Abstract
This review of the central nervous control systems for voice and swallowing has suggested that the traditional concepts of a separation between cortical and limbic and brain stem control should be refined and more integrative. For voice production, a separation of the non-human vocalization system from the human learned voice production system has been posited based primarily on studies of non-human primates. However, recent humans studies of emotionally based vocalizations and human volitional voice production has shown more integration between these two systems than previously proposed. Recent human studies have shown that reflexive vocalization as well as learned voice production not involving speech, involve a common integrative system. On the other hand, recent studies of non-human primates have provided evidence of some cortical activity during vocalization and cortical changes with training during vocal behavior. For swallowing, evidence from the macaque and functional brain imaging in humans indicates that the control for the pharyngeal phase of swallowing is not primarily under brain stem mechanisms as previously proposed. Studies suggest that the initiation and patterning of swallowing for the pharyngeal phase is also under active cortical control for both spontaneous as well as volitional swallowing in awake humans and non-human primates.
INTRODUCTION TO THE VOICE AND SWALLOWING SYSTEMS
Voice and swallowing functions in humans have both similarities and differences in the task demands and their neural control. Volitional voice production in humans includes cry, humming, speech and singing, but can also be involuntary in response to pain, fright, and emotional expression. Swallowing is often reflexive for salivary control but can also be volitional for eating, rapid drinking and pill swallowing. Both systems have mammalian brain stem and midbrain patterning control systems as well as cortical and subcortical volitional control. The extent to which the cortical control alters brain stem and midbrain patterning may vary between voice and swallowing and will be discussed in this review.
Both vocalization and swallowing involve laryngeal control and both systems are impacted when there is unilateral vocal fold paralysis as a result of recurrent laryngeal paralysis (Flint, Purcell and Cummings, 1997; Perie, Laccourreye, Bou-Malhab, et al., 1998). Without bilateral laryngeal muscle control to bring the vocal folds close to the midline, air flow will not be able to induce vibration for voice production; in severe cases only a whisper can be produced with unilateral vocal fold paralysis. During swallowing, muscles in the vocal folds, the ventricular (or false vocal folds) and the aryepiglottic folds contract to protect the airway along with hyoid and laryngeal elevation to move the larynx underneath the epiglottis (Kawasaki, Fukuda, Shiotani, et al., 2001). Hyo-laryngeal elevation likely assists with opening the upper esophageal sphincter to safely move the bolus through the hypopharynx and into the esophagus (Kahrilas, Dodds, Dent, et al., 1988; Medda, Kern, Ren, et al., 2003; Pearson, Hindson, Langmore, et al., 2013). With vocal fold paralysis, closure of the vocal folds may be slow and incomplete allowing aspiration of the substances through the vocal folds and into the trachea when swallowing liquids placing a patient at risk of aspiration pneumonia if aspiration is repeated over a prolonged period (Finck, 2006).
Both systems modulate respiratory control; during swallowing there is a resetting of the respiratory rhythm to produce an apnea most likely due to resetting of the respiratory system (Bongianni, Mutolo, Carfi, et al., 2000; Paydarfar, Gilbert, Poppel, et al., 1995). For vocalization, inspiration increases lung volume before the onset of vocalization which requires a prolonged expiratory phase to provide adequate subglottal air pressure and flow essential for inducing vocal fold vibration (Jiang, O’mara, Conley, et al., 1999).
Oral and lingual control are also inherent in both vocalization and swallowing. For both non-speech vocalization and speech, mouth opening, velar elevation and upright head position are required for sound projection. For speech, lips, jaw and tongue coordination not only shape the oral pharyngeal cavity for vowel resonances but also produce full or partial obstructions for consonants which must be coordinated with voice onset and offset (Borden and Harris, 1984).
During swallowing, jaw and tongue control is required for chewing food, during lip closure and posterior tongue propulsion is required to move the bolus into the oropharynx to initiate the pharyngeal phase of upper airway closure, hyo-laryngeal elevation, positive pressure and upper esophageal sphincter opening (Jacob, Kahrilas, Logemann, et al., 1989). Thus both voice and swallowing are complex coordinate systems requiring precise integration of oral, pharyngeal, laryngeal, and respiratory musculature in the upper airway.
Developmental Aspects
Both vocalization and swallowing are present at birth and become further differentiated as the infant develops and begins speaking and eating. Neither swallowing or vocalization are acquired through explicit instruction, rather these are implicitly acquired through a gradual process of increased adaptation resulting in more complex behaviors. Voice in humans starts with the birth cry in newborns in response to pain, hunger, and isolation. Vocalization functions gradually begin to integrate with oral control during babbling which is then shaped by speech in the environment and language development providing the emergence of first words for communication around one year of age. Speech motor control becomes increasingly skilled with exact timing of voice onset and offset being used to differentiate between speech sounds early in childhood.
Swallowing is present at birth and a pattern of suck requiring lip closure and tongue motion to create a negative intra-oral pressure, resulting in movement of liquid through the posterior oral cavity and pharynx, and into the esophagus. Infant patterning of suck, swallow and breathing integration are apparent in normal infants during suckling. As the infant moves to foods with soft texture further tongue control for moving and manipulating the bolus into the back of the mouth emerges. With solid food ingestion chewing is coordinated with tongue manipulation prior to the pharyngeal stage of upper airway closure and propulsion through the upper esophageal sphincter.
Sensory Systems
Both vocalization and swallowing are systems evident at birth based on initial brainstem or midbrain control with cortical control for volitional control emerging during childhood. These two systems depend upon sensory input and can be modified by volitional control. The auditory system provides the target for volitional control of voice. The young child implicitly develops an inner feedforward model of proprioceptive properties needed to reach the auditory target with increased accuracy for voice during communication. Without auditory reception, voice control for speech does not develop normally.
In swallowing, the target is bolus formation and movement from the oral cavity with tongue propulsion into the hypopharynx and clearance into the esophagus without entry into the airway. Here glossopharyngeal and hypopharyngeal tactile stimulation and taste reception play a role in facilitating the swallow (Jafari, Prince, Kim, et al., 2003; Kitagawa, Shingai, Takahashi, et al., 2002). A bilateral block of the superior laryngeal nerve in healthy volunteers had a profound effect on swallowing. Not only did the participants report increased effort required to initiate swallowing, videofluoroscopy studies showed increased penetration of the bolus into the laryngeal vestibule and aspiration in a quarter of the swallows during the block. No instances of aspiration were found on swallows without a sensory block in these healthy volunteers (Jafari, Prince, Kim, et al., 2003). Tracheal receptors likely play an important role by inducing coughing when there is an abnormal aspiration into the trachea. Coughing will eliminate the bolus from the trachea (Undem, Carr and Kollarik, 2002) and provides feedback on the safety of swallowing. The degree to which an internal proprioceptive model is developed to guide safe swallowing behavior is unknown. The results of Jafari et al., (2003) suggest that an internal model develops by adulthood which depends upon sensory feedback to initiate and modulate the swallowing pattern to prevent aspiration and penetration in normal adults.
Integrated Systems for Voice and Swallowing
Central nervous system control for both systems includes both relatively automatic behaviors present from birth and volitional control acquired with development. In the human, volitional control is evident when subjects respond to verbal instruction such as, “prolong a vowel” or “swallow hard”.
The relatively automatic brain stem and midbrain control for voice have been studied in decerebrate or anesthetized mammals and non-human primates and in some cases chronically implanted normally behaving non-human primates (Hage and Jurgens, 2006a; Hage, Jurgens and Ehret, 2006; Jurgens, 2000; Jurgens, 2002; Luthe, Hausler and Jurgens, 2000). Similarly, studies of brain stem and midbrain control of swallowing has been extensively studied in decerebrate or anesthetized mammals (Amirali, Tsai, Schrader, et al., 2001; Jean, 2001; Lang, Dean, Medda, et al., 2004) as well as chronically implanted awake non-human primates (Martin, Kemppainen, Masuda, et al., 1999; Narita, Yamamura, Yao, et al., 1999; Yajima and Larson, 1993).
Central Nervous System Control of the Laryngeal Musculature in the Brain Stem
Innervation of the laryngeal muscles is essential for both voice and swallowing. For voice, both vocal folds must be moved to the midline so they can be set into vibration by airflow from the lungs. The relationship between cricothyroid (lengthening of the vocal fold) and thyroarytenoid (shortening of the vocal fold) determines the tension and rate of vibration or fundamental frequency (Titze, Luschei and Hirano, 1989). This is precisely controlled by auditory feedback during voice production (Larson, Burnett, Kiran, et al., 2000). For swallowing, vocal fold and ventricular or false fold closure prevent the aspiration of food or liquid into the trachea and lungs. Thus bilateral vocal fold function is essential to both systems.
Cortical control of the laryngeal musculature is bilateral within each hemisphere in non-human primates; electrical stimulation of the laryngeal muscle area in the primary motor cortex in either hemisphere will induce bilateral vocal fold closure in anesthetized animals (Hast and Milojevic, 1966; Hast, Fischer, Wetzel, et al., 1974; Simonyan and Jurgens, 2003). Infarcts affecting the cortical primary motor on one side can induce paralysis in the contralateral limb, brain injury affecting the cortical primary motor area for the laryngeal muscles on one side do not produce vocal fold paralysis likely due to bilateral cortical control of the laryngeal musculature in each hemisphere. In fact vocal fold paralysis due to central nervous system lesions only occur in Wallenberg syndrome with infarction of the vertebral artery or the posterior inferior cerebellar artery on one side in the medial and lateral medulla (Kim, Lee, Suh, et al., 1994).
The descending connections to the laryngeal motoneurons in the nucleus ambiguus in non-human primates was clarified in a study of the squirrel monkey using glutaminergic blockade with kynurenic acid while stimulating the laryngeal muscle area in the primary motor cortex on one side (Jurgens and Ehrenreich, 2007). Only kynurenic injections to the reticular nucleus dorsal to the nucleus retroambiguus produced either bilateral vocal fold paralysis to unilateral cortical stimulation while kynurenic injections adjacent or involving the nucleus ambiguus blocked vocal fold movements on the side ipsilateral to the cortical stimulation. The authors concluded that there were multiple input regions to the laryngeal motoneurons in the nucleus ambiguus: the ipsilateral dorsal reticular nucleus to the ipsilateral nucleus ambiguous as well as inputs from the ipsilateral peri-ambigual reticular formation to the contralateral nucleus ambiguus (Jurgens and Ehrenreich, 2007).
Lesions in the medulla producing both dysphonia and swallowing disorders with aspiration involve the lower middle levels of the medulla in the inferior dorsolateral, and mid-lateral regions affecting inputs to the motor neurons for the laryngeal, facial and soft palate musculature (Kwon, Lee and Kim, 2005). When electrophysiological techniques were used to quantify laryngeal movements and submental muscle activation associated with hyo-laryngeal elevation for the pharyngeal phase of swallowing, the effects of a lateral medial medullary infarct was to delay and discoordinate the pharyngeal component of the swallowing pattern bilaterally (Aydogdu, Ertekin, Tarlaci, et al., 2001). This suggested that lateral medullary lesions interfered with both unilateral and contralateral premotor inputs to the motoneurons involving muscles active in the pharyngeal phase of swallowing. The pattern of slowed and discoordinated pharyngeal swallowing in Wallenberg syndrome differed from the delay in initiation of the pharyngeal phase seen in patients with cortical lesions affecting swallowing (Aydogdu, Ertekin, Tarlaci, et al., 2001). Thus the cortical control of swallowing may be more involved in volitional control of the oral phase of swallowing involving bolus manipulation and chewing, and the initiation of the pharyngeal phase. On the other hand brain stem control involves the patterning within the pharyngeal phase and the premotor inputs to the pharyngeal and laryngeal motoneurons on both sides. A unilateral lesion in the dorsolateral medulla situated in the rostral third of the medulla affecting the nucleus ambiguus and the nucleus tractus solitarius with their surrounding reticular formation may disrupt premotor inputs to both the ipsilateral and contralateral motor neurons producing dysphagia (Prosiegel, Holing, Heintze, et al., 2005). Dysphonia in Wallenberg syndrome is usually due to unilateral vocal fold paralysis. Only one case in the literature has reported bilateral vocal fold paralysis with dorsolateral medial medullary injury resulting in stridor and severe dysnea as the two flaccid folds are sucked towards the midline with each inspiration requiring emergency tracheostomy (Giordano, Gonella, Macchieraldo, et al., 1992). The integration of the laryngeal motoneurons in swallowing at the brain stem level is evident. However, although voice production may be hoarse due to unilateral paralysis in dorsolateral lesions medullary lesions in humans, no reports have indicated that the rapid pattern of voice for speech remains intact indicating that precise patterning of voice onsets and offsets for speech in humans is not controlled at the brain stem level.
VOICE CNS CONTROL
Methodological Considerations for Studying CNS Voice Control in Humans
Speech versus Language Tasks
Several methodological issues need to be considered when studying central control of voice in humans and may account for some of the differences in findings reported in the literature. First, voice is usually used for speech by most adults. Speech conveys meaning, and involves the formulation of meaningful phrases through lexical selection and grammatical relationships requiring language processing. If adults are producing sentences, retrieving words, repeating sentences or reading sentences, the phonological, lexical, syntactic aspects of language processing are involved which are well known to be left hemisphere dominant functions in close to 90% of persons; reversed language laterality is rare although more frequent in left handed persons (Kimura, 1983). To study voice control without confounding the effects of language, then, many investigators have confined their tasks to voice production that doesn’t include language meaning. Examples of non-language voice production tasks are syllables that primarily involve voice changes and not require use of lips, tongue and jaw movements for speech sound articulation. Examples of voice changes that are primarily changes involving the larynx and do not contain other articulators, such as lip, jaw and tongue are glottal stops (designated by “?”) where voice production is interrupted by closing the vocal folds abruptly to produce an interruption between vowels such as ?i?i?i?i (Brown, Ngan and Liotti, 2008; Loucks, Poletto, Simonyan, et al., 2007). This requires precise control at the larynx but does not convey language meaning, and for English speakers is a learned voice gesture. Another voice control syllable for speech is the syllable /hi/ where the /h/ is produced between vowels when the vocal folds are held apart and turbulence is heard as an /h/. Although the word “he” has meaning and in isolation would have language meaning, when speakers are asked to produce a series of /hihihihih/ the language content is not apparent. Thus repeated vowels interspersed with glottal stops or /h/ is a voice production task without language meaning but skilled and contains the laryngeal motor patterns learned for speech production without language meaning.
Covert or Whispered versus Spoken Speech
One approach to determining CNS control for voice production is to compare voice production for speech with covert speech for similar utterances. One such study showed that an intensity difference in the primary motor area that was greater over the jaw, lips and tongue region with overt speech that was not present during covert speech in both the right and left hemispheres to the same degree (Huang, Carr and Cao, 2002). Another approach to identifying brain activation for voice production was conducted using PET to compare speech with whispered speech (Schulz, Varga, Jeffires, et al., 2005). Although this was narrative speech with language, as the language content was similar in both the whispered and spoken narrative speech, and the two conditions differed in that only speech contained voice with rapid onsets and offsets while whisper was constant.
The contrasting of two conditions is based on the assumption that the differences are additive which has been questioned by others (Sidtis, Strother, Anderson, et al., 1999). Sidtis et al (1999) used four tasks with positron emission tomography; baseline rest, syllable repetition of either /pa/, /ta/ or /ka/, simple sustained phonation and repetitive lip closure. Comparison of each of the three tasks with a baseline condition differed from decomposition by subtraction. The phonation task was a sustained vowel which is much less complex and difficult than rapid alterations between voice onsets after plosive voiceless consonants. Thus it is easily understood how these tasks would not be additive. This then demonstrates the care that is needed when designing studies to examine a central nervous system for a particular complex function such as voice, speech, and swallowing.
Voice for Speech versus Non-speech
Another aspect of voice is whether the gestures are learned and skilled speech such as the vowels with glottal stops or /h/ or whether they are non-speech vocalizations, such as sigh, cough, and throat clear. These non-speech sounds involve voice but are not learned as part of speech expression. Comparisons between speech and non-speech sounds were conducted to determine if there are differences in humans’ central nervous system control for these non-speech voice productions and speech syllables (Chang, Kenney, Loucks, et al., 2009). Surprisingly, left hemisphere dominance was similar for speech and non-speech systems using BOLD fMRI suggesting that familiar vocalizations with auditory targets for speech or non-speech sounds involve similar brain mechanisms to those for voice control for speech. However, more examination of this issues is needed.
Learned Voice Productions versus Emotional Vocalization
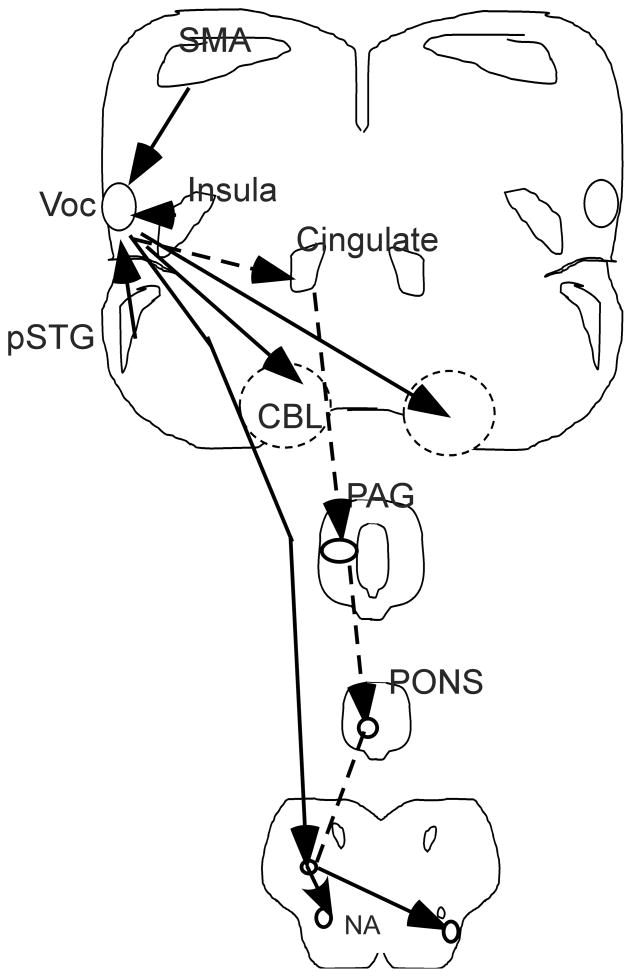
Jurgens and colleagues posited that the mammalian vocalization system which produces isolation cries in newborns, barks, cries to pain, and danger calls is innate and differs in central nervous system control from voice production for speech in humans (Jurgens, 2002; Jurgens, 2009). The mammalian vocalization system found through a series of careful experiments in the squirrel monkey, was identified as an innate system involving the cingulate, periaqueductal gray, pons and brain stem (Figure 2). It has been proposed that this is an innate emotional voice system in humans and differs from the CNS system that supports the development of learned voice productions for speech and non-speech that is cortically based (Jurgens, 2002; Jurgens, 2009). The degree to which the innate mammalian non-human vocalization system and learned non-speech and speech voice production systems interrelate in humans is not well understood. One difficulty with addressing this issue is the challenge of reliably producing spontaneous emotional voice expression such as laughter or crying in a neuroimaging session.
Figure 2.
A schematic diagram of central nervous system control for the human voice. Connections are only shown on one side but the same system is present on both the left and right sides. Input to the primary integrative vocalization center (Voc) and ongoing modulation is from the posterior superior temporal gyrus (pSTG) as well as inputs from the supplementary motor area (SMA), and the insula. Output from the primary integrative vocalization center is shown by solid lines for the direct pathway via the corticobulbar pathway and the cerebellum bilaterally while hatched lines show the pathway from the primary integrative vocalization center to the cingulate, the peri-aquaductal grey (PAG), the pons, and the reticular area in the medulla which then has input to the nucleus ambiguus (NA) on the ipsilateral and contralateral sides.
Difficulties with Motion Artifact
The most popular neuroimaging system is fMRI BOLD as it does not involve radiation exposure, can be repeated for multiple experiments in individuals in contrast with Positron Emission Tomography which requires the injection of radiolabelled ligands. However, BOLD fMRI is highly susceptible to motion artifact such as head movement which is difficult to control during voice, speech and swallowing as well as changes in the oral cavity that may produce magnetic field disturbances (Birn, Bandettini, Cox, et al., 1998b). The development of event-related sparse sampling was a significant technological advance allowing the use of fMRI for the study of speech, voice and swallowing (Birn, Bandettini, Cox, et al., 1999). Prior to that most of the speech studies using fMRI were “thought” experiments having the participants think of saying words or making sounds. There was a subsequent rapid increase in functional neuroimaging using fMRI for study CNS control for speech, voice and swallowing starting in 2000.
Event-Related Sparse Sampling
The event-related sparse sampling technique takes advantage of the relatively slow hemodynamic response to neuronal activation for a brief behavior or stimulus of a few seconds (Friston, Fletcher, Josephs, et al., 1998; Henson, Price, Rugg, et al., 2002). The hemodynamic response to a stimulus or a brief behavior starts just after the onset of the stimulation or behavior and peaks at 5 seconds. If the speech or swallowing are short in duration (less than 3 s) then the movement is finished and the motion artifact is limited to an interval before the peak response occurs. If a whole brain scan is then conducted between 4 and 6.5 s after the onset of the behavior then the BOLD measures are made without motion artifact during the interval when the blood oxygenation level increase is greatest for that behavior. This approach was first demonstrated for swallowing (Birn, Bandettini, Cox, et al., 1998a) and has been used extensively for voice (Loucks, Poletto, Simonyan, et al., 2007) and speech (Birn, Bandettini, Cox, et al., 1999; Golfinopoulos, Tourville, Bohland, et al., 2011).
Research Examining Dual Parallel Pathways for Vocal Production
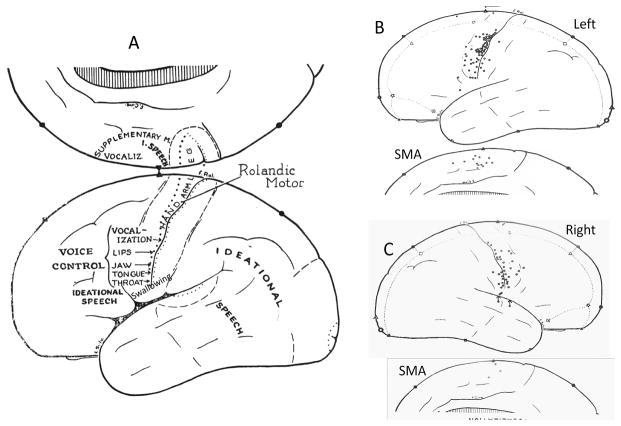
The difference between human volitional voice production for cry, humming, speech and singing, and involuntary vocalizations in response to pain, fright, and emotional expression has been a paramount issue in the study of human voice. Based on many decades of research on the CNS pathway for vocalization in the squirrel monkey, Jurgens determined that the vocalization system in involved the cingulate cortex, periaquaductal gray, pons and brain stem nuclei (Hage and Jurgens, 2006b; Jurgens, 1974; Jurgens, 1976; Jurgens and Ploog, 1970; Jurgens, Maurus, Ploog, et al., 1967; Muller-Preuss and Jurgens, 1976). Although stimulation of the primary motor laryngeal area was able to produce vocal fold movement in anesthetized monkey, stimulation in that area did not elicit vocalization in the same monkeys when awake (Jurgens, 1974). This is in contrast with the report by Penfield and Roberts that electrical stimulation in the primary motor area just above the representation of muscles in the jaw, lips and tongue was an integrative area that could elicit vocalization in awake humans during epilepsy surgery (Penfield and Roberts, 1959)(Figure 1.) Stimulation in this region in M1 just below the region for the hand muscles, elicited vocalization much more frequently when the same region was stimulated in the left hemisphere than on the right (Figure 1). This region is considered an integrative area involving respiratory, lip, jaw and tongue and laryngeal musculature for voice production in humans.
Figure 1.
Regions of the cerebral cortex in humans active for voice production and swallowing based on the studies by Penfield and Roberts (1959). In A the location of regions where muscular contractions were obtained in the lips, jaw, and tongue and larynx and pharynx (designated as throat). The location of an integrated area of vocalization on the primary motor area above the lips and jaw is designated. Swallowing was indicated as being evoked by stimulation lateral to the tongue and jaw. B. Sites in the left hemisphere and the left supplemental motor area which elicited vocalization in humans. C. Sites in the right hemisphere and the right supplementary motor area which elicited vocalization during electrical stimulation. SMA is the supplementary motor area. Adapted from (Penfield and Roberts, 1959).
Differences in the cortical control of vocalization in humans versus the lack of vocalization with cortical stimulation in non-human primates (Jurgens, 1974) was hypothesized to be due to the lack of a direct cortico-bulbar pathway from the cortex to the brain stem in the squirrel monkey (Jurgens, 1976) and in the rhesus monkey (Simonyan and Jurgens, 2003). Anatomical tracing studies conducted in humans used the Nauta-Gygax selective silver impregnation technique in patients with cortical stroke involving the motor areas examined for degenerating axons in the brain stem (Iwatsubo, Kuzuhara, Kanemitsu, et al., 1990; Kuypers, 1958). In both studies, the labeling of cells in or around the nucleus ambiguus was sparse in contrast with labeling of cells in the facial and hypoglossal nuclei and primarily on the contralateral side. Labeling of only a single cell in the n. ambiguus can be seen in a figure in one paper (Kuypers, 1958).
Recent Evidence Regarding Cortical Control in Non-Human Primates
Some results have suggested that cortical control of motor production of voice may occur in certain non-human primates. One study compared cortical expression of the immediate early gene of Egr-1in marmosets following only vocal reception of other marmoset calls versus marmosets involved in both reception and production of vocal calls. The ventrolateral prefrontal cortex, a region corresponding to Broca’s area in humans, had increased bilateral expression of Egr-1 in the vocalizing animals as opposed to the animals hearing but not vocalizing calls. Other evidence has been found of cortical neurons controlling vocalization have been found in the macaque (Coude, Ferrari, Roda, et al., 2011; Hage and Nieder, 2013). Recently, rhesus monkeys were shown with training to be able to switch volitionally between two vocalizations based on arbitrary visual stimuli (Hage, Gavrilov and Nieder, 2013). Although such evidence of voluntary control over vocalization engaging premotor cortical regions is far removed from the development of speech articulation learned in humans (Ackermann, Hage and Ziegler, 2014), it reduces the distinction between human and non-human primate vocalizations in the cortical control of vocalizations as posited by Jurgens (Jurgens, 2002; Jurgens, 2009).
Evidence Regarding a Dual-Pathway for Voice Production in Humans
Certainly a separation between emotional vocal expression and speech has been suggested by clinical observations of patients with global aphasia who retain emotional vocal expression such as cry and laughter (Landis, 2006). Further the disassociation between voice symptoms (phonation breaks) occurring in speech in spasmodic dysphonia while the patients are able to laugh and cry normally has also suggested a separation between CNS control of voice for speech and emotional expression (Bloch, Hirano and Gould, 1985).
Studies aimed at differentiating between volitional vocalization and emotional vocal control in humans have not clearly differentiated between these two types of vocalization. A study of laughter evoked by tickling, compared to suppression of laughter to tickling, and volitional production of laughter in normal humans compared brain activation patterns between these three conditions (Wattendorf, Westermann, Fiedler, et al., 2013). All three conditions activated the same primary sensori-motor and premotor regions involving cortical representation of the face, tongue, laryngeal and pharyngeal musculature as was identified as the vocalization region by Penfield (Penfield and Roberts, 1959). This was the same region identified by Brown in his fMRI study of voice production (Brown, Ngan and Liotti, 2008), and found to be active in both hemispheres during voice production either for speech or for singing (Riecker, Ackermann, Wildgruber, et al., 2000a). This area has been referred to as the laryngeal motor cortex and hypothesized to be the human center for voluntary laryngeal control for speech, breathing and swallowing (Simonyan and Horwitz, 2011). However, involuntary laughter activated by tickling also activated this region bilaterally. Further, the regions that were involved in laughter elicited by tickling and not by volitional laughter or laughter suppression were the lateral hypothalamus, parietal operculum, amygdala and the right cerebellum (Wattendorf, Westermann, Fiedler, et al., 2013). The PAG was active in the ticklish laughter condition but the anterior cingulate gyrus was active only in the laughter suppression and volitional laughter conditions. Although additional studies of emotional vocalization are needed, this one study did not confirm that involuntary laughter in the human was restricted to the same limbic vocalization system as identified by Jurgens and colleagues in the squirrel monkey (Jurgens, 2009).
Few studies have examined voice production without speech in humans (Brown, Ngan and Liotti, 2008; Huang, Carr and Cao, 2002; Loucks, Poletto, Simonyan, et al., 2007; Olthoff, Baudewig, Kruse, et al., 2008; Schulz, Varga, Jeffires, et al., 2005). In one, the primary vocalization motor cortex, the supplementary motor area and anterior cingulate were active bilaterally during humming without pitch intonation in normal humans (Olthoff, Baudewig, Kruse, et al., 2008). Thus voluntary voice production (humming) involved activation of some of the limbic vocalization system. In another study, simple glottal stop production without voice was contrasted with sustained phonation of a schwa vowel, a lip protrusion task and a vertical tongue movement inside the mouth without voice (Brown, Ngan and Liotti, 2008). The phonation only task was contrasted with the other simple movements to map the cortical representation of the laryngeal, lip and tongue musculature and demonstrated that the peak of activation for phonation (voice ) alone was the same area as the laryngeal task (a glottal stop) and dorsomedial to the primary motor representation of the lips and tongue. Activation in laryngeal voice area was bilateral and similar in both the left and right hemispheres. Few differences were found between the glottal stop task brain activation and phonation/voice; the right and left superior temporal gyrus was activated bilaterally only during phonation/voice as would be expected and the subthalamic nucleus was only activated during phonation/voice. Both tasks activated the right and left cerebellum although a greater region was activated in the cerebellum on both sides by phonation/voice. Neither task activated the anterior cingulate or peri-aquaductal grey.
Two studies have contrasted whispered or covert speech with voiced speech to identify which regions of the brain are active for voice without speech through subtraction of whisper or covert speech from voiced speech (Huang, Carr and Cao, 2002; Schulz, Varga, Jeffires, et al., 2005). When narrative speech was contrasted with whispered narrative speech by task subtraction to determine the system active for voice using positron emission tomography, activation of the peri-aquaductal grey was associated with vocalization and not whisper (Schulz, Varga, Jeffires, et al., 2005). The peri-aquaductal grey had high connectivity with the ventral frontal operculum, anterior medial temporal gyrus bilaterally, the left posterior medial temporal gyrus, left globus pallidum, right thalamus and right anterior cingulate. Negative relationships were found with the sensory II cortices in both hemispheres. The other study contrasted covert and overt speech and found greater activity in the medial lateral cortex over the primary motor cortex when speech was voiced versus thinking of speaking (Huang, Carr and Cao, 2002). This difference in activation was bilateral, the same on the left as on the right hemisphere in the medial lateral primary motor cortex. In summary, when only vocalization was studied independent of speech, bilateral activation was found in the premotor, motor and auditory areas. In some studies, voluntary voice production was also associated with activity in the limbic vocalization regions of the anterior cingulate and periaqueductal grey.
CNS Control of Voice Production During Speech
When voice for laryngeal articulated syllables such as glottal stops with vowels /?i?i?i?i?i/, or /h/ with vowels as in/hihihihi/ and no words, activation of the ventrolateral primary sensori-motor cortex was bilateral but considerably greater in the left hemisphere (Loucks, Poletto, Simonyan, et al., 2007). Here voice was combined with laryngeal movement for speech sounds of glottal stops and /h/ and left hemisphere dominance became evident although it wasn’t evident when only sustained vowels were studied (Brown, Ngan and Liotti, 2008). Thus rapid and precise laryngeal articulations that were learned as part of speech were predominantly controlled on the left side in the laryngeal/voice region of the primary motor cortex. When contrasted with rest and with prolonged exhalation, the only other differences were increased activation in the left superior temporal gyrus that occurred whether the speakers could hear their voice or not, suggesting that auditory areas containing the targets of voice for speech were activated during the production of learned laryngeal control tasks for speech (Loucks, Poletto, Simonyan, et al., 2007).
CNS Control for Speech Syllable Articulation
Similar patterns of left hemisphere predominance involving the left laryngeal/phonatory area occurs during nonsense speech syllable production (Bohland and Guenther, 2006; Brendel, Hertrich, Erb, et al., 2010; Ghosh, Tourville and Guenther, 2008; Riecker, Ackermann, Wildgruber, et al., 2000b). Additional areas of activation predominantly on the left were activated during studies of nonsense speech syllable productions when compared with previous studies of phonation/voice without speech reviewed above. These include the supplementary motor area, anterior insula, medial cingulate, thalamus, caudate and pallidum all bilaterally but greater on the left and the cerebellum bilaterally but greater on the right. Several of these areas have been proposed to involve the planning or preparation for speech production including the supplementary motor area, the anterior insula, dorsolateral frontal cortex (Brodman area 44) and the superior cerebellum while the speech execution loop may consist of the primary motor area, the thalamus, putamen/pallidum, caudate and interior cerebellum (Riecker, Mathiak, Wildgruber, et al., 2005). A carefully timed fMRI study confirmed these different loops for the planning, preparation and motor execution of speech sound syllables (Brendel, Hertrich, Erb, et al., 2010). The role of the left anterior insula in speech programming involving multiple vocal tract muscles has been proposed (Ackermann and Riecker, 2004) and injury to the left anterior insula has been associated with verbal apraxia when the complex sequencing of rapid automatic speech syllables is disrupted (Dronkers, 1996). Left hemisphere lesions affecting the insula and Broca’s area can impact the use voice for speech in verbal apraxia (Yadegari, Azimian, Rahgozar, et al., 2014) but such patients can continue to vocalize for emotional expression. Although the timing of voice for speech is disturbed (Freeman, Sands and Harris, 1978), patients rarely lose their ability to use voice with unilateral cortical lesions to the speech system. Vocalization for pain, discomfort and emotional distress continue unless there is extensive damage to cortical and subcortical systems (Nagaratnam, Nagaratnam, Ng, et al., 2004).
Auditory Control of Human Voice Production
The human vocal control system involves the primary motor area integrating the larynx, and oral facial musculature and is bilateral (Brown, Ngan and Liotti, 2008). Voice production is also highly integrated with activation with the superior temporal gyrus (Loucks, Poletto, Simonyan, et al., 2007). Several years ago it was demonstrated that humans will rapidly compensate for perturbations in the auditory feedback of their pitch during prolonged phonation of a vowel (Larson, Burnett, Kiran, et al., 2000). When BOLD responses on fMRI contrasted pitch shifted and non-shifted vocalization, activation differences were only found in the superior temporal gyrus which were somewhat greater on the left in the superior and medial temporal gyri (Parkinson, Flagmeier, Manes, et al., 2012).
Thus the voice production system in humans independent from speech is bilateral and involves the laryngeal/voice motor region originally identified by Penfield (Penfield and Roberts, 1959) (Figure 2). In contrast the human vocal system for speech involves a predominant activation in the left hemisphere involving the superior temporal gyrus, anterior insula, basal ganglia and the cerebellum more on the right. This system also employs activity in the anterior cingulate and periaqueductal grey to varying degrees. Further research is needed to determine the interactions between the proposed limbic vocalization system and the learned voice control for speech and singing.
SWALLOWING CNS CONTROL
Animal Studies of Brain Stem Control for Swallowing
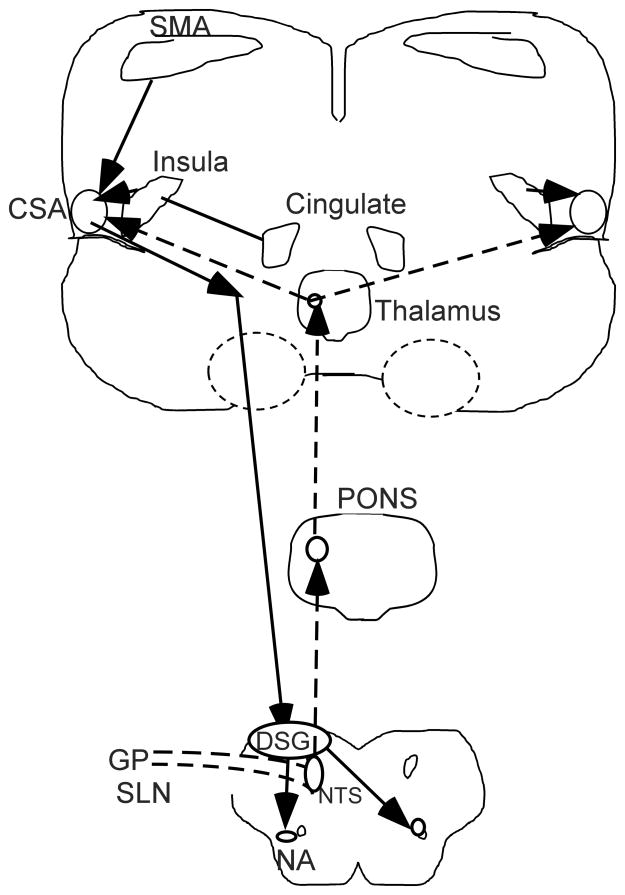
Extensive work has been conducted in mammals on the brain stem pathways involved in swallowing (Jean, 2001). The pharyngeal phase of swallowing has been studied extensively in the rat and two regions found involving central patterning for swallowing; one in the dorsal medulla activated by inputs to nucleus tractus solitarius referred to as the dorsal swallowing group and the other involving more ventral regions with inputs to the nucleus ambiguus, hypoglossal and other cranial muscle nuclei (Kessler and Jean, 1985) (Figure 3).
Figure 3.
A schematic diagram of central nervous system control for the human swallowing. Connections are only shown on one side but the same system is present on both the left and right sides. Afferent input to the swallowing system are shown in dashed lines and come from the glossopharyngeal (GP) and superior laryngeal nerves (SLN) into the nucleus tractus solitarius (NTS) and to the dorsal swallowing group of neurons (DSG) which provide patterning to the premotor neurons in the ventral swallowing group and motoneurons in the brain stem, the nucleus ambiguus (NA) is where the premotor neurons have inputs to the laryngeal motoneurons as one example. The sensory inputs in the NTS are relayed to the pons and interact with the taste inputs in the pons and then have input to the ventral posterior nucleus in the thalamus which has input to the oral facial regions in the sensory cortex adjacent to the central swallowing area (CSA) in the pericentral lateral motor and sensory cortex. Inputs to the CSA are also from the supplementary motor area (SMA) and the anterior insula. Output form the CSA is via the corticobulbar pathway to the dorsal swallowing group (DSG) which provides patterning for the pharyngeal phase of swallowing and has inputs to premotor neurons to activate motoneurons on both sides of the brain stem as shown for the nucleus ambiguus to control the laryngeal muscles during swallowing. This system also connects to many other motoneurons for the facial, tongue, jaw, lips and pharynx to control the patterning of multiple muscles during swallowing.
The interaction with respiratory control during swallowing has also been studied as a centrally controlled apnea during swallowing resets respiratory cycling (Paydarfar, Gilbert, Poppel, et al., 1995). While recording changes in membrane potentials in respiratory related neurons in the ventrolateral nucleus tractus solitarius activities in the same neurons during breathing and swallowing were examined (Gestreau, Milano, Bianchi, et al., 1996). Different patterns of firing were recorded in the same neurons during breathing and swallowing indicating that neurons in a central pattern generator for respiration also fired during swallowing pattern generation showing “functional flexibility” (Gestreau, Milano, Bianchi, et al., 1996). Similarly, laryngeal motoneurons in the nucleus ambiguus, the ventral swallowing group, were shown to be active in swallowing and breathing. Laryngeal motoneurons active during expiration became hyperpolarized and then depolarized with bursting during swallowing (Gestreau, Grelot and Bianchi, 2000). Thus the same neurons are involved in central pattern generator for respiration and swallowing. Depending upon the frequency of inputs from afferents in the superior laryngeal nerve the firing patterning of these neurons can change between breathing, cough and swallowing (Gestreau, Milano, Bianchi, et al., 1996; Jean, 1984; Jean, 2001).
Sensory Inputs for Triggering and Modulating Swallowing
The ability to induce fictive coughing, swallowing or respiratory apnea with increasing rates of electrical stimulation of the superior laryngeal nerve is one indication of the role that sensory input can have in modulating swallowing (Baekey, Morris, Gestreau, et al., 2001; Gestreau, Bianchi and Grelot, 1997; Gestreau, Grelot and Bianchi, 2000; Gestreau, Milano, Bianchi, et al., 1996). Stimulation of glossopharyngeal afferents can also induce fictive swallowing both in animals and humans (Chi-Fishman, Capra and Mccall, 1994; Fujiu, Toleikis, Logemann, et al., 1994; Kitagawa, Shingai, Takahashi, et al., 2002; Kitagawa, Nakagawa, Hasegawa, et al., 2009). Normally swallowing occurs spontaneously every few minutes and controls salivary flow. The rate of swallowing can be modulated by both superior laryngeal nerve and glossopharyngeal sensory inputs associated with water and citric acid (Kajii, Shingai, Kitagawa, et al., 2002)
Cortical Control of Swallowing
Penfield identified a region lateral to the primary face motor cortex as evoking swallowing with electrical stimulation in humans (Penfield and Rasmussen, 1950) ( Figure 1). Detailed mapping studies using intracortical microstimulation in macaques showed that swallowing could be evoked from a large area including four regions related to facial musculature (Martin, Kemppainen, Masuda, et al., 1999). These regions included the lateral region of the face in M1, the lateral region of the face in S1, an area immediately lateral and anterior to face in M1 and deep regions in the underlying white matter. This cortical swallowing area was similar in the right and left hemispheres and most sites evoking swallowing also evoked repetitive jaw movements similar to chewing. As continuous trains of stimulation were required to evoke swallowing it was concluded that swallowing depended upon the integration of large areas of cortex and may depend upon multiple inputs from the cortex to evoke the brain stem swallowing system. Only a relatively few sites evoked swallowing alone particularly in a region lateral to the facial M1 area, which could be a primary swallowing area (Martin, Kemppainen, Masuda, et al., 1999). Bilateral reversible cold block of this lateral pericentral swallow cortex reduced the occurrences of swallowing associated with chewing and a solid bolus or sucking a liquid bolus indicating: that cortical integrity in the central swallowing area was required to initiate swallowing (Narita, Yamamura, Yao, et al., 1999). In addition, with bilateral cold block, the motor patterning of muscle onsets of the genioglossus, geniohyoid, anterior digastric, masseter with the thyrohyoid muscle during swallowing was disrupted indicating that cortical control is necessary for normal patterning even when the brain stem regions were intact (Narita, Yamamura, Yao, et al., 1999).
Human Studies of Swallowing
The studies in the Macaque demonstrated that cortical control may play an active role in the spontaneous swallowing and may be essential for both initiating and patterning within swallowing. These results refute the concept that the control of the pharyngeal pattern of swallowing is reflexive and primarily based on brain stem based central pattern generator in the dorsolateral region of the medulla. Clinical swallowing intervention has been based on the assumption that only the oral phase of chewing and bolus manipulation is under cortical control and that cortical role for the pharyngeal phase is limited to the initiation of the pharyngeal phase (Logemann, 1998).
Functional brain imaging studies of swallowing in humans has shown a similar pattern of brain activation involving not only the lateral pericentral region similar to the swallowing cortical area in the macaque but also activation in the anterior insula, inferior parietal area and bilateral anterior cingulate (Soros, Inamoto and Martin, 2009). When spontaneous saliva swallowing, volitional saliva swallowing and water swallowing were contrasted using event-related fMRI, a similar pattern of brain activation was found for all three tasks involving the lateral precentral gyrus, lateral postcentral gyrus, and right insula. Activation foci within the superior temporal gyrus, middle and inferior frontal gyri, and frontal operculum also were identified for all swallowing tasks. Activation of the caudal anterior cingulate cortex and the right anterior insula was greater with the voluntary saliva swallow and water bolus swallow than the spontaneous saliva (Martin, Goodyear, Gati, et al., 2001). These results provide further support for the concept that cortical activation is actively involved in the initiation and modulation of both spontaneous and volitional swallowing in awake humans.
Manipulation of the Pharyngeal Phase of Swallowing
Behavioral studies have shown that humans can learn to modify the pharyngeal phase using techniques such as the Mendelsohn maneuver to raise and close the vocal folds before the pharyngeal phase (Mccullough and Kim, 2013). Experimental studies have also shown that with feedback during training healthy subjects can learn to change their airway protection during the pharyngeal phase of swallowing (Macrae, Anderson, Taylor-Kamara, et al., 2014).
Role of Sensory Stimulation for Modulating Human Swallowing
Evidence that changes in sensory stimulation can alter the frequency of swallowing is available (Theurer, Bihari, Barr, et al., 2005) and studies have shown that different types of stimuli can enhance cortical activation in the same regions that are active for swallowing (Lowell, Poletto, Knorr-Chung, et al., 2008). Brain stem studies cited earlier have shown that sensory stimulation can invoke fictive swallowing in anesthetized animals; however, the degree to which sensory stimulation also modulated cortical activity and central control of swallowing has both theoretical and practical importance. A study in dysphagia early post stroke demonstrated that recovery of swallowing was enhanced by electrical stimulation in the pharynx providing enhanced sensation from that region (Jayasekeran, Singh, Tyrrell, et al., 2010). The role of sensory function in executing swallowing was also demonstrated by the disruption of swallowing in healthy volunteers due to a bilateral dysruption of sensory input from the superior laryngeal nerve (Jafari, Prince, Kim, et al., 2003).
CONCLUSIONS
This review of the central nervous control systems for voice and swallowing has suggested that the traditional concepts of a separation between cortical and limbic and brain stem control should be refined and more integrative. A separation between limbic control of emotional vocalizations and human volitional voice production is less clear than has been proposed. Recent human studies have shown that some of the limbic vocalization system may be active during human voice production not involving speech. On the other hand recent studies of non-human primates have provided evidence that some cortical activity and change with training occurs during vocal behavior. For swallowing, evidence from the macaque and functional brain imaging in humans indicates that the control for the pharyngeal phase of swallowing is not only contributed by brain stem mechanisms. Studies suggest that the initiation and patterning of swallowing for the pharyngeal phase is under active cortical control for both spontaneous as well as volitional swallowing in awake humans and non-human primates.
Acknowledgments
Support for writing of this manuscript was provided by U54 NS 065701.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Hage SR, Ziegler W. Brain mechanisms of acoustic communication in humans and nonhuman primates: an evolutionary perspective. Behav Brain Sci. 2014;37:529–546. doi: 10.1017/S0140525X13003099. [DOI] [PubMed] [Google Scholar]
- Amirali A, Tsai G, Schrader N, Weisz D, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol. 2001;110:502–513. doi: 10.1177/000348940111000603. [DOI] [PubMed] [Google Scholar]
- Aydogdu I, Ertekin C, Tarlaci S, Turman B, Kiylioglu N, Secil Y. Dysphagia in lateral medullary infarction (Wallenberg’s syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke. 2001;32:2081–2087. doi: 10.1161/hs0901.094278. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol. 2001;534:565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Improved technique for study of brain activity during swallowing by functional magnetic resonance imaging (FMRI) Gastroenterology. 1998a;114:G2984. [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Human Brain Mapping. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magnetic Resonance in Medicine. 1998b;40:55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Bloch CS, Hirano M, Gould WJ. Symptom improvement of spastic dysphonia in response to phonatory tasks. Annals of Otology, Rhinology and Laryngology. 1985;94:51–54. doi: 10.1177/000348948509400111. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Carfi M, Fontana GA, Pantaleo T. Respiratory neuronal activity during apnea and poststimulatory effects of laryngeal origin in the cat. J Appl Physiol. 2000;89:917–925. doi: 10.1152/jappl.2000.89.3.917. [DOI] [PubMed] [Google Scholar]
- Borden GJ, Harris KS. Speech science primer: Physiology, acoustics, and perception of speech. 2. Baltimore, MD: Williams and Wilkins; 1984. [Google Scholar]
- Brendel B, Hertrich I, Erb M, et al. The contribution of mesiofrontal cortex to the preparation and execution of repetitive syllable productions: an fMRI study. Neuroimage. 2010;50:1219–1230. doi: 10.1016/j.neuroimage.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. 2008;18:837–845. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- Chang SE, Kenney MK, Loucks TM, Poletto CJ, Ludlow CL. Common neural substrates support speech and non-speech vocal tract gestures. NeuroImage. 2009;47:314–325. doi: 10.1016/j.neuroimage.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi-Fishman G, Capra NF, Mccall GN. Thermomechanical facilitation of swallowing evoked by electrical nerve stimulation in cats. Dysphagia. 1994;9:149–155. doi: 10.1007/BF00341258. [DOI] [PubMed] [Google Scholar]
- Coude G, Ferrari PF, Roda F, et al. Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS One. 2011;6:e26822. doi: 10.1371/journal.pone.0026822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Finck C. Laryngeal dysfunction after thyroid surgery: diagnosis, evaluation and treatment. Acta chirurgica Belgica. 2006;106:378–387. doi: 10.1080/00015458.2006.11679911. [DOI] [PubMed] [Google Scholar]
- Flint PW, Purcell LL, Cummings CW. Pathophysiology and indications for medialization thyroplasty in patients with dysphagia and aspiration. Otolaryngol Head Neck Surg. 1997;116:349–354. doi: 10.1016/S0194-59989770272-9. [DOI] [PubMed] [Google Scholar]
- Freeman FJ, Sands ES, Harris KS. Temporal coordination of phonation and articulation in a case of verbal apraxia: A voice onset time study. Brain Lang. 1978;6:106–111. doi: 10.1016/0093-934x(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Fujiu M, Toleikis JR, Logemann JA, Larson CR. Glossopharyngeal evoked potentials in normal subjects following mechanical stimulation of the anterior faucial pillar. Electroencephalogr Clin Neurophysiol. 1994;92:183–195. doi: 10.1016/0168-5597(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Bianchi AL, Grelot L. Differential brainstem fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosc. 1997;17:9340–9352. doi: 10.1523/JNEUROSCI.17-23-09340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp Brain Res. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Milano S, Bianchi AL, Grelot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Exp Brain Res. 1996;108:247–256. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51:1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Gonella ML, Macchieraldo A, Fornaseri V, Bosio C, Juliani E. Wallenberg’s syndrome: an assessment of the dysphagic and postural symptomatology. Acta Otorhinolaryngol Ital. 1992;12:165–174. [PubMed] [Google Scholar]
- Golfinopoulos E, Tourville JA, Bohland JW, Ghosh SS, Nieto-Castanon A, Guenther FH. fMRI investigation of unexpected somatosensory feedback perturbation during speech. Neuroimage. 2011;55:1324–1338. doi: 10.1016/j.neuroimage.2010.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Jurgens U. On the role of the pontine brainstem in vocal pattern generation: a telemetric single-unit recording study in the squirrel monkey. J Neurosci. 2006a;26:7105–7115. doi: 10.1523/JNEUROSCI.1024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Jurgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. Eur J Neurosci. 2006b;23:840–844. doi: 10.1111/j.1460-9568.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- Hage SR, Nieder A. Single neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nat Commun. 2013;4:2409. doi: 10.1038/ncomms3409. [DOI] [PubMed] [Google Scholar]
- Hage SR, Jurgens U, Ehret G. Audio-vocal interaction in the pontine brainstem during self-initiated vocalization in the squirrel monkey. Eur J Neurosci. 2006;23:3297–3308. doi: 10.1111/j.1460-9568.2006.04835.x. [DOI] [PubMed] [Google Scholar]
- Hage SR, Gavrilov N, Nieder A. Cognitive control of distinct vocalizations in rhesus monkeys. J Cogn Neurosci. 2013;25:1692–1701. doi: 10.1162/jocn_a_00428. [DOI] [PubMed] [Google Scholar]
- Hast MH, Milojevic B. The response of the vocal folds to electrical stimulation of the inferior frontal cortex of the squirrel monkey. Acta Otolaryngol. 1966;61:196–204. doi: 10.3109/00016486609127056. [DOI] [PubMed] [Google Scholar]
- Hast MH, Fischer JM, Wetzel AB, Thompson VE. Cortical motor representation of the laryngeal muscles in Macaca mulatta. Brain Res. 1974;73:229–240. doi: 10.1016/0006-8993(74)91046-4. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Human Brain Mapping. 2002;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T, Kuzuhara S, Kanemitsu A, Shimada H, Toyokura Y. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology. 1990;40:309–312. doi: 10.1212/wnl.40.2.309. [DOI] [PubMed] [Google Scholar]
- Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550:287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasekeran V, Singh S, Tyrrell P, et al. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138:1737–1746. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jiang J, O’mara T, Conley D, Hanson D. Phonation threshold pressure measurements during phonation by airflow interruption. Laryngoscope. 1999;109:425–432. doi: 10.1097/00005537-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Jurgens U. On the elicitability of vocalization from the cortical larynx area. Brain Res. 1974;81:564–566. doi: 10.1016/0006-8993(74)90853-1. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Projections from the cortical larynx area in the squirrel monkey. Exp Brain Res. 1976;25:401–411. doi: 10.1007/BF00241730. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Localization of a pontine vocalization-controlling area. J Acoust Soc Am. 2000;108:1393–1396. doi: 10.1121/1.1289204. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neuroscience and biobehavioral reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Ploog D. Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Research. 2007;1148:90–95. doi: 10.1016/j.brainres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Maurus M, Ploog D, Winter P. Vocalization in the squirrel monkey (Saimiri sciureus) elicited by brain stimulation. Exp Brain Res. 1967;4:114–117. doi: 10.1007/BF00240356. [DOI] [PubMed] [Google Scholar]
- Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- Kajii Y, Shingai T, Kitagawa J, et al. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol Behav. 2002;77:321–325. doi: 10.1016/s0031-9384(02)00854-5. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Fukuda H, Shiotani A, Kanzaki J. Study of movements of individual structures of the larynx during swallowing. Auris Nasus Larynx. 2001;28:75–84. doi: 10.1016/s0385-8146(00)00087-0. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Identification of the medullary swallowing regions of the rat. Exp Brain Res. 1985;57:256–263. doi: 10.1007/BF00236530. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee JH, Suh DC, Lee MC. Spectrum of lateral medullary syndrome. Correlation between clinical findings and magnetic resonance imaging in 33 subjects. Stroke. 1994;25:1405–1410. doi: 10.1161/01.str.25.7.1405. [DOI] [PubMed] [Google Scholar]
- Kimura D. Speech representation in an unbiased sample of left-handers. Human neurobiology. 1983;2:147–154. [PubMed] [Google Scholar]
- Kitagawa J, Shingai T, Takahashi Y, Yamada Y. Pharyngeal branch of the glossopharyngeal nerve plays a major role in reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1342–1347. doi: 10.1152/ajpregu.00556.2001. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Nakagawa K, Hasegawa M, et al. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci. 2009;51:167–171. doi: 10.2334/josnusd.51.167. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Cortico-bulbar connexions to the pons and lower brainstem in man. An anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology. 2005;65:714–718. doi: 10.1212/01.wnl.0000174441.39903.d8. [DOI] [PubMed] [Google Scholar]
- Landis T. Emotional words: what’s so different from just words? Cortex; a journal devoted to the study of the nervous system and behavior. 2006;42:823–830. doi: 10.1016/s0010-9452(08)70424-6. [DOI] [PubMed] [Google Scholar]
- Lang IM, Dean C, Medda BK, Aslam M, Shaker R. Differential activation of medullary vagal nuclei during different phases of swallowing in the cat. Brain Res. 2004;1014:145–163. doi: 10.1016/j.brainres.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Larson CR, Burnett TA, Kiran S, Hain TC. Effects of pitch-shift velocity on voice Fo responses. J Acoust Soc Am. 2000;107:559–564. doi: 10.1121/1.428323. [DOI] [PubMed] [Google Scholar]
- Logemann JA. Evaluation and treatment of swallowing disorders. 2. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. Neuroimage. 2007;36:131–143. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. NeuroImage. 2008;42:285–295. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe L, Hausler U, Jurgens U. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav Brain Res. 2000;116:197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- Macrae P, Anderson C, Taylor-Kamara I, Humbert I. The effects of feedback on volitional manipulation of airway protection during swallowing. J Mot Behav. 2014;46:133–139. doi: 10.1080/00222895.2013.878303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of Cortically Evoked Swallowing in the Awake Primate (Macaca fascicularis) J Neurophysiol. 1999;82:1529–1541. doi: 10.1152/jn.1999.82.3.1529. [DOI] [PubMed] [Google Scholar]
- Mccullough GH, Kim Y. Effects of the Mendelsohn Maneuver on Extent of Hyoid Movement and UES Opening Post-Stroke. Dysphagia. 2013:1–9. doi: 10.1007/s00455-013-9461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda BK, Kern M, Ren J, et al. Relative contribution of various airway protective mechanisms to prevention of aspiration during swallowing. Am J Physiol Gastrointest Liver Physiol. 2003;284:G933–939. doi: 10.1152/ajpgi.00395.2002. [DOI] [PubMed] [Google Scholar]
- Muller-Preuss P, Jurgens U. Projections from the ‘cingular’ vocalization area in the squirrel monkey. Brain Res. 1976;103:29–43. doi: 10.1016/0006-8993(76)90684-3. [DOI] [PubMed] [Google Scholar]
- Nagaratnam N, Nagaratnam K, Ng K, Diu P. Akinetic mutism following stroke. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2004;11:25–30. doi: 10.1016/j.jocn.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res. 1999;824:140–145. doi: 10.1016/s0006-8993(99)01151-8. [DOI] [PubMed] [Google Scholar]
- Olthoff A, Baudewig J, Kruse E, Dechent P. Cortical sensorimotor control in vocalization: a functional magnetic resonance imaging study. Laryngoscope. 2008;118:2091–2096. doi: 10.1097/MLG.0b013e31817fd40f. [DOI] [PubMed] [Google Scholar]
- Parkinson AL, Flagmeier SG, Manes JL, Larson CR, Rogers B, Robin DA. Understanding the neural mechanisms involved in sensory control of voice production. Neuroimage. 2012;61:314–322. doi: 10.1016/j.neuroimage.2012.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483 ( Pt 1):273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: MacMillan; 1950. [Google Scholar]
- Penfield W, Roberts L. Speech and brain mechanisms. Princeton, N.J: Princeton University press; 1959. [Google Scholar]
- Perie S, Laccourreye O, Bou-Malhab F, Brasnu D. Aspiration in unilateral recurrent laryngeal nerve paralysis after surgery. Am J Otolaryngol. 1998;19:18–23. doi: 10.1016/s0196-0709(98)90060-6. [DOI] [PubMed] [Google Scholar]
- Prosiegel M, Holing R, Heintze M, Wagner-Sonntag E, Wiseman K. The localization of central pattern generators for swallowing in humans--a clinical-anatomical study on patients with unilateral paresis of the vagal nerve, Avellis’ syndrome, Wallenberg’s syndrome, posterior fossa tumours and cerebellar hemorrhage. Acta Neurochir Suppl. 2005;93:85–88. doi: 10.1007/3-211-27577-0_13. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000a;11:1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, et al. Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex: a functional magnetic resonance imaging (fMRI) study. Brain Lang. 2000b;75:259–276. doi: 10.1006/brln.2000.2356. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, et al. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H215O PET study. Cereb Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Anderson JR, Rottenberg DA. Are brain functions really additive? Neuroimage. 1999;9:490–496. doi: 10.1006/nimg.1999.0423. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B. Laryngeal motor cortex and control of speech in humans. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2011;17:197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurer JA, Bihari F, Barr AM, Martin RE. Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia. 2005;20:254–260. doi: 10.1007/s00455-005-0021-1. [DOI] [PubMed] [Google Scholar]
- Titze IR, Luschei ES, Hirano M. Role of the thyroarytenoid muscle in regulation of fundamental frequency. Journal of Voice. 1989;3(3):213–224. [Google Scholar]
- Undem BJ, Carr MJ, Kollarik M. Physiology and plasticity of putative cough fibres in the Guinea pig. Pulmonary pharmacology & therapeutics. 2002;15:193–198. doi: 10.1006/pupt.2002.0362. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Westermann B, Fiedler K, Kaza E, Lotze M, Celio MR. Exploration of the neural correlates of ticklish laughter by functional magnetic resonance imaging. Cereb Cortex. 2013;23:1280–1289. doi: 10.1093/cercor/bhs094. [DOI] [PubMed] [Google Scholar]
- Yadegari F, Azimian M, Rahgozar M, Shekarchi B. Brain areas impaired in oral and verbal apraxic patients. Iranian journal of neurology. 2014;13:77–82. [PMC free article] [PubMed] [Google Scholar]
- Yajima Y, Larson CR. Multifunctional properties of ambiguous neurons identified electrophysiologically during vocalization in the awake monkey. J Neurophysiol. 1993;70:529–540. doi: 10.1152/jn.1993.70.2.529. [DOI] [PubMed] [Google Scholar]