblastoma | 3 complete response (60)1 complete response, 3 partial responses (61) |
| mesothelin | Mesothelin CAR T cells in patients with metastatic mesothelin+ cancer | NCT01583686 |
| EGFRvIII | EGFRvIII CAR T cells in patients with glioma | NCT01454596 |
| Engager T cells | ||
| None to date | None to date | |
Table 3. Modalities of T-cell therapy.
| Cell product | Generation | Mechanism | Advantages | Disadvantages | Enhancements |
|---|---|---|---|---|---|
| Tumor Infiltrating Lymphocytes (TILs) | Natural T cells isolated from autologous tumor tissue & cytokine expanded ex vivo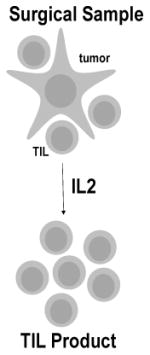
|
Native TCR recognizes processed TAA peptides presented on MHC-I molecules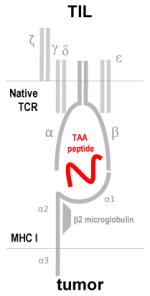
|
Recognize intracellular & extracellular antigens Broad TAA specificity |
Difficult to isolate & expand MHC-restricted operation Low frequency of TAA specific cells Autoimmunity due to shared epitopes |
TILs expressing chemokine receptors to enhance homing |
| Cytotoxic T Lymphocytes (CTLs) | Natural circulating tumor specific T cells enriched & expanded ex vivo from the patients' peripheral blood using APCs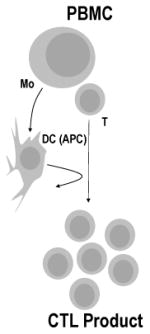
|
Native TCR recognizes processed TAA peptides presented on MHC-I molecules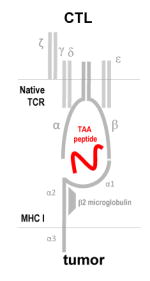
|
Specific to viral or non-viral TAA Take advantage of Th and APC augmentation of survival & persistence in vivo |
MHC-restricted Low affinity of TAA TCR Low frequency of TAA specific cells Limited efficacy in non-viral TAA |
Use of third party donor CTLs |
| Transgenic T Cell Receptor (tgTCR) T cells | Engineered T cells expressing an optimized transgenic TCR with specificity to TAAs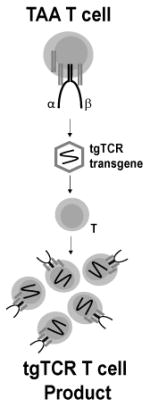
|
tgTCR recognizes processed TAA presented on MHC-I molecules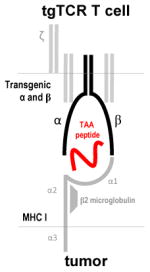
|
Target extracellular or intracellular TAAs Enhanced specificity |
MHC-restricted Mis-pairing with native TCR Usually restricted to HLA-A2 patients Rare recognition of native antigens |
Affinity matured tgTCR T cells DNR to resist TGFβ Expression in a γδ T cells or HSCs platform |
|
| |||||
| Chimeric Antigen Receptor (CAR) T cells | T cells engineered to express a CAR, an artificial molecule with a TAA specific scFv exodomain and a ζ-chain intracellular domain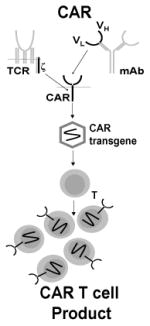
|
CAR exodomain recognizes intact TAA through the stereo-electrical properties of the exodomain (similar to a mAb Fv)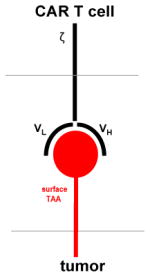
|
MHC-unrestricted Reliable production High frequency of TAA specificity |
Largely restricted to extracellular antigens Massive T cell proliferation results in CRS |
Affinity matured CAR molecules Conditional CAR T cells Multispecific CAR molecules, TanCAR DNR to resist TGFβ Safety switches |
Abbreviations: TILs, tumor infiltrating lymphocytes; TCR, T cell receptor; IL2, interleukin 2; TAA, tumor associated antigen; Th, helper T cells; Mo, monocyte; MHC-I, major histocompatibility class I; PBMC, peripheral blood mononuclear cells; APCs, antigen presenting cells; tgTCR, transgenic TCR; TGFβ, transformation growth factor beta; HLA, human leukocyte antigen; HSCs, hematopoietic stem cells; DNR, dominant negative receptor.
Natural components are grey, transgenic components are black, and tumor targets are red.
While TILs have shown promise in melanoma patients, cytotoxic T lymphocytes (CTLs) yield promising results in patients with virus-associated malignancies (Table 2). CTLs can be effectively isolated from the peripheral blood then enriched or rendered tumor specific ex vivo. Following infusion, CTLs engage the TCR with a target antigen-derived peptide presented on MHC I molecules, resulting T cell activation and anti-tumor activity (Table 3). Ex vivo generated Epstein-Barr Virus (EBV)-specific CTLs have been used for the treatment of post-transplant lymphoproliferative disease, nasopharyngeal carcinoma (NPC), and lymphoma with varied success (9-11). Similarly, cytomegalovirus (CMV)-specific CTLs have been efficacious in CMV-infected autologous glioblastoma (GBM) in pre-clinical work (12) and have been explored in clinical trials for GBM (13) (NCT01109095, NCT01205334, NCT00693095, Table 1, Table 2).
Although adoptive transfer of TILs and CTLs has shown promise, their broader application has been quite limited. There are prohibitive difficulties isolating and expanding TILs, which are present at the tumor site at very low frequency (14). In fact, the success of TIL transfer has been limited largely to malignant melanoma (6, 7). While CTLs are more extensive in their application than TILs, their tumor associated antigen (TAA) recognition is MHC-restricted. MHC-restriction is a major limitation, as the majority of solid tumors employ immune escape mechanisms such as altering their MHC expression and TAA processing (15). Genetically engineered T cells have emerged as an alternative to TIL and CTL therapies, overcoming some of the challenges associated with solid tumors.
T-cell Engineering: The Next Generation
Engineering T cells to recognize and target specific TAAs has been achieved through the production of transgenic T cell receptors and chimeric antigen receptors (Table 3). Transgenic T cell receptors (tgTCR) are produced by introducing genetic information encoding α and β chains of a TCR with specificity to a TAA into activated T cells (16). Tumor-specific T cells are isolated from a patient. After expansion, the genes encoding TAA-specific TCRs can be isolated, cloned, and transduced into T cells isolated from the patient's peripheral blood (16, 17). These T cells with optimal TCRs can be expanded ex vivo and subsequently administered to the patient. TgTCRs redirect T cells to target extracellular or intracellular TAAs by pairing with the endogenous CD3 complex and activating T cell signaling upon encounter of their respective antigen presented by MHC. Autologous T cells transduced with a TCR specific to NY-ESO-1 resulted in objective clinical responses in 60% of patients with synovial cell sarcoma and 45% of patients with melanoma (17, 18). This approach, nevertheless, faces some substantial challenges. First, the affinity of the TCR to its peptide/MHC complex can greatly affect efficacy. If the TCR has low affinity or the tumor downregulates MHC expression, tgTCR T cells become ineffective. Second, the combination of Human Leukocyte Antigen (HLA) alleles expressed by various individuals is expansive, making it imperative to either produce tgTCR T cells for each individual patient or develop a vast library of cells capable of recognizing TAAs within the MHC context of many different HLA types. Since this is unrealistic, tgTCR T cells have been limited primarily to HLA-A2 individuals (16). Lastly, some studies have demonstrated the ability of the transgenic α and β chains to “mis-pair” by forming heterodimers with native TCR chains (19). Strategies under investigation to circumvent these limitations include structural modifications of TCRs (20-22), transduction of γδ T cells (23) or hematopoietic stem cells (HSCs) later differentiated into T cells (24), and knocking down the expression of the endogenous TCR (25). On the other hand, some groups have chosen to completely bypass the restrictions imparted by MHC by producing an artificial design, the chimeric antigen receptor.
Chimeric antigen receptors (CAR) are fusion proteins composed of an antigen-recognition exodomain, commonly derived from an antibody, and a signaling endodomain traditionally consisting of the TCR zeta (ζ) chain that mediates T cell activation (26). CAR-encoding transgenes can be introduced to T cells, CTLs, or other immune effectors using a variety of transfer technologies (16). CAR functionality differs radically from traditional or transgenic T cells in that target recognition is MHC-unrestricted (27). CAR exodomain/TAA binding is thought to be comparable to the dynamics of antibody binding in both avidity and orientation. Although the exact activation mechanism is not known, it is thought that CARs activate canonical signaling pathways mediated by moieties present in their intracellular signaling domain (27).
CAR T cell investigations began by treating hematological malignancies, partly because the lineage-restricted surface expression of antigens was better understood and modified T cells could be more easily delivered to tumor sites within the blood. One of the most successfully targeted antigens to date is CD19 (cluster of differentiation 19). There are currently 27 active clinical trials employing CAR-based immunotherapies for the treatment of CD19+ hematological cancers (28). In contrast to the major successes in hematologic malignancies, the impact of CAR T cells in solid tumors remains limited. The most primitive CARs yielded short-lived antitumor activity, prompting studies addressing the role of cytokines (27), importance of T cell subset and phenotype (27), and design of CARs capable of providing co-stimulatory signals required for complete T cell activation (29).
While the expression of tgTCRs or CARs has greatly expanded the therapeutic reach of adoptively transferred T cells, neither strategy takes advantage of resident T cells. The adoptive transfer of ex vivo manipulated resident T cells (TILs) has been quite transformational in melanoma. Thus, interest in how to additionally redirect the specificity of T cells already present within the tumor microenvironment has grown (Table 1).
Redirecting Resident T cells to Tumor-Associated Antigens
Resident T cells represent a potentially powerful source of immune effectors readily available within the tumor. A number of technologies to harness these cells as a source of anti-tumor activity have been explored. Several bi-specific antibody (BsAb) platforms have been developed to redirect T cell activity to TAAs. The fusion of two single chain variable fragments (ScFv) via a flexible linker peptide yields tandem ScFvs (TaFv), chimeras that provide the minimal protein sequence needed for antigenic recognition as well as the flexibility necessary to enable interaction with multiple targets (30). TaFv molecules can be designed to target any two antigens that may exist within close proximity to each other, and also may be utilized to target an antigenic peptide and simultaneously activate an immune response. The latter approach has exhibited promising results: TaFv molecules have been engineered to include specificity to the TCR molecule CD3 as well as a TAA, termed bi-specific T cell engagers (BiTEs). BiTEs can redirect polyclonal T cells within the tumor to elicit a TAA-specific response, potentially activating multiple cycles of target cell killing (30). Most notably, a phase I clinical trial employing MT110, a CD3/EpCAM (epithelial cell adhesion molecule) BiTE for the treatment of solid tumors including lung, gastric, colorectal, breast, prostate, and ovarian cancers, was recently completed (NCT00635596). The results of this study have not yet been published. Moreover, as shown in pre-clinical studies, BiTE-secreting T cells, or engager T cells, take advantage of the innate avidity of T cells for the tumor site and locally secrete CD3/EphA2 (ephran type-A receptor 2) BiTEs, redirecting bystander T cells to the EphA2 TAAs expressed in glioma and lung cancer models (31).
A significant amount of research has focused on enhancing the activated T cell response against tumor cells. Each of these technologies resulted in varied success in pre-clinical and clinical studies of solid tumors. Such differential results inspired further investigation of challenges associated specifically with solid tumors, as well as ways to overcome the tumor microenvironment and regulatory T cells' immunosuppressive activity.
Challenges Specific to Solid Tumors
Solid tumors typically exhibit a highly heterogeneous landscape of TAAs, promoting antigen escape. Moreover, solid tumors are supported by a complex microenvironment capable of suppressing the immune response, and are often found in areas within the body that are difficult to access for treatment. These complexities mean that progressively more sophisticated T cell products are necessary for the treatment of solid tumors.
Solid tumor heterogeneity and strategies to offset antigen escape have been a particular interest of ours. Bi-specific T cells expressing distinct CARs for both HER2 (human epidermal growth factor receptor 2) and IL13Rα2 (interleukin-13 receptor subunit alpha-2), two highly prevalent GBM antigens, were able to offset antigen escape, enhance T cell activation, and provide a significant overall survival advantage in an orthotopic mouse model (32). Proof-of-concept experiments demonstrated the feasibility of targeting multiple TAAs simultaneously using a single CAR molecule with an exodomain incorporating two antigen recognition moieties joined in tandem (TanCAR) (33). TanCAR T cells can target each antigen individually and provide synergistic T cell activation upon encounter of both antigens (33). This CAR design was expanded to include elements from the tumor microenvironment as well as a tumor antigen (34), yielding a broad-spectrum product. Targeting a tumor profile rather than a specific TAA could represent a more viable strategy to control solid tumors.
T cells face a hostile tumor microenvironment featuring immunosuppressive molecules such as TGF-β (transforming growth factor beta) and IL-10 (interleukin 10). The development of a dominant negative TGF-β receptor (DNR; a human TGF-β receptor with a truncated endodomain) has conferred T cells with resistance to TGF-β (35). Autologous HER2+ DNR EBV CTLs are currently being tested in phase I/II trials for HER2 expressing malignancies (NCT00889954). Converting an immunosuppressive signal into a positive signal has also been pursued to actively enhance the proliferation of transduced T cells. An artificial molecule composed of the exodomain of the anti-proliferative Th2 cytokine, IL-4 (interleukin 4), fused to the endodomain of the Th1 proliferative cytokine, IL-7 (interleukin 7), demonstrated activation of the Th1 polarizing STAT5 pathway, enhanced proliferative capacity in vitro, and improved survival in mice engrafted with EBV-positive IL-4 producing tumors (36).
Unlike hematologic tumors, solid tumors often reside in heavily restricted areas of the body. For instance, gliomas and other central nervous system (CNS)-derived tumors are difficult to treat because cells infused systemically must be able to cross the blood brain barrier (BBB) in order to access the tumor. Therefore, the cell dose effectively reaching the tumor site may be significantly reduced from the dose originally administered (37). Studies have thus aimed to improve the homing capacity of T cells by expressing chemokine receptors. An ongoing phase I/II trial in which chemokine receptor CXCR2 (chemokine C-X-C motif receptor 2) and nerve growth factor receptor (NGFR) are transduced onto TILs is recruiting patients with metastatic melanoma. This novel therapy in conjunction with preparatory lymphodepletion and chemotherapy regimens will be assessed for safety and anti-tumor response (NCT01740557).
Even as T cell therapies improve in their ability to access and kill solid tumors, tumors can adapt, resulting in further immune evasion. Tumor cells can suppress an immune response by increasing their expression of key anti-inflammatory signals. Overcoming this adaptation and enabling T cells to continuously execute their anti-tumor effects is an extensive area of study.
Liberated: Strategies to Unleash T-cell Therapies
The balance between positive and negative signals, known as immune checkpoints, regulates T cell activation. These immune checkpoints are important in the maintenance of normal tolerance, though tumor cells are able to take advantage of this system by dysregulating the expression of ligands that bind to the receptors on T cells, halting the immune response (38).
A number of stimulatory and inhibitory receptors have been well characterized. For example, the “stimulatory” cluster of differentiation 28 (CD28) binds its ligand and provides one of the key interactions to enable full activation of T cells. On the other hand, the “inhibitory” cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is upregulated after T cell activation and can bind to the same cell ligand as CD28, leading to inhibitory signaling to shut down the T cell activation (39). Early clinical investigations of immune checkpoint blockade developed antibodies to block CTLA-4 (38). Ipilimumab, a human monoclonal antibody specific to CTLA-4, improved median overall survival by 3.4 months in patients with metastatic melanoma in a randomized phase III clinical trial and is now FDA-approved for the treatment of advanced melanoma (40).
More recently, the inhibitory interaction between programmed cell death protein 1 (PD-1) on T cells and programmed death-ligand 1 (PD-L1) on tumor cells has been under investigation. PD-1 is primarily responsible for the regulation of T cell activation in peripheral tissues, making it of increased importance in the context of solid tumors. PD-L1 may be constitutively expressed in some types of tumor cells, while it is known to be up-regulated as an evasion mechanism in other tumor cells (39). Recent clinical trials utilizing either a blocking monoclonal antibody specific to PD-1 (41) or a monoclonal antibody blocking PD-L1 (42) demonstrated improvements in objective response rate as well as a prolonged stabilization of disease state in patients with solid tumors, including melanoma, non-small cell lung cancer, and renal-cell cancer (41, 42). In September 2014, the first PD-1 inhibitor, pembrolizumab (Keytruda®), was approved by the FDA for treatment of metastatic melanoma. Adoptively transferred T cells could potentially exhibit enhanced functionality if administered in conjunction with compounds capable of regulating inhibitory immune checkpoint signals. Furthermore, as strategies are developed to maximize the antitumor activity of T cell products, evaluating the safety of these potent immune effectors will be crucial.
Improving the Safety of T-cell Therapies
Among the early success of T cells expressing tgTCRs in treating solid tumors, there have been clinical reports of on- and off-target toxicities (43-45). A high affinity TCR targeting MAGE-A3 in myeloma and melanoma demonstrated an unexpected cross-reactivity by recognizing titin, an unrelated protein expressed in cardiac tissue, ultimately resulting in cardiovascular toxicities and leading to the death of two patients (44). Similarly, there have been clinical reports of adverse effects following CAR T cell therapy (45, 46). A patient with lung and liver metastatic colon cancer was treated with third-generation HER2-specific CAR T cells after lymphodepletion and cytokine administration, only to experience respiratory distress and a cytokine release syndrome (CRS), a potentially life-threatening condition observed in patients who receive adoptive T cell transfer. In pathologically high quantities, immunostimulatory cytokines released after T cell activation and expansion can induce acute organ injury. In this case, either the dose of T cells administered to this lymphodeplete recipient or the resulting cytokines presumably caused lung injury from which the patient was unable to recover (45). In another study, multiple doses of mesothelin-specific CAR T cells developed with an RNA-based platform were infused to three patients with malignant pleural mesothelioma and one with pancreatic adenocarcinoma. A single patient developed an anaphylaxis reaction to the infused cells upon receipt of the third dose. This study concluded that the reaction resulted from the development of IgE antibodies specific to the CAR, warranting further study into the dosing schedule when administering repeated infusions of CAR T cells (46). To avoid such disastrous outcomes, choosing an appropriate target TAA is key, as is refining the affinity and specificity of the tgTCR or CAR, cell dose, and preparatory regimens utilized prior to cell therapy.
The incorporation of suicide genes into effector cells as a countermeasure has been evaluated clinically in two caspase-based systems, Herpes Simplex Virus-derived enzyme thymidine kinase (HSV-tk) and inducible caspase-9 (iC9). Both systems have demonstrated selective and efficient elimination of infused cells (47, 48). Splitting the signals needed to activate the transduced T cell between two separate CAR molecules has been used as an alternative to suicide genes. In pre-clinical models of prostate cancer, it was shown that T cells expressing two CARs, specific to antigens PSMA and PCMA, can be conditionally activated only in the presence of both antigens, but not by either antigen alone (49), enhancing on-tumor specificity.
Finally, administering tocilizumab, an anti-IL-6 (interleukin 6) antibody, steroid therapy, or targeted immunosuppressive agents, can control CRS fairly well (50). Overall with a means to control CRS and new systems incorporated into CAR molecules to prevent on-target off-tumor activity or eliminate overly activated cells the safety profile of next generation T cell therapies has improved greatly. As we move into the future these safety measures will enable more extensive use of CAR T cell therapies to target a multitude of solid tumors.
The Future of T-cell Therapy
While much of the success of T cell transfer has been in the treatment of hematological malignancies, therapies for the treatment of solid tumors are coming of age. T cell therapies have developed from the initial collection, expansion, and re-introduction of TILs and CTLs to the production and transfer of a number of engineered T cell types including those expressing tgTCRs, CARs, or BiTEs. To expand the effectiveness of T cell therapy, addressing concerns specific to the treatment of solid tumors such as tumor heterogeneity, antigen escape, an immunosuppressive microenvironment, and accessibility challenges will be imperative. New combinations of TAA targeting within CAR technology as well as multimodal therapies including the combination of checkpoint inhibitors, vaccines, or other antibodies with T cells targeting TAAs and/or components of the tumor niche will be pivotal in the future.
Additionally, as T cell products have become more sophisticated, concerns about their therapeutic safety have grown as well. In the future, rational design of pre-clinical and clinical studies to assess any potential on-target off-tumor effects will continue to be of utmost importance. Incorporating added measures into cell products such as suicide genes, which can eliminate all transferred cells in the event of an adverse situation, will help to ensure safety. Also, studying the optimal regimens to prepare patients for cell transfer, the appropriate dose(s) and dosing schedule, and routes of administration for therapeutic T cells will be critical to ensure optimal outcomes with minimal toxicities in patients with solid tumors.
Acknowledgments
The authors thank Catherine Gillespie for assistance in critical review of the manuscript.
Grant Support: K. Fousek was supported by the National Institute of General Medical Sciences of the NIH under award numbers T32HL092332 and T32GM088129. N. Ahmed was supported by the Alliance for Cancer Gene Therapy (ACGT), Alex's Lemonade Stand Foundation for Childhood Cancer, and a Stand Up To Cancer – St. Baldrick's Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest: The Center for Cell and Gene Therapy has a research collaboration with Celgene to develop chimeric antigen receptor (CAR)-based therapeutics that is administered by Baylor College of Medicine. N. Ahmed is listed as an inventor on patent applications in the field of T-cell and gene-modified T-cell therapy for cancer. No potential conflicts of interest were disclosed by the other author.
Disclaimer: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
References
- 1.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168–74. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 2.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 5.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16:5458–68. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 11.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 12.Ghazi A, Ashoori A, Hanley PJ, Brawley VS, Shaffer DR, Kew Y, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012;35:159–68. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuessler A, Smith C, Beagley L, Boyle GM, Rehan S, Matthews K, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74:3466–76. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 16.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heemskerk MH, Hagedoorn RS, van der Hoorn MA, van der Veken LT, Hoogeboom M, Kester MG, et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109:235–43. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 20.Arber C, Feng X, Abhyankar H, Romero E, Wu MF, Heslop HE, et al. Survivin-specific T cell receptor targets tumor but not T cells. J Clin Invest. 2015;125:157–68. doi: 10.1172/JCI75876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–8. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebestyen Z, Schooten E, Sals T, Zaldivar I, San Jose E, Alarcon B, et al. Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer. J Immunol. 2008;180:7736–46. doi: 10.4049/jimmunol.180.11.7736. [DOI] [PubMed] [Google Scholar]
- 23.van der Veken LT, Hagedoorn RS, van Loenen MM, Willemze R, Falkenburg JH, Heemskerk MH. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66:3331–7. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 24.Starck L, Popp K, Pircher H, Uckert W. Immunotherapy with TCR-redirected T cells: comparison of TCR-transduced and TCR-engineered hematopoietic stem cell-derived T cells. J Immunol. 2014;192:206–13. doi: 10.4049/jimmunol.1202591. [DOI] [PubMed] [Google Scholar]
- 25.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–15. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lameris R, de Bruin RC, Schneiders FL, van Bergen En, Henegouwen PM, Verheul HM, de Gruijl TD, et al. Bispecific antibody platforms for cancer immunotherapy. Crit Rev Oncol Hematol. 2014 doi: 10.1016/j.critrevonc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Iwahori K, Kakarla S, Velasquez MP, Yu F, Yi Z, Gerken C, et al. Engager T Cells: A New Class of Antigen-specific T Cells That Redirect Bystander T Cells. Mol Ther. 2015;23:171–8. doi: 10.1038/mt.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational Targeting Offsets Antigen Escape and Enhances Effector Functions of Adoptively Transferred T Cells in Glioblastoma. Mol Ther. 2013 doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21:1611–20. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31:500–5. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther. 2014;22:1211–20. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doolittle ND, Abrey LE, Bleyer WA, Brem S, Davis TP, Dore-Duffy P, et al. New frontiers in translational research in neuro-oncology and the blood-brain barrier: report of the tenth annual Blood-Brain Barrier Disruption Consortium Meeting. Clin Cancer Res. 2005;11:421–8. [PubMed] [Google Scholar]
- 38.Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014 doi: 10.1038/bjc.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 48.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartels CJ, Rosenberg SA, Yang JC. Adoptive cellular immunotherapy of cancer in mice using allogeneic T-cells. Ann Surg Oncol. 1996;3:67–73. doi: 10.1007/BF02409054. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–64. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16:474–85. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–87. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D'Apuzzo M, et al. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res. 2012;18:2199–209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629–37. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hegde M, Wakefield A, Brawley VS, Grada Z, Byrd TT, Chow KK, et al. Genetic modification of T cells with a novel bispecific chimeric antigen receptor to enhance the control of high-grade glioma (HGG) Journal of Clinical Oncology, 2014 ASCO Annual Meeting. 2014 Abstracts. [Google Scholar]
- 58.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]


