Abstract
OBJECTIVE
To describe the relationship between objectively assessed sleep and blood glucose in a prospective cohort of women recently diagnosed with gestational diabetes mellitus (GDM).
METHODS
Women with GDM were enrolled immediately after attending a GDM education class. All patients were recruited during their first week of attempted dietary management of GDM. They were instructed on the use of a glucometer and on the principles of a GDM diet. Women wore an actigraph and completed a sleep log for 7 consecutive days. Glucose records were compared against the objective sleep data. Linear mixed model analysis was used to estimate the association of sleep duration on morning fasting and one-hour postprandial blood glucose concentrations.
RESULTS
Thirty-seven participants provided data for 213 sleep-intervals that corresponded to at least one glucose reading. Sleep duration was negatively associated with fasting and one-hour postprandial blood glucose concentrations In analyses adjusted for age, gestational age and BMI, a one-hour increase in sleep time was associated with statistically significant reductions in fasting glucose (−2.09 mg/dL, 95% CI −3.98, −0.20) as well as postprandial glucose concentrations [lunch −4.62 mg/dL (95% CI −8.75, −0.50), dinner −6.07 mg/dL (95% CI −9.40, −2.73)].
CONCLUSION
Short sleep durations are associated with worsened glucose control in women with gestational diabetes. Educating women on healthy sleep and screening for and treating sleep disorders during pregnancy may have a role in optimizing blood glucose control in gestational diabetes.
INTRODUCTION
There is emerging interest in evaluating whether poor sleep during pregnancy affects maternal glucose metabolism. Diabetes mellitus complicates 6–7% of all pregnancies with 90% representing gestational diabetes mellitus (GDM)[1]. GDM is associated with an increased risk of preeclampsia, fetal macrosomia, birth trauma, and neonatal metabolic complications. Treatment of GDM during pregnancy has benefits including a decrease in large for gestational age infants, cesarean delivery, shoulder dystocia, and hypertensive disorders of pregnancy[2].
Several studies have found associations between shortened sleep duration (less than 7 hours/night) and impaired glucose metabolism in pregnancy[3–5]. Women with self-reported shortened sleep durations during pregnancy have 2–10 times greater risk of GDM. In addition to short sleep, long sleep durations may also affect glucose metabolism in pregnancy[4]. A limitation of the currently published data is the use of self-reported sleep duration as the exposure variable. Self-reports of sleep duration generally overestimate objectively measured sleep[6].
The aim of this study was to describe the relationship between objectively measured sleep and glucose control in a cohort of women with recently diagnosed GDM. We hypothesized that short and long sleep durations would be associated with higher fasting and 1-hour postprandial glucose values and that greater degrees of sleep disruption would be associated higher glucose concentrations.
MATERIALS AND METHODS
This was a prospective cohort study conducted at Magee-Womens Hospital of the University of Pittsburgh Medical Center from December 2012 to May 2014 with Institutional Review Board approval. Pregnant women with a new diagnosis of GDM were enrolled after obtaining written informed consent. Inclusion criteria included maternal age between 18 and 50 years old and a new diagnosis of GDM, not on treatment with insulin or glyburide. Non-English speaking and women with multiple gestations were excluded. GDM was diagnosed by a fasting 3-hour, 100-gram glucose tolerance test (GTT) with 2 abnormal values: fasting ≥95 mg/dL, 1 hour ≥180mg/dL, 2 hour ≥155 mg/dL, 3 hour ≥140 mg/dL. If no 3-hour GTT was performed (e.g., patient refusal or intolerance) a diagnosis of GDM was made based on a glucose concentration greater than 180 from a 1-hour 50-gram glucose challenge test or greater than three elevated fasting (>95mg/dL) and/or 1-hour postprandial (>140 mg/dL) fingerstick values after 1 week of glucose monitoring.
Participants were enrolled immediately after standardized group education on GDM conducted by diabetic educators. They were instructed on the use of a glucometer, timing of fingerstick glucose testing, and nutritional principles of a GDM diet. They were asked to follow the recommended diet and measure their fasting and 1-hour postprandial glucose values for 1 week. Participants wore an actigraph to objectively assess sleep and completed a sleep diary for the 7 days immediately after their GDM education class. The sleep diary recorded the participant’s subjective bedtime, wake time, and total sleep time and was used to inform the scoring of the actigraph data. Demographic data was obtained as well as obstetrical, medical, and surgical history, current medications, and height and weight information (current and pre-pregnancy). After a week of glucose and sleep monitoring participants met with a physician to determine whether medical management of GDM was warranted. Participants were not on insulin or glyburide during the week of glucose and sleep monitoring. The actigraph and sleep diary were returned and glucose measurements were received at this time.
Actigraphy is established as a valid and objective method of assessing sleep-wake parameters in natural settings[7]. Actigraph devices are worn on the wrist and record movements that can be used to estimate sleep parameters[8]. We used the Actiwatch Spectrum (Respironics) actigraphy device (Figure 1). Participants marked the beginning and end of each rest period by pressing an event marker button on the side of the Actiwatch. The actigraphy data was examined to ensure sufficient quality with no off-wrist time during each sleep interval. Actigraphy data was processed using Actiware software (version 6.0), with default settings (wake threshold medium, 10 minutes immobile for both sleep onset and offset criteria). Rest intervals were set by a single, trained scorer after examination of the event marker and sleep log entries. The event marker was used if a given rest interval had an event marker and sleep log data that were within 15-minutes of each other and reflective of activity in the actogram. If the event marker was present but the actigraphy device was not recording or if the event marker was absent altogether then the sleep log was used if the rest interval and sleep log were in agreement with the actogram data. In situations where event markers and sleep logs were completely absent or present, but with low agreement with activity, then the interval was set by the technician’s judgment[9]. All sleep durations of < 5 hours and > 9 hours were confirmed by a separate examiner. Figure 2 is a representative actigraphy recording from our study.
Figure 1.
Actiwatch Spectrum actigraphy device.
Figure 2.
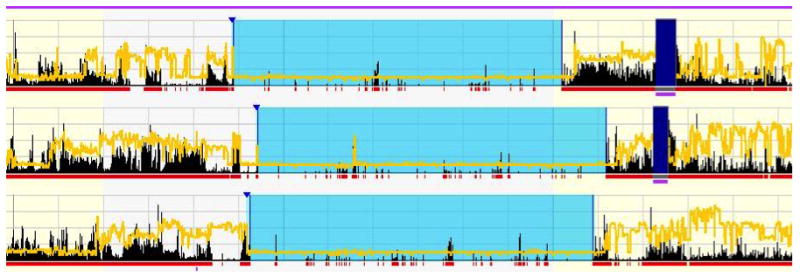
Representative actigraphy recording from a study participant. Rest or sleep intervals are marked in light blue, off-wrist time is indicated by dark purple, activity counts are indicated by black bars, and recorded light exposure is shown by the yellow lines.
Our primary sleep exposure variable was sleep duration during the primary sleep period. Other sleep variables measured were sleep midpoint (sleep onset time-wakeup time/2), wake after sleep onset (minutes spent awake after sleep has been initiated and before final awakening), and sleep fragmentation (sum of percent mobile and percent one minute immobile bouts divided by the number of immobile bouts for the sleep interval). Higher levels of wake after sleep onset and sleep fragmentation indicate worse sleep continuity[8].
We aimed to recruit enough women to have sufficient nights with sleep durations <7 hours to allow for a meaningful analysis. Referring to data from national survey studies[10], we expected that 30% of women would sleep less than 7 hours per night. We aimed for 200 nights of data, which would provide 60 nights with sleep durations of <7 hours. We estimated that we needed to recruit between 40–45 women to achieve this goal.
Relationships between sleep exposures and glucose outcomes were assessed using linear mixed models to provide the proper covariance structure to account for repeated measurements on the same patients. Four glucose outcomes were evaluated (fasting and 1-hour post-breakfast, lunch, and dinner) in each analysis. All models were computed first with no covariates to estimate the unadjusted effects of each sleep exposure on the glucose outcomes; then potential confounders of the sleep-glucose relationship (maternal age, pre-pregnancy BMI, and gestational age at enrollment) were introduced. We performed models that considered sleep exposures as continuous and as categorical variables to account for the possibility of a nonlinear relationship. All statistical analyses were performed using SAS version 9.4 (Cary, NC). P-values <0.05 are considered statistically significant.
RESULTS
Forty-five women were enrolled in the study. To contribute to the analysis a participant must have had at least one day with complete sleep data and at least one glucose measurement the following day. Sufficient data was available for 37 of the 45 enrolled participants (Table 1). Most of the 37 participants who contributed data had nearly complete glucose data. The median number of glucose values that correlated to a night of sleep was 6 fasting, 6 breakfast, 5.5 lunch, and 5.5 dinner.
Table 1.
Descriptive Statistics of Study Participants
| Characteristic | Sufficient Data* For Analysis | Glucose and/or Sleep Data | P-Value† |
|---|---|---|---|
| # Study Participants | 37 | 8 | |
| Age, years | 31 (20–43) | 30 (23–38) | 0.88 |
| Gravidity | 2 (1–7) | 3.5 (2–7) | 0.06 |
| Pre-Pregnancy BMI, kg/m2 | 31 (19–51) | 28 (21–48) | 0.77 |
| Gestational Age at Enrollment, weeks | 28 (6–33) | 28 (10–32) | 0.26 |
| BMI @ Enrollment, kg/m2 | 34 (22–52) | 32 (24–49) | 0.94 |
| Race, n (%) | 0.91 | ||
| White | 26 (70.3%) | 5 (62.5%) | |
| Black | 5 (13.5%) | 1 (12.5%) | |
| Other | 6 (16.2%) | 2 (25.0%) | |
| Tobacco Use, n (%) | 5 (13.5%) | 0 (0.0%) | 0.27 |
| Quit Tobacco During Pregnancy, n (%) | 3 (8.1%) | 0 (0.0%) | 0.40 |
| Prior gestational diabetes, n (%) | 6 (16.2%) | 4 (50.0%) | 0.04 |
| Glucose Screening Results | |||
| 1 hr glucose challenge test, mg/dL | 158 (138–280) | 181 (154–199) | 0.46 |
| Fasting GTT, mg/dL | 90 (63–151) | 82 (64–177) | 0.72 |
| 1-hour GTT, mg/dL | 196 (158–319) | 185 (181–205) | 0.25 |
| 2-hour GTT, mg/dL | 176 (128–317) | 180 (159–215) | 0.83 |
| 3-hour GTT, mg/dL | 117 (59–235) | 119 (69–202) | 0.75 |
Continuous variables reported as median (range); categorical variables reported as n (%)
Sufficient data = participants with at least one day that has both sleep and glucose data
P-values compare N=37 participants vs. N=8 with insufficient data using Wilcoxon tests (continuous) or Chi-Squared Tests (Categorical)
Sleep and blood glucose correlation was possible for 209 fasting glucose readings, 196 breakfast, 188 lunch, and 204 dinner postprandial readings. Median fasting glucose was 92 (range 34–199). Median postprandial glucose values were 122 (84–417), 119 (83–350), and 123 (76–376) for 1-hour post-breakfast, lunch, and dinner, respectively.
There were 213 sleep-nights from 37 women that corresponded to at least one glucose reading. The median bedtime was 11:30 PM (range 8:30 PM – 6 AM) with 61% of the days having a bedtime before midnight. The median sleep time was 6.77 hours (range 1.1–12.3 hours) and women mostly slept somewhere between >5–≤7 hours (46.3%) or >7–≤9 hours (37.8%). Eleven percent of study days had sleep duration less than 5 hours and 4.8% had sleep durations greater than 9 hours.
Increased sleep time was associated with lower glucose at all time points in unadjusted analyses (Table 2). When adjusting for maternal age, pre-pregnancy BMI, and gestational age at enrollment, there were significant associations between sleep and lower fasting glucose (β= −2.09 mg/dl per one-hour increase in sleep, p=0.03), post-lunch glucose (β= −4.62 mg/dl per one-hour increase in sleep, p=0.03), and post-dinner glucose (β= −6.07 mg/dl per one-hour increase in sleep, p=0.001). (Table 2.) The relationship was in the same direction for post-breakfast but was not statistically significant.
Table 2.
Relationships Between Continuous Sleep Exposures and Glucose
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| Glucose Outcome | Exposure | β† | 95% CI | P-Val | β | 95% CI | P-Val |
| Fasting | Bedtime | −0.39 | −1.72 – 0.93 | 0.56 | −1.50 | −2.98 – −0.02 | 0.05 |
| Breakfast | Bedtime | 2.63 | 0.20 – 5.07 | 0.04 | 1.81 | −1.11 – 4.72 | 0.23 |
| Lunch | Bedtime | 0.40 | −2.35 – 3.17 | 0.77 | 0.11 | −3.16 – 3.38 | 0.95 |
| Dinner | Bedtime | 3.68 | 1.28 – 6.07 | 0.003 | 3.49 | 0.67 – 6.31 | 0.02 |
| Glucose Outcome | Exposure | ||||||
| Fasting | Sleep Time | −2.52 | −4.35 – −0.69 | 0.008 | −2.09 | −3.98 – −0.20 | 0.03 |
| Breakfast | Sleep Time | −3.72 | −7.01 – −0.43 | 0.03 | −3.05 | −6.52 – 0.42 | 0.09 |
| Lunch | Sleep Time | −4.32 | −8.16 – −0.48 | 0.03 | −4.62 | −8.75 – −0.50 | 0.03 |
| Dinner | Sleep Time | −5.97 | −9.14 – −2.79 | <0.001 | −6.07 | −9.42 – −2.73 | 0.001 |
| Glucose Outcome | Exposure | ||||||
| Fasting | Sleep Midpoint | −1.27 | −2.82 – 0.28 | 0.11 | −2.51 | −4.18 – −0.85 | 0.004 |
| Breakfast | Sleep Midpoint | 1.85 | −0.99 – 4.70 | 0.21 | 0.69 | −2.59 – 3.97 | 0.68 |
| Lunch | Sleep Midpoint | −1.30 | −4.68 – 2.09 | 0.46 | −1.92 | −5.77 – 1.94 | 0.33 |
| Dinner | Sleep Midpoint | 2.52 | −0.32 – 5.36 | 0.08 | 2.03 | −1.19 – 5.25 | 0.22 |
| Glucose Outcome | Exposure | ||||||
| Fasting | Fragmentation | 0.26 | −0.06 – 0.57 | 0.12 | 0.15 | −0.20 – 0.49 | 0.40 |
| Breakfast | Fragmentation | 0.36 | −0.26 – 0.97 | 0.26 | 0.13 | −0.54 – 0.81 | 0.70 |
| Lunch | Fragmentation | 0.71 | 0.03 – 1.39 | 0.04 | 0.81 | 0.06 – 1.56 | 0.04 |
| Dinner | Fragmentation | 0.64 | 0.03 – 1.25 | 0.04 | 0.64 | −0.03 – 1.30 | 0.06 |
| Glucose Outcome | Exposure | ||||||
| Fasting | WASO‡ | 0.10 | −0.007 – 0.21 | 0.07 | 0.08 | −0.04 – 0.19 | 0.20 |
| Breakfast | WASO | 0.03 | −0.23 – 0.28 | 0.84 | −0.07 | −0.34 – 0.20 | 0.60 |
| Lunch | WASO | 0.12 | −0.14 – 0.38 | 0.38 | 0.11 | −0.17 – 0.38 | 0.44 |
| Dinner | WASO | 0.01 | −0.21 – 0.24 | 0.92 | −0.01 | −0.25 – 0.22 | 0.92 |
Sleep and blood glucose correlation was possible for 209 fasting glucose readings, 196 breakfast, 188 lunch, and 204 dinner postprandial readings
Adjusted for maternal age, gestational age at enrollment, and pre-pregnancy BMI
β coefficients represent the change in glucose with one-unit increase in each predictor variable (i.e. 1 hour of sleep time)
WASO=wake time after sleep onset
A later sleep midpoint was significantly associated with lower fasting glucose but no relationships were observed between sleep midpoint and postprandial blood glucose. Increasing sleep fragmentation was also associated with slightly higher post-lunch and post-dinner glucose. There was no relationship seen between wake after sleep onset and glucose.
We repeated the previous analysis using categories for sleep time (Table 3) using ≥7–<9 hours as the reference category. This analysis revealed that women with extremely short sleep (< 5 hours) significantly drove the effects from the prior set of models. It was associated with significantly higher glucose values at all measurement time points, with marked increases in postprandial glucose values.
Table 3.
Relationships Between Categorical Sleep Times and Glucose
| Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|
| Glucose Outcome | Exposure | β† | 95% CI | P-Val | β | 95% CI | P-Val |
| Fasting | Sleep < 5 Hrs | 17.42 | 6.92 – 27.92 | 0.003 | 15.87 | 5.13 – 26.61 | 0.007 |
| Sleep ≥5–<7 Hrs | 3.12 | −2.62 – 8.85 | 0.30 | 2.23 | −3.96 – 8.43 | 0.49 | |
| Sleep ≥7–<9 Hrs | 0.00 | Ref | Ref | 0.00 | Ref | Ref | |
| Sleep ≥9 Hrs | −3.02 | −15.32 – 9.28 | 0.63 | −2.90 | −15.35 – 9.55 | 0.65 | |
| Breakfast | Sleep < 5 Hrs | 35.25 | 17.18–53.33 | 0.001 | 32.31 | 13.10 – 51.52 | 0.003 |
| Sleep ≥5–<7 Hrs | 1.64 | −8.96 – 12.23 | 0.76 | −1.75 | −13.40 – 9.90 | 0.77 | |
| Sleep ≥7–<9 Hrs | 0.00 | Ref | Ref | 0.00 | Ref | Ref | |
| Sleep ≥9 Hrs | 16.11 | −5.70 – 37.92 | 0.16 | 12.97 | −9.74 – 35.69 | 0.27 | |
| Lunch | Sleep < 5 Hrs | 40.14 | 20.65 – 59.63 | <0.001 | 42.43 | 21.51 – 63.34 | <0.001 |
| Sleep ≥5–<7 Hrs | 4.90 | −7.41 – 17.20 | 0.44 | 5.86 | −8.15 – 19.87 | 0.42 | |
| Sleep ≥7–<9 Hrs | 0.00 | Ref | Ref | 0.00 | Ref | Ref | |
| Sleep ≥9 Hrs | 8.70 | −17.35 – 34.76 | 0.52 | 9.68 | −17.76 – 37.12 | 0.50 | |
| Dinner | Sleep < 5 Hrs | 43.78 | 26.97 – 60.59 | <0.001 | 43.28 | 25.31 – 61.24 | <0.001 |
| Sleep ≥5–<7 Hrs | −2.77 | −13.24 – 7.71 | 0.61 | −2.78 | −14.67 – 9.12 | 0.65 | |
| Sleep ≥7–<9 Hrs | 0.00 | Ref | Ref | 0.00 | Ref | Ref | |
| Sleep ≥9 Hrs | −2.10 | −24.07 – 19.88 | 0.85 | −2.37 | −25.47 – 20.73 | 0.84 | |
Sleep and blood glucose correlation was possible for 209 fasting glucose readings, 196 breakfast, 188 lunch, and 204 dinner postprandial readings
Adjusted for maternal age, gestational age at enrollment, and pre-pregnancy BMI
β coefficients represent the change in glucose compared to reference group of ≥7–<9 hrs of sleep
DISCUSSION
This study demonstrates that shorter sleep durations are associated with poorer glucose control in women with gestational diabetes. Sleep disorders are prevalent in pregnancy, and pregnancy itself has been linked to alterations in sleep[11–14]. Additionally, data suggests that the United States population is sleeping less over time. Since 1985, age-adjusted mean sleep duration has decreased and the percentage of adults sleeping less than 6 hours has increased by 31%, with an estimated 70.1 million Americans sleeping less than 6 hours in 2012[15]. Disturbed sleep is associated with oxidative stress, metabolic dysregulation, endothelial dysfunction and inflammation[16–18]. These same pathophysiologic mechanisms have been implicated in the pathogenesis of adverse pregnancy outcomes, including gestational diabetes[19, 20].
Data from the Sleep Heart Health Study suggest that compared to individuals sleeping 7–8 hours/night, individuals who report sleeping ≤ 5 hours/night or less than 6 hours/night have an adjusted odds ratio for diabetes of 2.51 and 1.66, respectively[21]. Investigations of sleep duration in pregnancy have predominantly used subjective measures, but demonstrate an association between short sleep and gestational diabetes[3, 4]. In an actigraphy-based analysis of 63 women, shorter sleep was associated with a higher rate of abnormal values in routine 1-hour glucose tolerance testing[22].
Our study was designed to evaluate the association of objectively assessed sleep and glycemic control in pregnancy after gestational diabetes is diagnosed. Our results indicate that short sleep durations in pregnancy are associated with poorer glucose control. We demonstrated a 2–6 mg/dL increase in glucose per hour less of sleep. If these associations were entirely causal in nature (which is unlikely), increasing sleep time from 5 hours to 8 hours per night has the potential to result in a 6–18 mg/dL reduction in blood glucose throughout the day. Given the glucose values in our sample, this represents a 5–20% improvement. The magnitude of this effect is similar to that seen with the administration of 2.5–5mg of glyburide[23].
Our data did not demonstrate a robust relationship between sleep continuity measures and glycemic control. Additionally, our data regarding sleep timing did not reveal any consistent relationships between sleep midpoint and glucose control, but our analysis is limited secondary to not having data regarding meal timing and dietary intake. Just as appetite and calorie intake vary over the 24-hour period, so does the body’s ability to process and utilize energy. Altered circadian timing may lead to appetite dysregulation and staying up later may also affect the availability of and preference for certain foods[24, 25]. Our study is also limited by the number of participants with long sleep (≥ 9 hours), which prohibited a more in depth analysis of the possible curvilinear relationship between sleep and glycemic control.
In pregnancies complicated by GDM, failure to achieve glycemic control occurs in 10–20% of cases[26] and is associated with a higher rate of adverse pregnancy outcomes[27]. Our study was powered to examine the relationship between sleep and glucose control and not clinical outcomes related to GDM such as macrosomia, neonatal hypoglycemia, and rate of cesarean delivery. Furthermore, it is not known whether interventions to improve sleep in pregnancy could optimize glycemic control or improve clinical outcomes in women with GDM.
Our results support those of previous studies that demonstrated a positive association between self-reported short sleep duration and maternal metabolism[3–5] and strengthen these associations with the use of objective sleep data. Moreover, beyond being associated with an increased risk of GDM, short sleep may limit the ability to achieve glucose control during pregnancy once GDM is diagnosed.
Acknowledgments
Supported by a Magee Womens Research Institute-Clinical Trainee Research Award, K12HD043441.
Footnotes
Presented as a poster at the Society for Maternal-Fetal Medicine Conference; New Orleans, LA; February 3–8, 2014, and at the Society for Maternal-Fetal Medicine Conference; San Diego, CA; February 2–7, 2015.
References
- 1.Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 2.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142.e1–5. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34:2454–7. doi: 10.2337/dc11-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 8.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514–27. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyne K, Sherry DD, Gallagher PR, Olsen M, Brooks LJ. Accuracy of computer algorithms and the human eye in scoring actigraphy. Sleep Breath. 2013;17:411–7. doi: 10.1007/s11325-012-0709-z. [DOI] [PubMed] [Google Scholar]
- 10.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 12.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 13.Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003;9:477–83. doi: 10.1097/00063198-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration Among US Adults From 1985 to 2012. Sleep. 2014 doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–32. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 22.Herring SJ, Nelson DB, Pien GW, Homko C, Goetzl LM, Davey A, et al. Objectively measured sleep duration and hyperglycemia in pregnancy. Sleep Med. 2014;15:51–5. doi: 10.1016/j.sleep.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartor G, Melander A, Schersten B, Wahlin-Boll E. Serum glibenclamide in diabetic patients, and influence of food on the kinetics and effects of glibenclamide. Diabetologia. 1980;18:17–22. doi: 10.1007/BF01228296. [DOI] [PubMed] [Google Scholar]
- 24.Reinberg A, Migraine C, Apfelbaum M, Brigant L, Ghata J, Vieux N, et al. Circadian and ultradian rhythms in the feeding behaviour and nutrient intakes of oil refinery operators with shift-work every 3–4 days. Diabete Metab. 1979;5:33–41. [PubMed] [Google Scholar]
- 25.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33:1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–41. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 27.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]



