Abstract
Background
The Centers for Disease Control and Prevention (CDC) 12-Step Campaign to Prevent Antimicrobial Resistance was launched to educate clinicians about antimicrobial resistance and provide strategies to improve clinical practice, including antimicrobial utilization.
Methods
A multicenter retrospective observational study of antibiotic use was performed in 4 tertiary care NICUs to assess adherence to the guidelines defined by the CDC 12-Step Campaign using predetermined criteria. Fifty infants per NICU were identified who received intravenous antibiotics at greater than 72 hours of age. Antibiotic regimens, clinical and microbiologic data, and indications for initiation and continuation of antibiotics (after 72 hours of use) were recorded. Inappropriate utilization was characterized at initiation, continuation, by agent, and by CDC 12-Step.
Results
Two hundred neonates received 323 antibiotic courses totaling 3344 antibiotic-days. Ninety (28%) courses and 806 (24%) days were judged to be nonadherent to a CDC 12-Step. Inappropriate use was more common with continuation of antibiotics (39%) than with initiation (4%) of therapy. Vancomycin was the most commonly used drug (n = 895 antibiotic-days) of which 284 (32%) days were considered inappropriate. Carbapenems were used less frequently (n = 310 antibiotic-days), and 132 (43%) of these days were inappropriate. Common reasons for nonadherence at the time of continuation included failure to narrow antibiotic coverage after microbiologic results were known and prolonged antibiotic prophylaxis after surgery with chest tube placement.
Conclusions
The CDC 12-Step Campaign can be modified for neonatal populations. Inappropriate antibiotic prescribing was common in the study NICUs. Improvement efforts should target antibiotic use 72 hours after initiation, particularly focusing on narrowing therapy and instituting protocols to limit prophylaxis.
Keywords: antibiotic usage, neonates, resistance, intensive care units, CDC program
INTRODUCTION
In 2002, the Centers for Disease Control and Prevention (CDC) launched the 12-Step Campaign to Prevent Antimicrobial Resistance to educate clinicians about resistance and provide strategies to change clinical practices, including antimicrobial prescribing.[1] Several guidelines have been published to address different patient populations including hospitalized adults, hospitalized children, dialysis patients, surgical patients, and residents of long-term care facilities. The Campaign presents 4 major strategies: preventing infection, diagnosing and treating infection effectively, using antimicrobials wisely, and preventing transmission. Although 5 of the CDC 12-Steps address the strategy “Use antimicrobials wisely”, additional steps (eg, “Use appropriate methods of diagnosis” and “Target the pathogen”) also affect antimicrobial prescribing.
The CDC 12-Steps were not specifically developed for a neonatal intensive care unit (NICU), but most strategies are applicable to this population. Infants hospitalized in the NICU have high rates of health care associated infections and subsequently high rates of antibiotic use.[2,3] In a national point prevalence study of 29 NICUs in the United States done in 1999 and 2000, 43% of NICU patients were receiving antimicrobials on the survey date, with a median number of 2 agents.[3] Few studies have measured appropriateness of antibiotic use in the NICU. The aim of this study was to characterize antibiotic use in tertiary care NICUs and to assess use as appropriate versus inappropriate based on CDC 12-Step guidelines modified for neonates.
MATERIALS AND METHODS
Study Design and Study Sites
We performed a multicenter retrospective observational study of antibiotic use in 4 tertiary care NICUs to assess adherence to the guidelines in the CDC 12-Step Campaign to prevent antimicrobial resistance among Hospitalized Children. The study NICUs were located at: Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, New York, NY; Komansky Center for Children’s Health of New York-Presbyterian, Weill Cornell University Medical Center, New York, NY; Children’s Hospital of Philadelphia, Philadelphia, PA; and Christiana Care Health Systems, Newark, DE. Three sites perform neonatal surgery. Pediatric residents, neonatology fellows, and nurse practitioners work at all 4 NICUs. Additional site characteristics are shown in Table 1. Review Board approval was obtained for reviewing medical records at all 4 sites, including a waiver of informed consent.
Table 1.
Characteristics and Antibiotic Prescribing Resources at Participating NICUs
| Characteristics | NICU | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Acute beds (n) | 58 | 50 | 50 | 60 |
| Annual discharges (n) | 970 | 679 | 900 | 1100 |
| Average length of stay (days) | 18 | 18 | 20 | 15 |
| Admissions < 1000 g (birthweight) | 87 (9%) | 51 (8%) | 135 (15%) | 132 (12%) |
| Restricted antimicrobials require prior approval | Yes | No | Yes | No |
| 48 hour ‘pass’ for empiric vancomycin therapy for late onset sepsis | Yes | Yes | Yes | Yes |
| Approval to continue vancomycin >48 hours | Yes | No | Yes | NA |
| Vancomycin approver | ID fellow | NA | ID fellow/PharmD | NA |
Abbreviations: ID, infectious diseases; NA, not applicable
Study Subjects
Fifty consecutive eligible infants from each NICU were studied. Eligible subjects were those infants who received intravenous antibiotics for any indication at ≥72 hours of age. Infants were included whether they were born at the institution, transferred from another facility, or admitted from the community. An infant was excluded if death occurred within 24 hours of administration of antibiotics. Nonintravenous antibiotics, antifungal agents, and antiviral agents were excluded. Eligible infants were consecutively selected from NICU admission records beginning from September 1st, 2005. Accrual required 9 to 16 weeks depending on the site.
Data Collection
The following data were collected from eligible infants: receipt of all intravenous antibiotics confirmed by nursing records from 72 hours of age until hospital day 120, discharge, or death; demographic characteristics, clinical characteristics, and microbiology results. For all blood stream infections, the presence of a central venous catheter was recorded. Data were collected from paper and from electronic medical records. The indications for antibiotics were derived from physician progress notes. Primary data collection was performed by SJP at all 4 sites, and a 10% convenience sample of randomly selected infants was reviewed by another member of the study team to determine the joint probability of agreement on 5 key data elements (88%, data not shown). Disagreement was resolved by consensus.
Assessing Adherence with CDC 12-Step Recommendations
The primary unit of analysis was an antibiotic course, defined as one or more antibiotics given concurrently for one or more days and the indication for use. The indication for an antibiotic could change if the clinical status of the patient changed or microbiologic data became available. Therefore, the indications for antibiotic use were recorded at 2 points, initiation of the course and continuation of the course, measured 72 hours after initiation, if applicable. Indications for initiation and for continuation were categorized as previously described:[4] (1) definitive use (treating a pathogen with known susceptibility); (2) empiric use (treating for signs of infection, or known positive culture with pending susceptibility; or (3) prophylaxis. For example, vancomycin and gentamicin might have been initiated empirically for increased episodes of apnea and bradycardia and suspected late onset sepsis, but vancomycin was continued for definitive use for a positive blood culture for methicillin-resistantStaphylococcus aureus.
Adherence with selected CDC recommendations was determined for each course at initiation and continuation using criteria derived from common practice, society guidelines, and consensus, as displayed in Table 2.[5–7] A course was considered to be inappropriate if it deviated from a CDC 12-Step recommendation at initiation, continuation, or both. For example, an antibiotic course could be considered inappropriate despite appropriate initiation of vancomycin and gentamicin for suspected late onset sepsis if these 2 agents were continued for treating a positive blood culture for methicillin-susceptible S. aureus. With the exception of the examples provided in Table 2, courses initiated for empiric treatment of suspected infection or continued for culture-negative sepsis were considered appropriate. Similarly, except for the examples listed in Table 2, diagnosis and treatment of body-site infections (eg, ventilator associated pneumonia, postoperative wound infection) was not deemed inappropriate unless there was an antibioticpathogen mismatch. If an antibiotic course deviated from more than 1 CDC recommendation, the hierarchy shown in Table 2 was used to assign the CDC 12-Step violated.
Table 2.
Hierarchy of Selected CDC 12 Step Recommendations and Examples of Inappropriate Use in the NICU
| CDC 12 Step | Examples of Inappropriate Use |
|---|---|
| 4. Target the pathogen | Continued use of broad spectrum agent when narrower agent is available |
| Use of vancomycin for methicillin-susceptible Staphylococcus aureus infection rather than oxacillin | |
| Inadequate therapy | |
| Use of gentamicin for a gentamicin-resistant pathogen | |
| Continued use of 2 agents when a single agent is adequate | |
| Use of vancomycin and gentamicin for Staphylococcus epidermidis blood stream infection | |
| 6. Practice antimicrobial control | Prolonged postoperative prophylaxis (eg, cefazolin: >24–48 h) |
| Chest tube prophylaxis (eg, cefazolin: >24–48 h) | |
| 8. Treat infection, not contamination or colonization | Colonization |
| Continued treatment of CONS blood stream infection with positive central venous catheter and negative peripheral blood culture in stable infant | |
| Contamination | |
| Treatment of positive arterial culture with negative blood cultures from other sites in stable infant | |
| Treatment of a urine culture positive for ≥2 organisms in stable infant | |
| 9. Know when to say “no” (to antibiotics) | Initial therapy with broad spectrum agent |
| Use of a carbapenem for empiric treatment of late onset sepsis (without evidence of necrotizing enterocolitis or multi-drug resistance) | |
| Redundant coverage | |
| Use of a carbapenem and metronidazole for treatment of anaerobic pathogens in necrotizing enterocolitis | |
| 10. Stop treatment when infection is cured or unlikely | Prolonged duration of therapy |
| Treatment of Staphylococcus epidermidis blood stream infection for >10 d since last positive culture and/or for longer duration than indicated in treatment plan | |
| Treatment of Staphylococcus aureus or gram negative bacilli blood stream infections >14 d since last positive culture and/or for longer duration then indicated in treatment plan |
For each antibiotic, the number of days of appropriate and inappropriate use was also calculated. If a subject received 5 days of inappropriate vancomycin and gentamicin that subject would be considered to have received 5 inappropriate days of each drug.
RESULTS
Study Subjects
Of the 200 study subjects, 108 (54%) were male and 42 (21%) were low birth weight infants (<1500 g) of whom 30 were <1000 g. The 200 study subjects accounted for 6255 hospital-days and their median duration of hospital stay was 33 days (median stay per NICU 26–36 days). While the median gestational age was 33 weeks, the median gestational ages at NICU 2 and NICU 4 were 29 and 31 weeks, respectively, while those at NICU 1 and NICU 3 were 37 and 38 weeks, respectively. The higher gestational age of infants at NICU 1 and 3 likely reflected a relatively larger proportion of full term infants requiring surgical or cardiac care. Of the 200 infants studied, 42 (21%) had hemodynamically significant congenital heart disease. Overall, 109 surgical procedures were performed (excluding laser retinoplasty); 62 (31%) infants, of whom 46 were full term infants, underwent at least 1 surgical procedure.
Overall Antibiotic Use
Subjects received 323 intravenous antibiotic courses and 3344 antibiotic-days, with a median of 1.5 courses per patient (range, 1–11 courses). The median duration of the antibiotic courses was 6 days (range, 1–23 days). The indications for antibiotic initiation were: definitive treatment (n = 16, 5%), empiric treatment (n = 212, 66%), and prophylaxis (n = 95, 29%). Overall, 204 (63%) of the 323 antibiotic courses were continued beyond 72 hours for the following indications: definitive treatment (n = 76, 37%), empiric treatment (n = 67, 33%), and prophylaxis (n = 61, 30%).
Of the 323 antibiotic courses, 90 (28%) courses and 806 (24%) antibiotic-days were judged to be nonadherent to at least 1 CDC 12-Step. Seventy infants (35%) received at least 1 inappropriate course. Inappropriate use was more common with continuation of antibiotics than with initiation (39% vs. 4%, respectively, P < 0.001) as shown in Figure 1. Differences in rates of inappropriate use between the 4 NICUs was not statistically significant.
Figure 1.
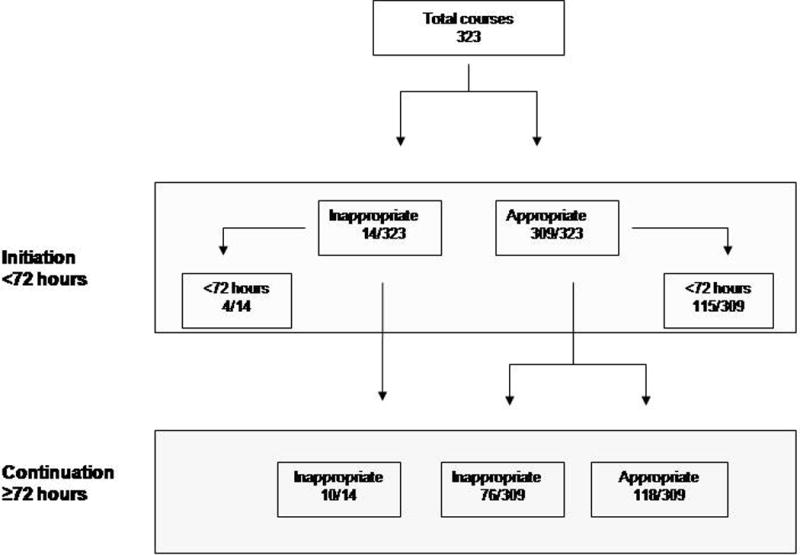
Flow chart of appropriate and inappropriate antibiotic courses.
Inappropriate Antibiotic Use by 12-Steps
Nonadherent courses classified by CDC 12-Step recommendation are listed in Table 3. “Target the pathogen” was the most common CDC 12-Step that was violated. Common examples of nonadherence included continuing vancomycin for methicillin-susceptible S. aureus or using a carbapenem to treat a Gram-negative rod susceptible to piperacillin/tazobactam. Nonadherence to “Practice antimicrobial control” was due largely to prolonged (>48 hours) postsurgical antibiotic or chest tube prophylaxis with cefazolin. Failure to “Treat infection, not contamination or colonization” included treatment of 17 episodes of a single positive blood culture for coagulase-negative staphylococci (CONS) despite a concurrent negative blood culture from another site before initiation of antibiotics and treatment of 7 episodes of a single positive blood culture of CONS despite concurrent positive cultures for another pathogen, eg, a Gram-negative rod or S. aureus. Of antibiotic use nonadherent to “Stop treatment when infection is cured or unlikely”, 26 days reflected use beyond the treatment duration planned in physician progress notes.
Table 3.
Inappropriate Antibiotic Days Classified by CDC 12-Step
| CDC 12-Step Recommendation Violation | Days | % |
|---|---|---|
| Target the pathogen | 309 | 38 |
| Practice antimicrobial control | 167 | 21 |
| Treat infection, not contamination or colonization | 130 | 16 |
| Say “no” to vancomycin or others | 140 | 17 |
| Stop treatment if infection cured or unlikely | 60 | 8 |
Although not necessarily indicative of inappropriate use, exploratory outcomes related to other 12-Step recommendations were also determined. For example, only 22 (35%) of the 62 antibiotic courses initiated for late onset sepsis and continued for a positive blood culture had 2 blood cultures obtained before the initiation of antibiotics (Step 3: “Use appropriate methods for diagnosis”) and only 13 infants had a written consultation from the Infectious Diseases (ID) service (Step 5: “Access the Experts”).
Inappropriate Antibiotic Use by Agents
Inappropriate and appropriate use was also categorized by antimicrobial agent as shown in Figure 2. Vancomycin and cefazolin contributed the most antibiotic days; 32% of each was used inappropriately. The 12-steps most commonly involved in inappropriate vancomycin use were Steps 4 and 8 while the step most commonly involved in inappropriate cefazolin use was Step 6. Carbapenem agents had the highest proportion of inappropriate antibiotic days (44%). While used less frequently, second and third generation cephalosporin and oxacillin use was most adherent with CDC guidelines (14% and 10% inappropriate days, respectively).
Figure 2.

Appropriate and inappropriate use classified by agent and antibiotic days. Other antibiotics (100% appropriate use) included piperacillin/tazobactam, ampicillin/sulbactam, clindamycin, rifampin, tobramycin, amikacin.
DISCUSSION
We think that this is the first study to use the CDC 12-Step guidelines for assessing antimicrobial prescribing in the NICU population. In the 4 NICUs studied, approximately 25% of antibiotic courses and antibiotic-days were considered inappropriate and affected approximately 35% of infants who received intravenous antibiotics after 72 hours of age. Several consistent patterns of inappropriate use were observed. First, most inappropriate antibiotic use occurred at continuation of antibiotics rather than at initiation, as seen in a previous study of vancomycin use in pediatric patients.[8] Nonadherence to the guideline “Target the pathogen” was most common, followed by the guideline “Practice antimicrobial control”. Inappropriate use of specific agents often centered around 12-Step violations. For example, inappropriate use of vancomycin commonly reflected failure to “Target the pathogen”, while virtually all inappropriate use of cefazolin reflected prolonged surgical prophylaxis or chest tube prophylaxis.
The 12-Step Campaign’s development, dissemination, or relevance to clinical practice have been studied using physician interviews and focus groups.[9] Pediatricians, 20% of whom were neonatologists, regarded the 12-Steps “Target the pathogen” and “Practice antimicrobial control” as among the most important to limit antimicrobial resistance.[10] The 12-Step strategies were also used to evaluate prospectively the antibiotic treatment of 540 hospitalized adults and feedback was given to prescribers.[11] Overall, 37% of antibiotic courses evaluated at 48 to 72 hours after initiation were considered inappropriate. Similar to our findings, nonadherence to the guideline “Target the pathogen” was the most common reason for nonadherence (39% of all inappropriate courses).
Antimicrobial stewardship is increasingly being promoted as a means to limit antimicrobial resistance and improve quality of care. The Infectious Disease Society of America reviewed potential strategies to improve antimicrobial use and developed evidence-based recommendations for antimicrobial stewardship programs.[12] Few studies of antimicrobial stewardship interventions have been performed in pediatric populations.[13] The results of our study suggest that interventions to improve antibiotic prescribing in the NICU should be implemented at continuation of antibiotics rather than initiation and should focus primarily on a limited number of clinical scenarios. For example, limiting vancomycin use for empiric therapy might be unacceptable in the NICU population, but changing therapy for a documented pathogen is universally acceptable. Similarly, evidence-based guidelines for perioperative prophylaxis could be widely acceptable at a given institution, supported by local epidemiology and surveillance for postoperative infections. Physicians are more likely to find guidelines agreeable when they are applied to specific clinical scenarios.[14] Although not universally used, adjuvant tests measuring C-reactive protein or interleukin-8 improve antimicrobial prescribing.[15,16] An antimicrobial stewardship program has been shown to effectively and safely reduce inappropriate antimicrobial prescribing in hospitalized children; interventions included: “ID consultation” (43%), “Target known or suspected pathogens” (20%), “Optimize antimicrobial treatment” (33%), and “Stop antimicrobial treatment” (4%).[17] Compliance with these recommendations was 89% in that study.
Our study had limitations. Infants were accrued consecutively rather than randomly; therefore, temporal clustering may have reflected the practice patterns of a few physicians. We did not assess baseline familiarity and acceptance of the CDC 12 Step Campaign recommendations among neonatologists at the study sites. NICU practitioners may disagree on specific 12-Step recommendations (eg, discordance of culture results as a sign of contamination or colonization). To maximize the acceptability of our criteria, we acknowledged the inherent uncertainty in diagnosing neonatal infections and accepted the clinical judgment of the prescribers to initiate empiric antibiotics for late onset sepsis and to continue antibiotics for treatment of culture negative sepsis.[18,19] Thus, such antibiotic courses were not considered inappropriate in our study. These relatively permissive criteria for evaluating empiric therapy may have decreased measured rates of inappropriate use at antibiotic initiation. Our study design did not allow us to determine whether infants had informal infectious disease consultations. The characteristics of our study sites and their prescribing practices may not be generalizable to other NICUs. While predetermined criteria were derived to assign inappropriate use to specific CDC 12-Steps, these criteria have not been externally validated. Future studies by other investigators are needed to validate these criteria.
Acknowledgments
Support: NIH P20 RR020616
References
- 1.Centers for Disease Control and Prevention. 12-Step Program to Prevent Antimicrobial Resistance in Health Care Settings. 2002 Available at: http://www.cdc.gov/drugresistance/healthcare/default.html. Accessed 2008.
- 2.Banerjee S, Grohskopf LA, Sinkowitz-Cochran RL, et al. National Nosocomial Infections Surveillance System. Pediatric Prevention Network Incidence of pediatric and neonatal intensive care unit-acquired infections. Infect Control Hosp Epidemiol. 2006;27:561–570. doi: 10.1086/503337. [DOI] [PubMed] [Google Scholar]
- 3.Grohskopf L, Huskins WC, Sinkowitz-Cochran RL, et al. Use of Antimicrobial agents in US neonatal and pediatric intensive patients. Pediatr Infect Dis J. 2005;24:766–773. doi: 10.1097/01.inf.0000178064.55193.1c. [DOI] [PubMed] [Google Scholar]
- 4.Jones DA, Pulver BL, Tai B, et al. Glycopeptide prescribing in an Australian tertiary paediatric hospital. J Paediatr Child Health. 2001;37:342–347. doi: 10.1046/j.1440-1754.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger PE, Gross PA, Barrett TL, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Clin Infect Dis. 1994;18:422–427. doi: 10.1093/clinids/18.3.422. [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 7.Edwards FH, Engelman RM, Houck P, et al. The society of thoracic surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part I: duration. Ann Thorac Surg. 2006;81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Bolon MK, Arnold AD, Feldman HA, et al. Evaluating vancomycin use at a pediatric hospital: new approaches and insights. Infect Control Hosp Epidemiol. 2005;26:47–55. doi: 10.1086/502486. [DOI] [PubMed] [Google Scholar]
- 9.Brinsley KJ, Sinkowitz-Cochran RL, Cardo DM. Assessing motivation for physicians to prevent antimicrobial resistance in hospitalized children using the health belief model as a framework. Am J Infect Control. 2005;33:175–181. doi: 10.1016/j.ajic.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Brinsley K, Sinkowitz-Cochran R, Cardo D. An assessment of issues surrounding implementation of the Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. Am J Infect Control. 2005;33:402–409. doi: 10.1016/j.ajic.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Cosgrove SE, Patel A, Song X, et al. Impact of different methods of feedback to clinicians after postprescription antimicrobial review based on the Centers For Disease Control and Prevention’s 12 Steps to Prevent Antimicrobial Resistance Among Hospitalized Adults. Infect Control Hosp Epidemiol. 2007;28:641–646. doi: 10.1086/518345. [DOI] [PubMed] [Google Scholar]
- 12.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America, Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 13.Patel SJ, Larson EL, Kubin CJ, et al. A review of antimicrobial stewardship control strategies in hospitalized and ambulatory pediatric populations. Pediatr Infect Dis J. 2007;26:531–537. doi: 10.1097/INF.0b013e3180593170. [DOI] [PubMed] [Google Scholar]
- 14.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;28:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 15.Ehl S, Gering B, Bartmann P, et al. C-reactive protein is a useful marker for guiding duration of antibiotic therapy in suspected neonatal bacterial infection. Pediatrics. 1997;99:216–221. doi: 10.1542/peds.99.2.216. [DOI] [PubMed] [Google Scholar]
- 16.Franz AR, Bauer K, Schalk A, et al. Measurement of interleukin 8 in combination with C-reactive protein reduced unnecessary antibiotic therapy in newborn infants: a multicenter, randomized, controlled trial. Pediatrics. 2004;114:1–8. doi: 10.1542/peds.114.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Metjian TA, Prasad PA, Kogon A, et al. Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital. Pediatr Infect Dis J. 2008;27:106–111. doi: 10.1097/INF.0b013e318158603a. [DOI] [PubMed] [Google Scholar]
- 18.Rubin LG, Sánchez PJ, Siegel J, et al. Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists’ practices. Pediatrics. 2002;110:e42. doi: 10.1542/peds.110.4.e42. [DOI] [PubMed] [Google Scholar]
- 19.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]


