Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that leads to the progressive deterioration of cognitive and memory functions. The deposition of extracellular beta-amyloid (Aβ) senile plaques and intracellular tau neurofibrillary tangles are considered the cardinal pathological hallmarks of AD, however, accumulating evidence indicates that immune cells may also play an important role in disease pathogenesis. Among these immune cells, blood-derived cells and their infiltration into the CNS towards Aβ plaques have been implicated in therapeutic strategies against AD. Here, we review the current literature on blood cell migration into the AD brain and the important players involved in this selective migration towards Aβ plaques.
1. AD pathology and β-amyloid plaques
Alzheimer’s disease (AD) is characterized by the presence of extracellular senile beta-amyloid (Aβ) plaques and intracellular neurofibrillary tau tangles, however, other disease pathology features include the loss of cholinergic neurons and synapses, the loss of white matter, congophilic/cerebral amyloid angiopathy (CAA), inflammation, oxidative stress and cerebrovascular dysfunction (Mufson et al., 2008; Perl, 2010; Querfurth and LaFerla, 2010; Serrano-Pozo et al., 2011). Senile plaques are primarily composed of Aβ peptides, byproducts of amyloid precursor protein (APP) metabolism following its sequential cleavage by the enzymes β- and γ-secretase, which results in the generation of two Aβ species: Aβ40 and Aβ42 (LaFerla et al., 2007). Aβ40 is the more prevalent isoform found in vivo and serves as a major component of CAA (LaFerla et al., 2007; Serrano-Pozo et al., 2011). Aβ42 makes up only 10% of the total Aβ, but is the more predominant toxic species found in plaques due to its enhanced hydrophobicity, aggregation and fibrillization potential. It can spontaneously self-aggregate to generate soluble neurotoxic oligomers or insoluble fibrils that form plaques (LaFerla et al., 2007; Querfurth and LaFerla, 2010). Neuritic or dense-core senile plaques contain Aβ fibrils arranged radially into a central core. More importantly, these plaques are typically surrounded by dystrophic neurites, reactive astrocytes, activated microglial cells, and synaptic loss, indicating that these cells may play an important role in disease pathology (Fig. 1A). Abnormal mitochondria and lysosomes have also been found within these activated cells, indicating that energy or protein degradation processes may be compromised. In addition, there is also evidence that Aβ plaques are directly associated with brain vessels, indicating that blood vessel proximity may play a role in plaque formation and/or remodeling (Kumar-Singh et al., 2005).
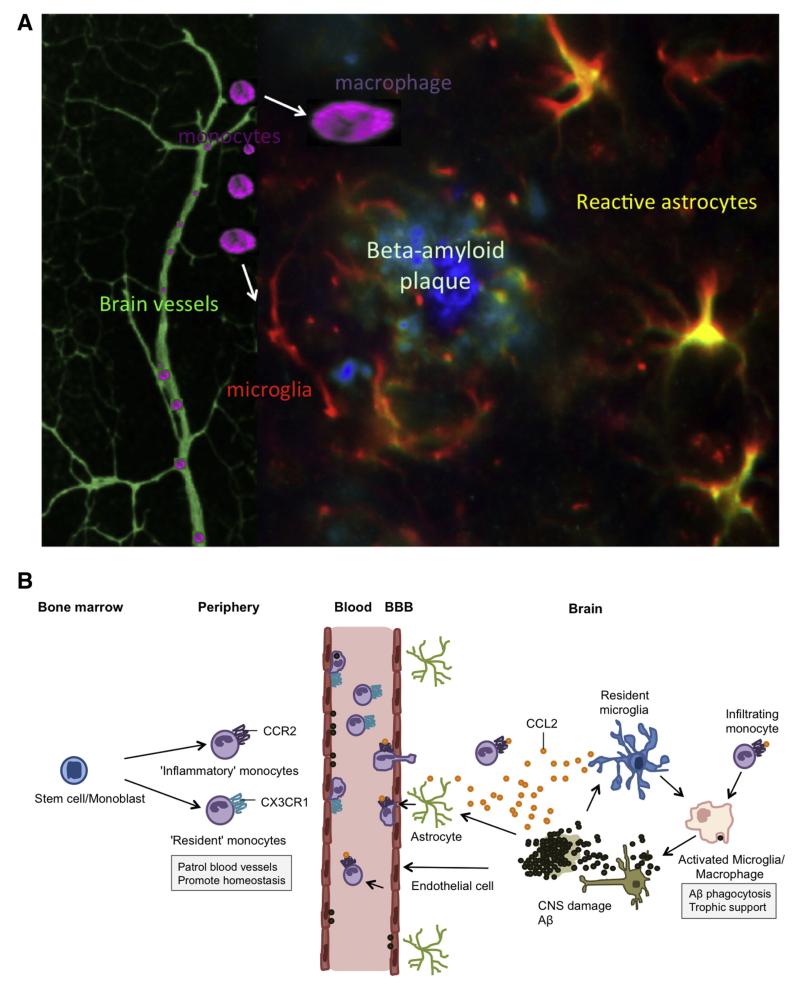
Fig. 1.
The migration of peripheral monocytes to beta-amyloid (Aβ) plaques in the AD brain. (A) A schematic rendering of fluorescent stainings (taken from our own laboratory) of an Aβ plaque with associated brain vessels. The Aβ core contains aggregated Aβ peptides, surrounded by reactive astrocytes and activated microglia. Monocytes migrate into the brain and may differentiate into macrophages or microglia. (B) A hypothetical rendering of monocyte recruitment into the AD brain. The recruitment of monocytes into the AD brain begins when Aβ deposition and associated neuronal damage triggers a local immune response activating astrocytes, endothelial cells, and microglia. This activation leads to the secretion of the chemokine CCL2, which recruits more immune effector cells (mainly CCR2+ monocytes) to the site of parenchymal Aβ deposition. Resident microglia appear to lose their ability to effectively phagocytose Aβ, however, blood-derived monocytes differentiate into macrophages, which are more effective at phagocytosis and clearing Aβ plaques. Although CCR2+ inflammatory monocytes have become the primary monocyte subpopulation implicated in providing therapeutic benefits to the AD brain, recent data indicates that CX3CR1hi resident monocytes may be responsible for clearing vascular Aβ deposition.
This cartoon B has been partly adapted and modified from others: Britschgi and Wyss-Coray (2007), El Khoury and Luster (2008), Gate et al. (2010), Hickman and El Khoury (2010), Malm et al. (2010), Michaud et al. (2013), Mildner et al. (2011).
2. Inflammatory processes around β-amyloid plaques in AD
In AD brains, Aβ plaques are surrounded by activated microglial cells and reactive astrocytes. In addition, several inflammation-related mediators have been found within plaques. Microglia are considered the resident macrophages of the CNS and serve as important players in driving the inflammatory response in AD (Wyss-Coray and Rogers, 2012). However, they also prove vital in promoting and maintaining a healthy CNS (Schwartz and Shechter, 2010). Using their highly motile processes they constantly sample and survey their surrounding microenvironment making them the first line of defense against pathogens and injury in the brain (Glass et al., 2010; Wyss-Coray and Rogers, 2012). Without physiological stress, microglia display a ramified or deactivated phenotype secreting anti-inflammatory and neurotrophic factors (Glass et al., 2010; Khandelwal et al., 2011). Histological studies have shown that activated (amoeboid) microglia surround senile plaques in AD brains, along with astrocytes, and that these cells stain positive for inflammatory markers including major histocompatibility complex (MHC) class II, cyclooxygenase (COX)-2, monocyte chemoattractant/chemotactic protein (MCP)-1, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, or IL-16 (Akiyama et al., 2000; Glass et al., 2010). However, the role of these cells is somewhat complex. On one side, data indicates that microglia are poor phagocytes of Aβ and thus cannot play a significant role in Aβ clearance or plaque remodeling, either promoting or protecting against Aβ-induced pathology (Gate et al., 2010; Grathwohl et al., 2009). On the other side, reports indicate that microglial cells are capable of remodeling and enhancing the clearance of Aβ plaques (Chakrabarty et al., 2010; Gate et al., 2010; Kiyota et al., 2009). Despite these findings, it is becoming more accepted that microglia activation (and the release of neurotoxic factors) is a secondary event following Aβ deposition and aggregation (Schwartz and Shechter, 2010).
3. The migration of blood cells into the CNS
The brain employs several immune control mechanisms to tightly regulate inflammation and its potential brain damage-promoting pathways (Rezai-Zadeh et al., 2009). One important feature of this immune privilege involves the blood–brain barrier (BBB), which in the context of inflammation, is responsible for restricting the entry of substrates and cells into the brain (Rezai-Zadeh et al., 2009). During an effective immune response, vascular permeability is increased along with blood flow to the site of inflammation or injury. This is accompanied by the selective entry of immune cells from the periphery, which help further propagate an inflammatory response by producing cytokines and carrying out other important effector cell functions (Yong and Rivest, 2009). Migration from the blood into the brain occurs at post capillary venules and involves a multiple-step process of events that are mediated by various chemokines, their associated receptors and adhesion molecules.
Peripheral immune cell migration into the CNS resembles typical leukocyte extravasation into other organs. There are four ‘classic’ steps of immune cell extravasation including: 1) capture (tethering) and rolling, which involves the interaction of selectins and mucins and/or integrins and IgG members; 2) activation, which is propagated by chemokines leading to integrin activation; 3) arrest, which is mediated through integrins and their counter-ligands; and 4) diapedesis or transmigration (Rossi et al., 2011). First, immune cells roll along the endothelium. This is initiated by selectin binding resulting in the ‘tethering’ of the immune cell to the endothelium (this involves e.g., P-selectin and very late antigen-4 (VLA-4)) (Malm et al., 2010). Interestingly, a subset of immune cells also patrols the resting endothelium circulating against the direction of blood flow; dependent on the interaction of a specific integrin, e.g., the chemokine receptor CX3CR1 (Prinz et al., 2011). During the process of rolling and tethering, engagement with a chemoattractant can lead to integrin activation on the immune cells and increased affinity of the integrin for its associated adhesion molecule on the endothelium. This ultimately leads to immune cell adhesion. Following adhesion, the immune cell undergoes transendothelial migration or extravasation from the blood vessel. These early transmigration events involve e.g., platelet endothelial cell adhesion molecule (PECAM-1), a cell adhesion molecule expressed on endothelial cells, and e.g., RAGE, which is a receptor for macrophage 1 (MAC-1; CD11b) (Malm et al., 2010). Once across the endothelium and basement membrane, the immune cell crosses the perivascular space and glia limitans and migrates towards the source of the chemoattractant (i.e., the site of inflammation). In vitro studies have demonstrated that Aβ promotes the expression of adhesion molecules and can enhance immune cell adhesion and subsequent transmigration across the BBB.
3.1. Red blood cells in the AD brain
Although alterations have been found in red blood cells isolated from AD patients (Blass et al., 1985; Cortes-Canteli et al., 2012; Mohanty et al., 2010; Sabolovic et al., 1997; Zhang et al., 2013), there is no indication that these cells infiltrate the AD brain nor that such infiltration plays a role in disease progression. It could, however, be possible that these cells affect leukocyte infiltration into the CNS. In AD, red blood cells show membrane alterations as well as impairments in their oxygen delivery capabilities (Mohanty et al., 2010), which could result in aggravating oxidative stress and vascular injury, ultimately leading to enhanced endothelial permeability and the infiltration of peripheral immune cells.
3.2. Platelets in the AD brain
Platelets are responsible for maintaining the integrity of blood vessels and promoting hemostasis. These cells are of particular interest in AD since they contain high amounts of APP and release Aβ40 into the blood stream (Evin et al., 2003). Furthermore, studies have shown that the larger 130 kDa APP isoform is significantly reduced in platelets isolated from AD patients, implicating their potential role in altered APP metabolism (or use in the development of AD biomarkers) (Padovani et al., 2001). Vascular injury or stimulation via Aβ could result in platelet activation and adhesion to the vessel wall. These studies along with others provide increasing evidence that platelet activation contributes to CAA. In this context, activated platelets in turn could produce more Aβ as well as chemokines (Zhang et al., 2013), promoting immune cell infiltration. In fact, platelets are known amplifiers of leukocyte adhesion (von Hundelshausen et al., 2009). A recent study by Thornton and colleagues demonstrated that platelets secrete IL-1α, which drives the transendothelial migration of neutrophils in vitro (Thornton et al., 2010). However, thus far there have been no indications that platelets migrate into the AD brain (Kniewallner et al., 2015).
3.3. Leukocytes in the AD brain
Leukocytes are considered the immune cells of the periphery and can be divided into granulocytes including basophils (<1%), eosinophils (<6%), and neutrophils (40–75%), and lymphocytes (20–45%) including T cells, B cells, and natural killer cells and monocytes (2–10%). Although the AD brain does not present prominent infiltration of peripheral leukocytes such as those seen in other neuroinflammatory diseases (e.g., multiple sclerosis), recent studies indicate that their infiltration does occur and is stimulated by Aβ (Rezai-Zadeh et al., 2009). For instance, early investigations demonstrated that Aβ infusion in rats results in the adhesion and migration of leukocytes across arteries, venules and cerebral vessels (Rossi et al., 2011). However, whether this infiltration does indeed occur in AD and by which particular cell type, remain under intense debate.
3.3.1. Granulocytes
Neutrophils are the most abundant population of cells in the peripheral blood accounting for 40–75% of leukocytes in human blood. These cells specialize in phagocytosis, rapidly mobilizing to areas of infection and known for their ability to work in anaerobic conditions. However, the lifespan and functional role of neutrophils during an innate immune response is relatively short-lived, compared to macrophages, which help propagate and sustain a local response. Neutrophil infiltration into the CNS has been implicated in CNS diseases involving an inflammatory reaction (e.g., bacterial meningitis) (Ransohoff and Brown, 2012), however, whether this migration takes place under chronic neuroinflammatory conditions (e.g., multiple sclerosis and AD) remains unknown. In AD, one recent study reports that Ly6C/G (Gr-1)+ (a marker for cells of the myeloid lineage) cells migrate towards Aβ plaques in an AD disease mouse model. Using 2-photon microscopy the authors observed dynamic extravasation of these cells from blood vessels into the brain parenchyma of 9- to 13-month-old 5XFAD mice (Baik et al., 2014). Although the underlying mechanism for this migration is still unclear, data from these experiments indicates that Aβ plaques, but not soluble Aβ, may play an important role in recruiting these cells from the blood. A previous study in neutrophils has shown that serpin-enzyme complex (SEC) receptor mediates the chemotaxis of these cells in response to the Aβ25–35 peptide (Joslin et al., 1992). Thus, it could be possible that depending on the species of Aβ, different inflammatory responses (i.e., an early and short proinflammatory burst response vs. a low and chronic response) may be generated based on the activation of specific receptors and pathways. However, a more detailed discussion of this issue can be found in the monocyte section.
3.3.2. Lymphocytes
Although peripheral lymphocyte migration is a not prominent feature in the AD brain like other neurodegenerative diseases (i.e., multiple sclerosis), T cell infiltration has been observed in the brains of AD patients (Itagaki et al., 1988; Rogers et al., 1988; Togo et al., 2002; Town et al., 2005), albeit at low numbers (Lucin and Wyss-Coray, 2009; Rossi et al., 2011). In addition to the brain parenchyma, alterations in T cells have been detected in the periphery of AD patients (Larbi et al., 2009; Monsonego et al., 2003, 2006) and CD4+ and CD8+ T cells have been found associated with CAA pathology, in addition to monocytes and macrophages in leptomeningeal and cortical vessels (Yamada et al., 1996). Previous investigations have shown that peripheral T cells in AD patients overexpress chemokines and receptors (i.e., MIP-1α and CXCR2) associated with T cell migration across the endothelium (Liu et al., 2010; Man et al., 2007). Furthermore, hippocampal Aβ injection in the rat results in the upregulation of receptors (i.e., RAGE, CCR5) involved in promoting T cell migration into the brain (Li et al., 2009).
Despite these findings, it still remains unclear how T cells enter the brain through the BBB and what specific stimulus is responsible for T cell accumulation in the AD brain (Monsonego et al., 2006, 2013; Rossi et al., 2011). Recent in vitro studies suggest that Aβ1–42-treated microglia release TNF-α to promote transendothelial migration of T cells via MHC I expression (Yang et al., 2013). In addition, studies involving Aβ immunization have indicated that CD11c+ dendritic cells may stimulate Aβ-specific T cells to target Aβ depositions in the brain (Fisher et al., 2014). Specific cytokines may also play an important role in T cell infiltration. A previous study by Buckwalter and colleagues showed that local expression of TGF-β1 increases both meningeal and parenchymal T cell number in a mouse model of AD following Aβ1–42 immunization (Buckwalter et al., 2006).
It also remains unclear what role these cells play in disease pathology and cognitive outcome. Previous studies indicate that T cells in the CNS can both promote and impair cognitive processes based on their given subtype (Estes and McAllister, 2014). Specifically, recent preliminary data indicates that transferring Aβ-specific Th1 cells enhances Aβ plaque development in APP-swe/PS1dE9 mice, whereas transferring Aβ-specific Th2 cells has no effect. Interestingly, the transfer of Th17 cells reduces Aβ concentrations in the hippocampus (Lynch and Mills, 2012). In another study, systemic transplantation of T regulatory cells ameliorates cognitive impairment and reduces Aβ plaque deposition and soluble Aβ levels. This treatment also resulted in a reduction in microglial activation and systemic inflammatory factors (Yang et al., 2013). Although not characterized from the periphery, a recent study showed that intracerebroventricular-injected Th1 CD4+ T cells are able to migrate within the brain parenchyma associated with increased expression of ICAM-1 and MHC II. This study also shows that these T cells effectively target Aβ plaques, increase Aβ uptake and promote neurogenesis in a mouse model of AD (Fisher et al., 2014). In addition, these authors have shown that T cell migration towards Aβ plaques and significant plaque clearance also occur in APP/IFN-γ double transgenic mice following Aβ immunization (Fisher et al., 2010). However, in both transgenic mice and AD patients this Aβ immunization results in meningoencephalitis (Buckwalter et al., 2006; Fisher et al., 2014).
3.3.3. Monocytes
Monocytes represent 2–10% of peripheral blood leukocytes in humans and rodents. They are generated from myeloid precursor cells in the bone marrow and released into the bloodstream where they circulate before entering organs and differentiating into tissue-specific macrophages and dendritic cells (Ziegler-Heitbrock, 2007). Monocytes are characterized morphologically as mononuclear cells with bean-shaped nuclei and phenotypically by their expression of surface markers. There are a wide variety of phenotypically and functionally heterogeneous monocyte subpopulations varying in maturation, differentiation, and activation states based on their differential expression of these surface markers (Auffray et al., 2009; Buckner et al., 2011; Shi and Pamer, 2011).
In humans, monocytes are identified by their expression of CD14 and CD11b and further characterized into heterogeneous subsets by their differential expression of CD14 and CD16. Mouse monocytes are defined by their expression of CD11b (membrane-activating complex 1 (Mac-1)), CD115 (M-CSF receptor), and F4/80 (Strauss-Ayali et al., 2007). In both humans and rodents, there are two main subpopulations that divide monocytes phenotypically and functionally: ‘classical’ or ‘inflammatory’ monocytes (Ly6C+ in mice), which express higher levels of CCR2, and ‘non-classical’ or ‘resident’ monocytes (Ly6C- in mice), which express higher levels of CX3 chemokine receptor 1 (CX3CR1; fractalkine) (Yrlid et al., 2006). Michaud et al. (2013) recently showed in mice that patrolling (Ly6Clo) monocytes are attracted to the walls of Aβ-positive veins. Although more functional studies are needed to help distinguish their distinct functional and physiological roles, recent studies in rodent models have concluded that CCR2-expressing monocytes are recruited to sites of inflamed or injured tissue, whereas CX3CR1-expressing cells exhibit more patrolling behavior and involvement in tissue maintenance (Gordon and Taylor, 2005; Strauss-Ayali et al., 2007).
Monocytes play an important role during innate and adaptive immune responses (Tacke and Randolph, 2006; Ziegler-Heitbrock, 2007). Inflammation and other injury lead to an increased recruitment of monocytes to peripheral tissues aiding in host immune system defense and repair. During an inflammatory response, monocytes are recruited to the sites of inflammation by CCL2 (classical monocytes) or CX3 chemokine ligand 1 (CX3CL1; resident monocytes) and subsequently differentiate into effector cells (Ziegler-Heitbrock, 2007). Depending on their surface marker expression, monocytes can further propagate the immune response by secreting inflammatory mediators including cytokines and chemokines (proinflammatory monocytes), by giving rise to antigen-presenting cells including macrophages and dendritic cells, or by performing antigen-presenting cell activity themselves (i.e., tissue repair and phagocytosis) (Khandelwal et al., 2011).
3.4. Monocyte-derived neurotoxicity
The role of monocytes and monocyte-derived cells in the propagation of neurodegenerative disease is still under intense debate. Similar to microglia, these cells may induce beneficial effects, but also contribute to neurodegeneration through uncontrolled inflammation and/or neurotoxic pathways. Previous studies have shown that microglia and THP-1 monocytes can interact with fibrillar Aβ and stimulate the production of proinflammatory cytokines, reactive oxygen species (ROS), and neurotoxic secretory molecules, ultimately resulting in neuronal apoptosis (Combs et al., 2001). Specifically, studies have demonstrated that CD36, a class B scavenger receptor, CD47, an integrin-associated protein, and α6β1-integrin form a cell surface receptor complex that mediates the binding of microglia or THP-1 monocytes to Aβ fibrils. This binding subsequently activates a tyrosine kinase intracellular signaling cascade stimulating IL-1β, TNF-α and ROS production (Bamberger et al., 2003). In line with these studies, El Khoury and colleagues have also shown that CD36 can mediate the response of microglia and macrophages to Aβ, including promoting the secretion of H2O2, cytokines, chemokines and ROS (Coraci et al., 2002; El Khoury et al., 2003). In addition to CD36, recent investigations have also identified the NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome as an important mediator of microglia/monocyte-induced neurotoxicity. These studies have demonstrated that fibrillar Aβ can act as an activator of inflammasomes, which promotes caspase-1 signaling and the subsequent release of IL-1β, IL-18, IL-1α proinflammatory cytokines (Sheedy et al., 2013). Converging evidence indicates that phagocytosis of Aβ (through the interaction of CD36 and a TLR heterodimer of TLR4-TLR6) and subsequent loss of lysosome integrity, triggered by aggregated or insoluble material, result in the release of lysosomal content (e.g., cathepsin B and/or ROS) and assembly of the inflammasome (Halle et al., 2008; Sheedy et al., 2013). Thus, it appears that the engulfment and endocytosis of Aβ by macrophages results in the conversion of soluble Aβ into its more fibrillar or pathogenic form, ultimately triggering dysfunctional degradation/lysosome damage and activating the inflammasome and release of proinflammatory and neurotoxic molecules (Sheedy et al., 2013). Interestingly, a recent study by Heneka and colleagues has shown that a deficiency in the NLRP3 inflammasome results in the skewing of microglial cells towards a more M2 or anti-inflammatory phenotype, as well as, attenuates amyloid accumulation in an AD mouse model (Heneka et al., 2013). Together, these studies indicate that microglia and monocyte-derived cells also play an important role in promoting proinflammatory and neurotoxic pathways. Thus, developing strategies against chronic monocyte/microglial cell inflammatory activation may prove beneficial for AD neurodegeneration. In support of this, recent investigations have shown that the use of peroxisome proliferator-activated receptor γ (PPARγ) agonists, which suppress inflammatory gene expression, ameliorate spatial memory performance in an AD mouse model. The authors report that treatment of a PPARγ agonist results in the enhanced phagocytosis of Aβ by microglial cells (mediated by increased CD36 expression) as well as the reduction of cortical and hippocampal Aβ levels (Yamanaka et al., 2012).
3.5. Implications for monocyte-derived recruitment, phagocytosis and therapeutic use in AD
Emerging evidence from studies in stroke, brain trauma, and AD indicate that these severe brain disorders can lead to BBB breakdown and subsequent migration of blood-derived monocytes into the CNS (Malm et al., 2010). Interestingly, post-mortem brain sections of AD patients with co-morbid stroke exhibit deposition of brain-infiltrating macrophages containing Aβ fibrils (Wiesniewski et al., 1991; Akiyama et al., 1996). Despite the detrimental role of leukocyte infiltration in some neurological disorders, accumulating data indicates that peripheral monocytes may be beneficial to AD in ameliorating disease pathology and progression.
One of the first studies to demonstrate the benefits of monocytic cells was the investigation performed by Simard and colleagues in mice, which showed that blood-derived microglia (now referred to as bone marrow-derived/blood-derived monocytes or macrophages) are recruited to sites of Aβ plaque deposition (triggered by Aβ40 and Aβ42) and can eliminate Aβ deposits via phagocytosis (Simard et al., 2006). Following this study, El Khoury et al. demonstrated that CCR2 deficiency, or disrupting the recruitment and accumulation of mononuclear phagocytes (i.e., microglia/blood-derived monocytes) to lesion sites, impairs the accumulation of these cells near Aβ plaques and accelerates Aβ plaque burden in AD transgenic mice (El Khoury et al., 2007). Together, these studies indicate that blood-derived monocytes play an important neuroprotective role via Aβ clearance. In support of these findings, others have shown that stimulating the infiltration or turnover of peripheral monocytic cells (e.g., blocking immunosuppressive TGF-β or administrating M-CSF, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), chitin, or LPS, or NGF) can attenuate AD pathology (i.e., reducing parenchymal and cerebrovascular Aβ deposition) and cognitive deficit in AD rodent models (Boissonneault et al., 2009; Hawkes and McLaurin, 2009; Malm et al., 2010; Naert and Rivest, 2013; Rezai-Zadeh et al., 2009; Town et al., 2008; Yong and Rivest, 2009; Hohsfield et al., 2014). In addition to eliminating Aβ deposits and preventing plaque formation, these cells (mainly the anti-inflammatory subset) are also capable of arresting the local production of proinflammatory factors (e.g., TNF), enhancing neurogenesis, releasing proteolytic enzymes and proteinases, and producing reparative growth factors (e.g., insulin-like growth factor I, IGF-I) (Gate et al., 2010; Malm et al., 2010; Schwartz and Shechter, 2010).
However, there is still some controversy regarding monocyte infiltration in the AD brain. Our lab and other researchers have provided convincing in vitro data indicating that the transmigration of monocytes into the CNS is a specific response to Aβ (Fiala et al., 1998; Giri et al., 2000; Humpel, 2008; Malm et al., 2010). In support of these findings, others have shown that this is also true in vivo. Lebson et al. recently reported that significant monocyte deposition is present only in the brains of AD transgenic mice, neighboring Aβ plaques, whereas little or no cell deposition is present in the brains of control animals (Lebson et al., 2010; Malm et al., 2005; Stalder et al., 2005). Unlike other studies, which show monocyte infiltration following irradiation and bone-marrow transplant, this study indicates that monocytes can infiltrate the AD mouse brain without prior irradiation-related BBB damage. Furthermore, Lampron and colleagues demonstrated that the formation of Aβ plaques in an AD mouse model is sufficient to induce bone marrow-derived cell entry into the CNS without irradiation, albeit at low numbers (Lampron et al., 2013).
In addition to chemokine receptors like CCR2, formylpeptide receptors (FPRs) are also recognized as another group of classical chemoattractant receptors involved in leukocyte trafficking (Chen et al., 2013). A previous investigation in human peripheral blood monocytes, demonstrated that chemotaxis towards Aβ and APP occurs via the activation of formyl peptide receptor like-1 (FPRL1), a low affinity receptor located on leukocytes and mononuclear phagocytes (Kaneider et al., 2004). Interaction of FPRL1 or its mouse homolog FPR2 with Aβ results in the internalization of Aβ by macrophages, enhanced chemotactic activity, as well as the release of neurotoxic substances (Iribarren et al., 2005).
Extensive evidence indicates that CCL2 and CX3CL1 play an important role in monocyte trafficking during inflammatory disorders including AD (Hickman and El Khoury, 2010). Some hypothesize that the CCL2/CCR2 pathway is involved in monocytic trafficking out of the bone marrow and into the blood, while the CX3CL1/CX3CR1 pathway is involved in the capture and adhesion of monocytes to the vessel wall near the barrier of the inflamed tissue (Hickman and El Khoury, 2010). Thus, it could be possible that FPRs may play a distinct role in local monocyte or mononuclear phagocyte recruitment, in which cells from the perivascular space or non-lesioned parenchyma (where Aβ deposition is not apparent) are recruited to areas of the parenchyma where senile plaques and Aβ accumulation are prevalent.
Extensive in vivo and in vitro evidence indicates that blood-derived monocytes can phagocytose Aβ (Hawkes and McLaurin, 2009; Malm et al., 2010; Town et al., 2008), however, the exact mechanism of how monocytes clear Aβ remains unclear. As previously mentioned, several studies have demonstrated that insoluble fibrillar Aβ, the aggregated form of Aβ found in senile plaques, can bind to receptors on microglia and activate these cells to produce cytokines, chemokines, and reactive oxygen and nitrogen species. The receptors involved in promoting this binding and/or phagocytosing fibrillar Aβ include: class A1 scavenger receptors (Scara1), class B2 scavenger receptors (Scara2), CD36, and RAGE (Frenkel et al., 2013). Other microglial receptors involved in Aβ uptake include TLR4/CD14, FPRL1, and complement receptors (Sokolowski and Mandell, 2011). These cells also produce a number of Aβ-degrading enzymes, including insulysin, neptrilysin, and others (Frenkel et al., 2013). However, again whether these same receptors and/or pathways are also involved in monocyte Aβ clearance remains unclear. Furthermore, it is important to consider the varying forms of Aβ and how these species stimulate differential activation of mononuclear phagocytes and their subsequent degradation of Aβ. Previous studies indicate that soluble Aβ is taken up via pinocytosis, whereas fibrillar Aβ is taken up by phagocytosis in macrophages/microglia (Sokolowski and Mandell, 2011). A recent study by Frenkel and colleagues indicates that Scara1, a scavenger receptor found on myeloid cells, serves as the main receptor for soluble Aβ clearance, rather than CD36 (Frenkel et al., 2013). Recent genome-wide association studies in AD patients have also identified a new susceptibility loci and possible receptor for altered Aβ processing: TREM2, a receptor involved in regulating phagocytosis and inflammatory responses in myeloid cells (Hickman and El Khoury, 2014). Further studies are needed to distinguish which receptors and pathways distinguish successful phagocytosis from ineffective phagocytosis and whether this is cell type dependent (i.e., improved in monocytes vs. microglia) or Aβ species dependent.
The question remains, however, if blood-derived monocytes are recruited to the AD brain (either as a specific response to Aβ or a leaky BBB caused by vascular injury) and are effective phagocytes of Aβ (based on animal studies), then why does AD continue to progress? Furthermore, could it be possible that AD patients suffer from faulty immune signaling functions in respect to the infiltration, patrolling and/or phagocytic (e.g., toll-like receptor, TLR) abilities of these monocytic cells? To our knowledge, there have been no reports on the immune defects seen in mouse AD macrophages, however, reports have shown that the expression of receptors involved in Aβ uptake and Aβ-degrading enzymes, does decrease with age in AD mouse models (Frenkel et al., 2013). Thus, it could be possible that microglia and/or monocyte-derived cells could become dysfunctional, lose their ability to degrade Aβ, produce proinflammatory cytokines and neurotoxins, which in turn promote further Aβ production and accumulation (Hickman and El Khoury, 2014).
Clinical investigations have shown that peripheral monocytic cells from AD patients appear ineffective at phagocytosing Aβ (altered TLR, MHC II, or COX-2, CCR2) and may even contribute to CAA (Fiala et al., 2007; Malm et al., 2010; Mildner et al., 2011; Zaghi et al., 2009). These patient studies using monocytes isolated from healthy individuals show that these cells can phagocytose and degrade Aβ as well as gain access to Aβ-positive vessels and ingest Aβ. Monocytes from AD patients, on the other hand, are unable to phagocytose Aβ as well as display lower expression of surface proteins involved in Aβ phagocytosis and pronounced apoptotic signaling (Fiala et al., 2005, 2007; Zaghi et al., 2009). These studies suggest that monocytes/macrophages may be contributing to CAA by releasing Aβ into the vessel walls following apoptosis (Malm et al., 2010). Thus, finding a way to promote effective Aβ clearance in these cells, while avoiding the activation of proinflammatory and neurotoxic pathways, should prove an attractive target for AD therapies. In a recent study by Mizwicki and colleagues, the authors treated peripheral blood mononuclear cells from AD patients with 1α,25-dihydroxyvitamin D3 (1,25D3), the active metabolite of vitamin D, and resolvin D1 (RvD1), a derivative of omega-3 fatty acids, in an effort to balance the activation of inflammation, specifically avoiding neurotoxic pathways and promoting effective Aβ phagocytosis. Their findings indicate that both 1,25D3 and RvD1 can enhance Aβ phagocytosis, inhibit soluble Aβ-induced production of proinflammatory cytokines and inhibit fibrillar Aβ-induced apoptosis (Mizwicki et al., 2013). These studies are promising, however, further investigation is needed in order to determine whether such treatments will translate into neuroprotection and/or cognitive improvement. It could also be possible that AD patients exhibit enhanced levels of CCL2, which some investigators believe could result in desensitization of cells and thus impairment in their ability to infiltrate, respond to inflammatory or neuronal insult, or phagocytose Aβ (Mildner et al., 2011; Naert and Rivest, 2013).
3.6. Concluding remarks
Taken together, these findings indicate that developing strategies to stimulate the production of new and functioning peripheral monocytic cells may prove an insightful avenue for further therapeutic development (Fig. 1B). One such approach could involve genetically altering these cells to express therapeutic genes (e.g., signals or neuroprotective factors that help promote neuronal survival or Aβ clearance) and using these cells to deliver therapeutic substances/capabilities to lesion sites by exploiting their ability to home to regions of brain damage and Aβ deposition. The selective migration of monocytes towards Aβ plaque deposition makes these cells optimal candidates for the delivery of therapeutic substances to the AD brain.
Footnotes
This work has been supported by the Austrian Science Funds (P24541-B24).
References
- Akiyama H, Kondo H, Mori H, Kametani F, Nishimura T, Ikeda K, Kato M, McGeer PL. The amino-terminally truncated forms of amyloid beta-protein in brain macrophages in the ischemic lesions of Alzheimer’s disease patients. Neurosci. Lett. 1996;219:115–118. doi: 10.1016/s0304-3940(96)13197-9. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, Han SH, Choi H, Kim KH, Moon M, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol. Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J. Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass JP, Hanin I, Barclay L, Kopp U, Reding MJ. Red blood cell abnormalities in Alzheimer disease. J. Am. Geriatr. Soc. 1985;33:401–405. doi: 10.1111/j.1532-5415.1985.tb07150.x. [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease. Brain. 2009;132:1078–1092. doi: 10.1093/brain/awn331. [DOI] [PubMed] [Google Scholar]
- Britschgi M, Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nat. Med. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood–brain barrier and infection by HIV: implications for NeuroAIDS. Cell. Immunol. 2011;267:109–123. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter MS, Coleman BS, Buttini M, Barbour R, Schenk D, Games D, Seubert P, Wyss-Coray T. Increased T cell recruitment to the CNS after amyloid beta 1–42 immunization in Alzheimer’s mice overproducing transforming growth factor-beta 1. J. Neurosci. 2006;26:11437–11441. doi: 10.1523/JNEUROSCI.2436-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Liu M, Liu Y, Wang C, Yoshimura T, Gong W, Le Y, Tessarollo L, Wang JM. Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway in-flammation. J. Biol. Chem. 2013;288:16262–16273. doi: 10.1074/jbc.M113.450635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris EH. Fibrinogen and altered hemostasis in Alzheimer’s disease. J. Alzheimers Dis. 2012;32:599–608. doi: 10.3233/JAD-2012-120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharm. Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. Brain Pathol. Vol. 24. Zurich, Switzerland: 2014. Alterations in immune cells and mediators in the brain: it’s not always neuroinflammation! pp. 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evin G, Zhu AHR, Masters CL, Li QX. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J. Neurosci. Res. 2003;74:386–392. doi: 10.1002/jnr.10745. [DOI] [PubMed] [Google Scholar]
- Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M, et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood–brain barrier model. Mol. Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Y, Nemirovsky A, Baron R, Monsonego A. T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e10830. doi: 10.1371/journal.pone.0010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A. Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance. J. Immunol. 2014;192:92–102. doi: 10.4049/jimmunol.1301707. [DOI] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, Farfara D, Kingery ND, Weiner HL, El Khoury J. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer’s disease: the blood-borne identity. J. Neural Transm. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. beta-Amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 2000;279:C1772–1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer’s disease. Curr. Drug Targets CNS Neurol. Disord. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, El Khoury J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014;88:495–498. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohsfield LA, Ehrlich D, Humpel C. Intravenous infusion of nerve growth factor-secreting monocytes supports the survival of cholinergic neurons in the nucleus basalis of Meynert in hypercholesterolemia Brown-Norway rats. J. Neurosci. Res. 2014;92:298–306. doi: 10.1002/jnr.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C. Basolateral aggregated rat amyloid beta(1–42) potentiates transmigration of primary rat monocytes through a rat blood–brain barrier. Curr. Neurovasc. Res. 2008;5:185–192. doi: 10.2174/156720208785425701. [DOI] [PubMed] [Google Scholar]
- Iribarren P, Zhou Y, Hu J, Le Y, Wang J. The role of formyl peptide receptor like 1 (FPRL1/MFPR2) in mononuclear phagocyte responses in Alzheimer’s disease. Immunol. Res. 2005;31:165–176. doi: 10.1385/IR:31:3:165. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci. Lett. 1988;91:259–264. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- Joslin G, Griffin GL, August AM, Adams S, Fallon RJ, Senior RM, Perlmutter DH. The serpin-enzyme complex (SEC) receptor mediates the neutrophil chemotactic effect of alpha-1 antitrypsin-elastase complexes and amyloid-beta peptide. J. Clin. Investig. 1992;90:1150–1154. doi: 10.1172/JCI115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneider NC, Lindner J, Feistritzer C, Sturn DH, Mosheimer BA, Djanani AM, Wiedermann CJ. The immune modulator FTY720 targets sphingosine-kinase-dependent migration of human monocytes in response to amyloid beta-protein and its precursor. FASEB J. 2004;18:1309–1311. doi: 10.1096/fj.03-1050fje. [DOI] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Yamamoto M, Xiong H, Lambert MP, Klein WL, Gendelman HE, Ransohoff RM, Ikezu T. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4:e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewallner KM, Ehrlich D, Kiefer A, Marksteiner J, Humpel C. Platelets in the Alzheimer’s disease brain: do they play a role in cerebral amyloid angiopathy? Curr. Neurovasc. Res. 2015 Jan 2; doi: 10.2174/1567202612666150102124703. 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lampron A, Pimentel-Coelho PM, Rivest S. Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration. J. Comp. Neurol. 2013;521:3863–3876. doi: 10.1002/cne.23363. [DOI] [PubMed] [Google Scholar]
- Larbi A, Pawelec G, Witkowski JM, Schipper HM, Derhovanessian E, Goldeck D, Fulop T. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 2009;17:91–103. doi: 10.3233/JAD-2009-1015. [DOI] [PubMed] [Google Scholar]
- Lebson L, Nash K, Kamath S, Herber D, Carty N, Lee DC, Li Q, Szekeres K, Jinwal U, Koren J, et al. Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J. Neurosci. 2010;30:9651–9658. doi: 10.1523/JNEUROSCI.0329-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Shang DS, Zhao WD, Tian L, Li B, Fang WG, Zhu L, Man SM, Chen YH. Amyloid beta interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood–brain barrier. J. Immunol. 2009;182:5778–5788. doi: 10.4049/jimmunol.0803013. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B, Fang WG, Zhu L, Chen YH. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging. 2010;31:175–188. doi: 10.1016/j.neurobiolaging.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA, Mills KH. Immunology meets neuroscience—opportunities for immune intervention in neurodegenerative diseases. Brain Behav. Immun. 2012;26:1–10. doi: 10.1016/j.bbi.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Malm T, Koistinaho M, Muona A, Magga J, Koistinaho J. The role and therapeutic potential of monocytic cells in Alzheimer’s disease. Glia. 2010;58:889–900. doi: 10.1002/glia.20973. [DOI] [PubMed] [Google Scholar]
- Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, Fang WG, Zhu L, Chen YH. Peripheral T cells overexpress MIP-1alpha to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol. Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Michaud JP, Bellavance MA, Prefontaine P, Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid Beta. Cell Rep. 2013;5:646–653. doi: 10.1016/j.celrep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 2011;31:11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, Mahanian M, Weitzman R, Hayden EY, Rosenthal MJ, Nemere I, Ringman J, Teplow DB. 1α,25-Dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-β phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty JG, Shukla HD, Williamson JD, Launer LJ, Saxena S, Rifkind JM. Alterations in the red blood cell membrane proteome in Alzheimer’s subjects reflect disease-related changes and provide insight into altered cell morphology. Proteome Sci. 2010;8:11. doi: 10.1186/1477-5956-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, Fisher Y, Owens T, Weiner HL. Abeta-induced meningoencephalitis is IFN-gamma-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Nemirovsky A, Harpaz I. CD4 T cells in immunity and immuno-therapy of Alzheimer’s disease. Immunology. 2013;139:438–446. doi: 10.1111/imm.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert. Rev. Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Rivest S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer’s disease. J. Mol. Cell Biol. 2013;5:284–293. doi: 10.1093/jmcb/mjt028. [DOI] [PubMed] [Google Scholar]
- Padovani A, Pastorino L, Borroni B, Colciaghi F, Rozzini L, Monastero R, Perez J, Pettenati C, Mussi M, Parrinello G, Cottini E, Lenzi L, Trabucchi M, Cattabeni F, Di Luca M. Amyloid precursor protein in platelets: a peripheral marker for the diagnosis of sporadic AD. Neurology. 2001;57:2243–2248. doi: 10.1212/wnl.57.12.2243. [DOI] [PubMed] [Google Scholar]
- Perl DP. Neuropathology of Alzheimer’s disease. Mt Sinai J. Med. New York. 2010;77:32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J. Clin. Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Gate D, Town T. CNS infiltration of peripheral immune cells: DDay for neurodegenerative disease? J. NeuroImmune Pharm. 2009;4:462–475. doi: 10.1007/s11481-009-9166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte–endothelial interactions. J. Leukoc. Biol. 2011;89:539–556. doi: 10.1189/jlb.0710432. [DOI] [PubMed] [Google Scholar]
- Sabolovic D, Roudier M, Boynard M, Pautou C, Sestier C, Fertil B, Geldwerth D, Roger J, Pons JN, Amri A, et al. Membrane modifications of red blood cells in Alzheimer’s disease. J. Gerontol. A: Biol. Med. Sci. 1997;52:B217–220. doi: 10.1093/gerona/52a.4.b217. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Mandell JW. Phagocytic clearance in neurodegeneration. Am. J. Pathol. 2011;178:1416–1428. doi: 10.1016/j.ajpath.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, Coomaraswamy J, Staufenbiel M, Landmann R, Jucker M. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002;124:83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- Town T, Tan J, Flavell RA, Mullan M. T-cells in Alzheimer’s disease. Neruomol. Med. 2005;7:255–264. doi: 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hundelshausen P, Koenen RR, Weber C. Platelet-mediated enhancement of leukocyte adhesion. Microcirculation. 2009;16:84–96. doi: 10.1080/10739680802564787. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta Neuropathol. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harbor Perspect. Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Itoh Y, Shintaku M, Kawamura J, Jensson Ó, Thorsteinsson L, Suematsu N, Matsushita M, Otomo E. Immune reactions associated with cerebral amyloid angiopathy. Stroke. 1996;27:1155–1162. doi: 10.1161/01.str.27.7.1155. [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Shang DS, Zhao WD, Fang WG, Chen YH. Microglial TNF-alpha-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem. Res. 2013;38:2295–2304. doi: 10.1007/s11064-013-1138-5. [DOI] [PubMed] [Google Scholar]
- Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J. Immunol. 2006;176:4155–4162. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, Mahanian M, Bernas M, Weinand M, Rosenthal MJ, Espinosa-Jeffrey A, de Vellis J, Teplow DB, Fiala M. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117:111–124. doi: 10.1007/s00401-008-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huang W, Jing F. Contribution of blood platelets to vascular pathology in Alzheimer’s disease. J. Blood Med. 2013;4:141–147. doi: 10.2147/JBM.S45071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]