Abstract
Neuroendocrine tumors are a heterogeneous group of neoplasms that are best worked up and managed using a variety of clinical and imaging studies. They are often diagnosed after they have already metastasized, though this does not necessarily preclude an attempt at curative surgical treatment or surgical debulking. Tumor burden assessment often requires use of multiple imaging modalities including computed tomography, magnetic resonance imaging and ultrasound. Somatostatin receptor-based imaging is also of great utility in looking for primaries and determining the extent of metastatic disease. This paper will review the most common imaging modalities used in the diagnosis and treatment of neuroendocrine tumors.
Keywords: 68Ga-DOTANOC, 68Ga-DOTATOC, CT, MRI, NET, neuroendocrine tumor, OctreoScan, PET, somatostatin imaging, ultrasound
Neuroendocrine tumors (NETs) arise from the diffuse neuroendocrine system, which is comprised of 17 different cell types that reside in skin, lung, the hepatobiliary system, the urogenital tract, thyroid and the gastrointestinal tract [1]. Though these tumors can arise in any of the aforementioned tissues, the most common primary sites are the lung, rectum and small bowel [2]. The hallmark of these tumors is their propensity for hormone secretion, which may cause symptoms such as diarrhea or flushing, and serve as biologic markers of the disease.
Historically, these tumors were thought to be extremely rare, though many recent studies have shown an increasing incidence [2–4]. This increase has been ascribed, in part, to improved methods of diagnosis and greater disease awareness [3]. Innovations in somatostatin receptor-based technologies have also pushed NET molecular diagnostics and therapeutics forward, improving the quality of life for many patients [5,6].
This review will focus on the imaging techniques used in the diagnosis, staging and follow-up of neuroendocrine tumors. A short review of conventional imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US) and 18FDG-PET will precede a more extensive review of the somatostatin-based imaging techniques that make the workup of NET patients unique.
Conventional imaging techniques
Computed tomography
CT scans are often the initial imaging study for a patient presenting with signs or symptoms suggestive of a NET. These studies are most useful for disease staging and surgical planning as they provide excellent anatomic detail of the tumors themselves and surrounding structures. Primary NETs (GI and lung NETs) and their metastases are generally hyperenhancing with IV contrast and are best seen in the arterial phase of a triple phase CT scan (Figure 1) [7,8].
Figure 1. Triple phase CT scan demonstrating approximately 70% replacement of the liver by enhancing neuroendocrine tumor metastases.
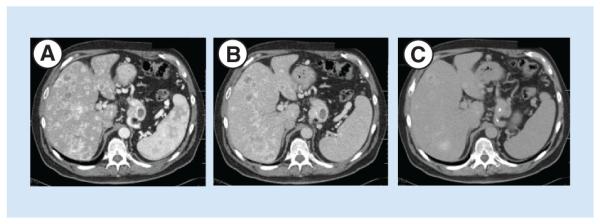
(A) Arterial phase, (B) venous phase and (C) equilibrium phase of delayed images. Note that hyperenhancing lesions are most clearly seen on arterial phase.
In primary NETs, the average sensitivity of a CT scan is 73% [9]. This modality is also useful when the primary tumor site is unknown. In a single-institution retrospective study, it was the most common study ordered to look for an unknown primary tumor site and was able to uncover the primary in 95% of cases [10]. CT scans have even better sensitivity in detecting NET metastases, as they demonstrate 80% sensitivity for hepatic metastases [9,11] and 75% sensitivity for extrahepatic metastases [9].
Magnetic resonance imaging
MRI is the best conventional study to detail hepatic metastases in NETs (Figure 2) [12]. It is not as useful as CT for the detection of primary small bowel lesions or their associated lymphadenopathy, but is good for the detection of primary pancreatic NETs. A study comparing MRI, CT and standard somatostatin receptor-based imaging (OctreoScan) reported 95.2% sensitivity for MRI, 78.5% sensitivity for CT and 49.3% sensitivity for the OctreoScan in detecting hepatic metastases. MRI also detected significantly more liver lesions than the other two modalities (p = 0.02 vs CT, p < 10−4 vs OctreoScan) [13].
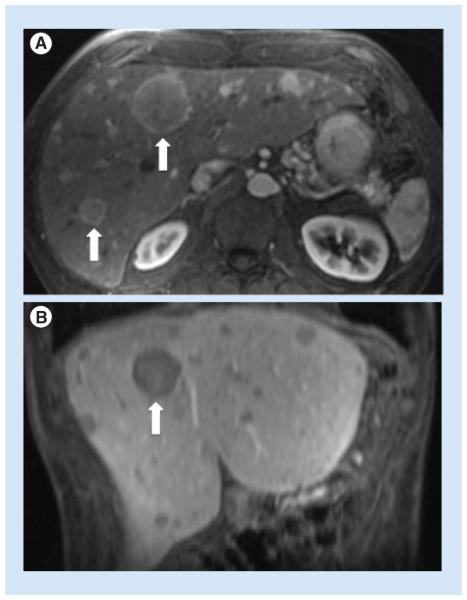
Figure 2. Hepatic neuroendocrine tumor metastases demonstrated on axial T1 weighted fat-suppressed postgadolinium images.
(A) Axial image, early arterial phase and (B) coronal image, portal venous phase. Arrows indicate large hepatic metastases.
Ultrasound
The primary role of conventional ultrasound in neuroendocrine disease is detection of hepatic metastases and estimation of total hepatic tumor burden. This technique has the advantages of near-universal availability, intraoperative utility, minimal expense and lack of radiation. Most US examinations are performed without contrast, which limits their sensitivity (compared with CT and MRI). A recent study showed that the use of a microbubble contrast agent improved the sensitivity of conventional US from 68 to 99% in detection of individual hepatic NET metastases (p < 0.0001) and reduced the rate of false positive calls [14].
18FDG positron emission scanning
18-fluoro-deoxy-glucose PET (FDG PET) is used to detect malignancy for a variety of tumor types. Unfortunately, its utility has not been borne out in NETs, as the majority of NETs tend to be relatively metabolically inactive and fail to take up the tracer well [15]. However, high-grade NETs are more likely to demon- strate avid uptake of 18FDG, giving these scans utility in identifying tumors likely to display more aggressive behavior [16]. The advantages and disadvantages of various imaging modalities are summarized in Table 1.
Table 1.
Comparison of the advantages and disadvantages of the major imaging modalities used in neuroendocrine
| Modality | Advantages | Disadvantages |
|---|---|---|
| CT |
|
|
| MRI |
|
|
| Ultrasound |
|
|
| Octreoscan |
|
|
| MIBG |
|
|
| FDG-PET |
|
|
| DOTA-PET |
|
|
Somatostatin receptor-based imaging techniques
Somatostatin is an endogenous peptide that is secreted by neuroendocrine cells, activated immune cells and inflammatory cells [17]. It affects its antiproliferative and antisecretory functions by binding to one of five types of somatostatin receptors (SSTR1- SSTR5). These are G-protein coupled receptors and are normally distributed in the brain, pituitary, pancreas, thyroid, spleen, kidney, gastrointestinal tract, vasculature, peripheral nervous system and on immune cells [18]. Expression of SSTRs is highest on well-differentiated NETs. Somatostatin receptor type 2 is the most highly expressed subtype, followed by SSTRs 1 and 5, SSTR3 and SSTR4.
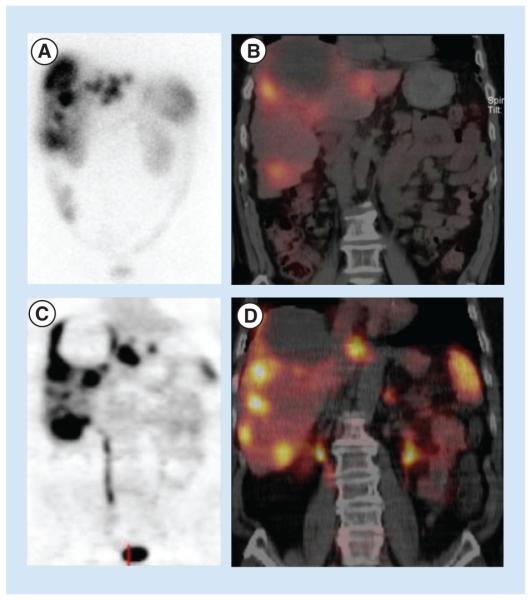
The ubiquity of SSTRs on NET cell surfaces makes them ideal targets for treatment and imaging [19]. There are two primary types of somatostatin receptorbased imaging available. The most common is the OctreoScan, which uses the ligand 111In-DPTA-D-Phe-1-octreotide and binds primarily to SSTR2 and SSTR5 [18]. In its original form, it provided a planar, full body image (Figure 3A). In modern practice, this image is fused with single photon emission computed tomography (SPECT) and CT (Figure 3B). This takes advantage of the specificity of the OctreoScan and the anatomic detail provided by SPECT/CT, improving OctreoScan’s diagnostic accuracy [20]. The newest somatostatin receptor-based imaging modality uses the positron emitter 68Ga to label various somatostatin analogs. The most common of these labeled analogs are 68Ga-DOTATOC (Figure 4), 68Ga-DOTANOC and 68Ga-DOTATATE [21]. These PET images are fused with CT to enhance the anatomic resolution of the study (Figure 3C & D) [22].
Figure 3. Comparison of (A) planar OctreoScan, (B) fused OctreoScan/SPECT/CT, (C) planar 68Ga-DOTATOC PET and (D) 68Ga-DOTATOC PET/CT in the same patient.
The patient has approximately 33% of their liver replaced by neuroendocrine tumor metastases. The images in (C) and (D) provide more precise delineation of lesions, compared with (A) and (B).
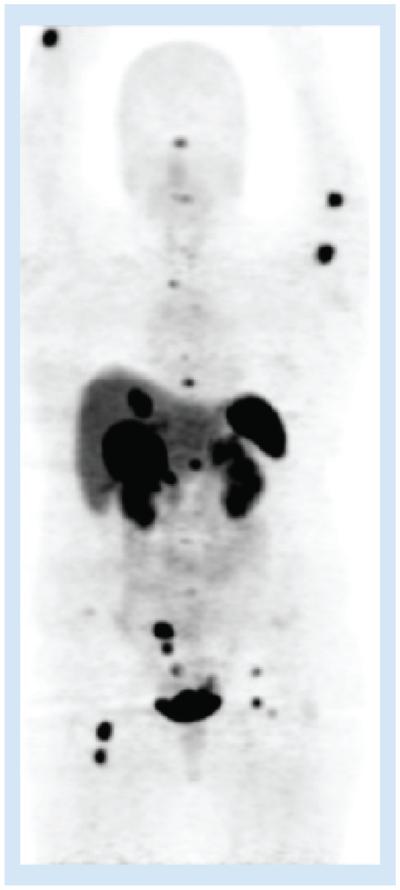
Figure 4. 68Ga-DOTATOC demonstrating multiple metastases in the upper and lower extremities, liver and right lower quadrant in a patient with an SBNET primary.
Physiologic uptake is seen in the pituitary, kidneys, spleen and bladder.
The indications for somatostatin receptor-based imaging are: detection and localization of primary NETs and their metastases, staging, follow-up for disease recurrence and to select patients for peptide receptor radionuclide therapy (PRRT). Long acting octreotide should be discontinued 4 to 6 weeks prior to imaging and the patient should be transitioned to a short acting regimen. For OctreoScan, patients need only to void prior to image acquisition. Though if the abdomen is the area of interest, use of an over-the-counter laxative the night prior to imaging may minimize the amount of nonpathologic uptake in the bowel, as approximately 2% of the labeled somatostatin analog is cleared via the hepatobiliary system and will end up in the intestine. The OctreoScan images are obtained 4 and 24 h after intravenous injection of the ligand [18,23]. The newer DOTA PET images are acquired once, at 60 min after radiotracer injection [22].
Octreotide analog studies
The first iteration of somatostatin receptor-based imaging used 123I-Tyr-3-octreotide as its SSTR-specific ligand. This radiotracer was tested in vivo in a small group of patients (n = 10) in 1989. Investigators reported that 50% of primary tumors from a variety of sites were identified correctly. In two patients, previously undetected metastases were demonstrated. These scans also appeared to predict whether patients would benefit from treatment with octreotide. Patients who had responded had positive scans and those that were considered nonresponders to octreotide had negative scans [24]. There were a few problems with this initial ligand. Not only was it difficult to prepare, but it had a short half-life and accumulated at high levels in the small intestine, limiting its utility for GI NETs.
The response to these problems was development of 111In-DTPA-D-Phe-1-octreotide, which is the ligand used by the modern OctreoScan. This tracer was easier to prepare, cleared primarily by the kidneys (solving the problem of accumulation within the GI tract), and had a much longer half-life (optimal image acquisition occurs at 24 h postinjection of the radiotracer). A small comparison study of the two tracers showed identical results in 4 of 6 patients. In the remaining 2 patients, the new 111In tracer was able to identify an insulinoma that the 123I tracer missed, and identified a greater total number of ‘carcinoid deposits’ compared with its predecessor [25].
A much larger series published by the same group in 1993 solidified 111In-DTPA-D-Phe-1-octreotide’s role as the best ligand for nuclear imaging. In this report, 735 patients had an OctreoScan using 111In-DTPA-D-Phe-1-octreotide and complete records available to confirm the findings of the study. Investigators found that the OctreoScan localized 86% of carcinoids, 89% of neuroblastomas, 86% of pheochromocytomas, 94% of paragangliomas and 80% of PNETs. It was less useful in detecting medullary thyroid carcinomas and pituitary tumors. No patient experienced adverse side effects from radiotracer injection [26].
In most centers, OctreoScan is combined with SPECT or SPECT/CT to improve its anatomic localization [27]. These fused images are obtained in thin slices, which minimizes obfuscation of the tumor by radioactivity emitted from surrounding normal tissues [28]. These improvements have been shown to alter the management in approximately 15% of cases, compared with planar OctreoScan images [29]. The sensitivity of the fused modality is comparable to standard imaging, though a wide range exists. In primary tumors, the OctreoScan’s sensitivity ranges from 35 to 80% [11,30–32], with its performance for unknown primary tumors dipping beneath the lower end of that range (24%) [11]. Its ability to detect the primary is limited by the size but not SSTR2 expression, as tumors less than 2 cm are significantly more likely not to localize but do not have significantly different SSTR2 expression than their larger counterparts [32].
One of the benefits to incorporating the OctreoScan into a workup for NETs is the ability to survey the entire body for metastases with a single study. The OctreoScan performs better in the detection of liver metastases than it does in primary tumor detection, and demonstrates sensitivities ranging from 49 to 91% in this context [11,13,31,33–34]. This compares relatively favorably with MRI (80 – 95%), the gold standard of liver lesion characterization [13,33]. Similar to the situation with primary tumor detection, the OctreoScan’s sensitivity appears to be limited by tumor size [13]. Due to OctreoScan being a functional (i.e., SSTR-specific) full body scan, it can often identify lesions that were missed by CT or MRI. Chiti et al. found that OctreoScan identified new lesions in 28% of patients [11]. In two studies focusing on identification of NET metastases, the OctreoScan identified new lesions in 47% [33] and 4.6% [13] of patients, respectively, whose lesions were otherwise missed by CT or MRI. A study performed in a set of 31 patients with bronchial carcinoids showed comparable results. In 22 cases, the OctreoScan results were identical to conventional imaging studies. Previously undiscovered lesions were detected by OctreoScan in 6 of 31 cases, and in 2 cases, the OctreoScan was the only modality to detect these lesions [35].
The OctreoScan is a useful adjunct in the workup of NETs, especially when fused with SPECT and CT, and is likely the most commonly used imaging study in the diagnosis and work up of NETs. It has moderate sensitivity as a stand-alone study, but excellent specificity and has the advantage of being the only functional imaging study available for the majority of these tumors. Further, its whole-body acquisition allows much information to be gathered with a straightforward protocol. Its advantages maintain its role in the workup of these tumors, while its disadvantages have prompted continued innovations to further improve utility. These include high uptake in the liver making smaller metastases difficult to visualize, excretion into the GI tract, insensitivity for small primaries, and low anatomic detail that limits its value in planning operations.
68Ga-labeled studies
The newest advance in somatostatin receptor-based imaging is the introduction of 68Ga-labeled radioligands, whose uptake is measured by PET scan (Figure 4). These peptides are easier and cheaper to synthesize than standard octreotide-analog based ligands, boast single time point image acquisition (1 h postinjection vs OctreoScan, which requires patients to be scanned at 4 and 24 h postinjection), and allow for quantifying the uptake of the ligand within the lesions. Its superior spatial resolution derives from the fact that it measures the radiation from two photons coincidentally. SPECT, in comparison, measures the gamma radiation emitted from one photon directly. This results in different limitations of detection – millimeters for 68Ga-PET compared with 1 cm or more for SPECT [28]. There are a few choices of ligands with this type of imaging, but the differences lie primarily in their SSTR affinities – all of the ligands bind with great affinity to SSTR2 and SSTR5. 68Ga-DOTANOC also binds to SSTR3. Despite these differences, no single 68Ga ligand has stood out as the clear choice for use in NETs [22]. As with standard somatostatin receptor-based imaging, these 68Ga-PET studies are fused with CT to improve anatomic localization.
Comparison studies between 68Ga-PET and standard imaging techniques (CT, OctreoScan) have universally demonstrated the superiority of 68Ga-PET in detection of NET primary tumors and metastases. Two early studies compared 68Ga-DOTATOC to standard somatostatin imaging (SRS)-SPECT and CT. Buchmann et al. reported that 68Ga-DOTATOC detected more than 279 NET lesions in 27 patients with histologically proven NETs, whereas SRS-SPECT detected only 157. The greatest number of lesions were detected in the liver. 68Ga-DOTATOC found more than 152 hepatic lesions, while SRS-SPECT found only 105, resulting in a 66% concordance rate between the two modalities. The concordance for abdominal lymph nodes was worse at 40.1%. In this set of patients, the greater sensitivity of 68Ga-DOTATOC resulted in an altered surgical plan in one patient [36].
Gabriel et al. reported similar conclusions to Buchmann et al. in their comparison study of 68Ga-DOTATOC, SRS-SPECT and CT in 84 patients. They found the sensitivity and specificity of 68Ga-DOTATOC to be 97 and 92%, respectively. For SRS-SPECT, the sensitivity was 52% and specificity 92%. The difference in sensitivities between these two modalities was statistically significant (p < 0.001). 68Ga-DOTATOC provided new clinical information in 21.4% of patients, which resulted in altered surgical plans in three patients (surgery declined due to previously unknown widespread metastases) [37].
Studies have also compared 68Ga-DOTANOC to standard imaging (CT, MRI, US, OctreoScan). In the smaller of these studies (n = 19), 68Ga-DOTANOC was compared with OctreoScan. In general, 68Ga-DOTANOC detected more numerous lesions than OctreoScan, but there were a fair number that were missed by 68Ga-DOTANOC and detected by OctreoScan [38]. In a larger study (n = 109), the sensitivity of 68Ga-DOTANOC was superior to CT, MRI, and US by almost every measure. In primary tumors, the sensitivity of 68Ga-DOTANOC was 78.3% compared with 63.8% sensitivity with conventional imaging (CT, MRI, US) (p < 0.001). In metastases, 68Ga-DOTANOC had a sensitivity of 97.4%, while conventional imaging demonstrated a sensitivity of 81.8%. Broken down by metastasis type, 68Ga-DOTANOC was significantly better at detecting lymph node metastases than conventional imaging (p < 0.0001), though there was no difference in these studies’ abilities to detect liver metastases (p = 1). 68Ga-DOTANOC improved the care of 19% of patients included in the study. The primary tumor was detected in 5.5% of patients where it was otherwise missed, which led to surgery in these patients. In 6.4%, a more extensive surgery was planned, as additional metastases were uncovered. Three patients were spared surgery because 68Ga-DOTANOC demonstrated more extensive metastatic disease than did conventional imaging [39].
68Ga-DOTA PET has also proven its utility in special situations, such as detection of unique NETs and in cases of unknown primary NETs. Neuroendocrine tumors can arise in a variety of tissues. Rare subtypes are frequently excluded from large series, and so little is known about how standard NET workup and treat- ments perform in these cases. Fanti et al. examined the question of how well 68Ga-DOTANOC detected ‘unique’ NET primaries in 2008. The study included 13 patients with NETs originating from uterus (n = 3), ovary (n = 1), kidney (n = 1), prostate (n = 3), breast (n = 1), ear (n = 1), as well as paragangliomas (n = 3). In this set, 68Ga-DOTANOC was deemed to have added useful clinical information in 50% of cases. In 57% of cases, the study was ‘the determinant of management.’ The study concluded that in the setting of unusual NET primaries, 68Ga-DOTANOC can add useful information to many clinical situations but that it is most useful for patients with paragangliomas [40].
For unknown primary sites, 68Ga-DOTANOC was able to localize the primary tumor in 35 of 59 patients (59%) with known neuroendocrine metastases. Primary sites (confirmed at surgery or by other imaging modalities) included pancreas (n = 16), small bowel (n = 15), lung (n = 2), rectum/colon (n = 2) and a paraganglioma (n = 1). CT localized the primary tumor in only 20% of patients [41]. In another study of patients with elevated serum markers and suspected NETs (n = 131), 68Ga-DOTANOC was true positive in 17 cases, true negative in 112 cases and false negative in 2 cases [42]. Taken together, these studies suggest that when a patient has histologically proven neuroendocrine disease, but prior workup has failed to uncover the primary, 68Ga-PET imaging has a high likelihood of uncovering the primary compared with conventional imaging. When neuroendocrine disease is suspected but not histologically proven, 68Ga-PET should not be used as the primary imaging modality, given its expense and the likelihood that it has a high probability of being (true) negative in patients with elevated serum markers as the only indication of disease [42]. Physiologic uptake in the uncinate process of the pancreas, pituitary, spleen (or accessory spleen) and kidneys may be misconstrued as a false positive result [43]. Its true utility lies in its ability to supplement the information gathered via clinical investigations and standard imaging (CT, OctreoScan) [42].
Just as the OctreoScan has been used to select patients for treatment with Octreotide, 68Ga-DOTA PET imaging can be used to determine which patients might benefit from Octreotide and PRRT. The benefit is determined not just by whether or not the tumors take up the radioligand. A recent report suggests that patients can be stratified into ‘responders’ or ‘non-responders’ to PRRT, based on their pretreatment maximum standard uptake value (SUVmax), where ‘responders’ have an SUVmax of greater than 17.9 [44]. However, this is not a universal finding, as in 2009, Gabriel et al. found that SUVmax was a poor measure of treatment response [45]. Larger studies will be required to resolve this issue.
Beyond prediction of treatment response, there have been questions as to how PRRT may affect the uptake of 68Ga radioligand in follow-up studies in normal and cancerous tissues, and possibly diminish its utility as a follow-up study. Quantitative differences in 68Ga uptake can be determined by comparing pre- and post-treatment maximum standard uptake values (SUVmax). The general conclusions from two recent studies were that treatment with PRRT did not seem to affect the accuracy of the follow-up 68Ga-PET studies. In one study, there was no significant difference in SUVmax in any of the tissues evaluated [46], and in the other, a significant difference in SUVmax was seen pre- and post-treatment in liver metastases (p < 0.02), but the uptake in benign liver tissue remained so low that the study maintained its clinical utility for these lesions [43].
123I-MIBG imaging
Radioiodinated (123I) metaiodobenzylguanidine (MIBG) is an analog of norepinephrine that is used to image catecholamine-secreting NETs such as pheochromocytomas, paragangliomas and glomus tumors [47]. In patients with functional pheochromocytomas or paragangliomas, this modality has a sensitivity of 90% [48,49] and positive predictive value of 100% [49]. However, it has limited use in GI NETs, as this modality was positive in only 49.1% of patients. In the same cohort of patients, OctreoScan was positive in 91.2% [50]. As an imaging tool, this study is best used to confirm a diagnosis of pheochromocytoma or paraganglioma and define the extent of metastatic disease in these tumors [51]. Its most practical use in GI NETs may be to determine whether patients with metastases may benefit from treatment with 131I-MIBG.
Conclusion
Neuroendocrine tumors are a unique malignant entity that can be evaluated by imaging in a variety of ways. Conventional imaging modalities such as CT, US, MRI and the OctreoScan are excellent initial studies to locate the primary tumor, evaluate extent of disease and plan for surgical management. However, in cases where these studies do not provide the detail required to make more complex management decisions, the 68Ga-PET studies have demonstrated their utility as excellent confirmatory studies, and have the capability to alter clinical decision making due to their superior sensitivity.
Future perspective
There are likely two areas in NET-specific imaging that will see improvement in the coming years. The first is access to PET imaging with 68Ga-DOTA octreopeptides. Though this modality is available in the majority of European centers, lack of FDA approval for the 68Ga radiotracer has hindered its use in the USA. However, as more randomized controlled trials are performed and clinical experience increases with this valuable modality, FDA approval for the 68Ga labeled octreotide analogs is expected. This approval will likely result in 68Ga-PET imaging supplanting the standard OctreoScan as the somatostatin-receptor based imaging modality of choice. The second area of interest in this field is discovery of novel targets for NET-specific imaging. Not all NETs have high expression of SSTR and may thus be missed by current somatostatin-based imaging. As molecular investigations of NETs have increased, there have been some recent advances in this area [52], but no doubt more are likely to come.
Executive summary.
Conventional imaging techniques
Contrast-enhanced CT scans are useful in the preoperative setting, as they provide excellent anatomic characterization of both malignant and normal tissues. Hepatic metastases are best visualized in the arterial phase, and will wash out in subsequent phases.
MRI is the ideal study to differentiate between malignant and benign hepatic lesions.
Ultrasound is a useful adjunct to CT and is used to identify hepatic metastases. Its advantages are its low cost, widespread availability and use as an intraoperative tool.
18FDG PET may identify high grade neuroendocrine tumors, as they tend to be more metabolically active.
Somatostatin-based imaging
OctreoScan is the current standard in somatostatin-based imaging. It can be fused with SPECT and CT to provide both functional and anatomic information.
68Ga-PET imaging has greater sensitivity detecting NETs than the OctreoScan. It is fused with CT to provide anatomic details. Despite its superior spatial resolution, it is only available in a small number of centers around the USA.
Conclusion
Optimal work up of neuroendocrine tumors requires use of a combination of conventional and somatostatin-based imaging techniques.
68Ga-PET imaging may supplant OctreoScan as the somatostatin-based imaging modality of choice, once FDA approval of the 68Ga radiotracer allows for greater access throughout the USA.
Acknowledgements
The authors would like to acknowledge the expert opinions lent by EJ Carolan and Y Menda during the preparation of this manuscript.
Financial & competing interests disclosure
JE Maxwell is supported by NIH grant 5T32#CA148062–05. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch. Surg. 2011;396(3):273–298. doi: 10.1007/s00423-011-0739-1. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Mocellin S, Nitti D. Gastrointestinal carcinoid: epidemiological and survival evidence from a large population-based study (n = 25 531) Ann. Oncol. 2013;24(12):3040–3044. doi: 10.1093/annonc/mdt377. [DOI] [PubMed] [Google Scholar]
- 4.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J. Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011;29(17):2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 6•.Kwekkeboom DJ, De Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26(13):2124–2130. doi: 10.1200/JCO.2007.15.2553. Seminal article on somatostatin-based imaging. [DOI] [PubMed] [Google Scholar]
- 7.Bushnell DL, Baum RP. Standard imaging techniques for neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011;40:153–162. doi: 10.1016/j.ecl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs MA, Weinstein S, Hope TA, Aslam R, Yee J, Coakley F. Neuroendocrine tumors: beyond the abdomen. J. Comput. Assist. Tomogr. 2014;38:898–914. doi: 10.1097/RCT.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 9••.Sundin A, Vullierme MP, Kaltsas G, Plockinger U. Mallorca Consensus Conference Participants, European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90(2):167–183. doi: 10.1159/000184855. The most recent guidelines for neuroendocrine tumors (NETs) set forth by the European Neuroendocrine Tumor Society (ENETS) [DOI] [PubMed] [Google Scholar]
- 10.Kirshbom PM, Kherani AR, Onaitis MW, Feldman JM, Tyler DS. Carcinoids of unknown origin: comparative analysis with foregut, midgut, and hindgut carcinoids. Surgery. 1998;124(6):1063–1070. doi: 10.1067/msy.1998.93105. [DOI] [PubMed] [Google Scholar]
- 11•.Chiti A, Fanti S, Savelli G, et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-enteropancreatic tumours. Eur. J. Nucl. Med. 1998;25:1396–1403. doi: 10.1007/s002590050314. An excellent early study comparing three methods of conventional imaging in NETs. [DOI] [PubMed] [Google Scholar]
- 12.Joseph S, Wang YZ, Boudreaux JP, et al. Neuroendocrine tumors: current recommendations for diagnosis and surgical management. Endocrinol. Metab. Clin. North Am. 2011;40(1):205–231. doi: 10.1016/j.ecl.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Dromain C, De Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J. Clin. Oncol. 2005;23(1):70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Hoeffel C, Job L, Ladam-Marcus V, Vitry F, Cadiot G, Marcus C. Detection of hepatic metastases from carcinoid tumor: prospective evaluation of contrast-enhanced ultrasonography. Dig. Dis. Sci. 2009;54(9):2040–2046. doi: 10.1007/s10620-008-0570-x. [DOI] [PubMed] [Google Scholar]
- 15.Sundin A, Eriksson B, Bergström M, Långström B, Öberg K, Örlefors H. PET in the diagnosis of neuroendocrine tumors. Ann. NY Acad. Sci. 2004;1014(1):246–257. doi: 10.1196/annals.1294.027. [DOI] [PubMed] [Google Scholar]
- 16.Pasquali C, Rubello D, Sperti C, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J. Surg. 1998;22:588–592. doi: 10.1007/s002689900439. [DOI] [PubMed] [Google Scholar]
- 17.Patel YC. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 18••.Kwekkeboom DJ, Krenning EP, Scheidhauer K, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology. 2009;90(2):184–189. doi: 10.1159/000225946. The most recent guidelines from the ENETS regarding imaging with OctreoScan. [DOI] [PubMed] [Google Scholar]
- 19.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl. 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 20.Lu SJ, Gnanasegaran G, Buscombe J, Navalkissoor S. Single photon emission computed tomography/computed tomography in the evaluation of neuroendocrine tumours: a review of the literature. Nucl. Med. Commun. 2013;34(2):98–107. doi: 10.1097/MNM.0b013e32835bd59d. [DOI] [PubMed] [Google Scholar]
- 21.Sundin A. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2012;26(6):803–818. doi: 10.1016/j.bpg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosini V, Campana D, Tomassetti P, Fanti S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:s52–s60. doi: 10.1007/s00259-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 23.Balon HR, Brown TL, Goldsmith SJ, et al. The SNM practice guideline for somatostatin receptor scintigraphy 2.0. J. Nucl. Med. Technol. 2011;39(4):317–324. doi: 10.2967/jnmt.111.098277. [DOI] [PubMed] [Google Scholar]
- 24.Krenning EP, Breeman WaP, Kooij PP, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1(8632):242–244. doi: 10.1016/s0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 25.Krenning EP, Bakker WH, Kooij PPM, et al. Somatostatin receptor scintigraphy with Indium-111-DTPA-D-Phe-1-oman: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J. Nucl. Med. 1992;33(5):652–658. [PubMed] [Google Scholar]
- 26•.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 1993;20(8):716–731. doi: 10.1007/BF00181765. A large series (written by the developer of the OctreoScan) summarizing one institution’s experience with somatostatin-based imaging. [DOI] [PubMed] [Google Scholar]
- 27.Bural GG, Muthukrishnan A, Oborski MJ, Mountz JM. Improved benefit of SPECT/CT compared to SPECT alone for the accurate localization of endocrine and neuroendocrine tumors. Mol. Imaging Radionucl. Ther. 2012;21(3):91–96. doi: 10.4274/Mirt.80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanzonico P. Principles of nuclear medicine imaging: planar, SPECT, PET, multi-modality, and autoradiography systems. Radiat. Res. 2012;177(4):349–364. doi: 10.1667/rr2577.1. [DOI] [PubMed] [Google Scholar]
- 29.Krausz Y, Keidar Z, Kogan I, et al. SPECT/CT hybrid imaging with 111In-pentetreotide in assessment of neuroendocrine tumours. Clin. Endocrinol. (Oxf.) 2003;59:565–573. doi: 10.1046/j.1365-2265.2003.01885.x. [DOI] [PubMed] [Google Scholar]
- 30.Savelli G, Lucignani G, Seregni E, et al. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumors. Nucl. Med. Commun. 2004;25(5):445–449. doi: 10.1097/00006231-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kumbasar B, Kamel IR, Tekes A, Eng J, Fishman EK, Wahl RL. Imaging of neuroendocrine tumors: accuracy of helical CT versus SRS. Abdom. Imaging. 2004;29(6):696–702. doi: 10.1007/s00261-003-0162-3. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell JE, Sherman SK, Menda Y, Wang D, O’dorisio TM, Howe JR. Limitations of somatostatin scintigraphy in primary small bowel neuroendocrine tumors. J. Surg. Res. 2014;190(2):548–553. doi: 10.1016/j.jss.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi W, Johnston CF, Buchanan KD, et al. Localization of neuroendocrine tumours with 111In-DTPA-octreotide scintigraphy (Octreoscan): a comparitive study with CT and MR imaging. Q. J. Med. 1998;91:295–301. doi: 10.1093/qjmed/91.4.295. [DOI] [PubMed] [Google Scholar]
- 34•.Dahdaleh FS, Lorenzen A, Rajput M, et al. The value of preoperative imaging in small bowel neuroendocrine tumors. Ann. Surg. Oncol. 2013;20(6):1912–1917. doi: 10.1245/s10434-012-2836-y. A retrospective comparison of the value of CT and the OctreoScan in the preoperative setting. [DOI] [PubMed] [Google Scholar]
- 35.Fanti S, Farsad M, Battista G, et al. Somatostatin receptor scintigraphy for bronchial carcinoid follow-up. Clin. Nucl. Med. 2003;28(7):548–552. doi: 10.1097/00003072-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2007;34(10):1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007;48(4):508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 38.Krausz Y, Freedman N, Rubinstein R, et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan) Mol. Imaging Biol. 2011;13:583–593. doi: 10.1007/s11307-010-0374-1. [DOI] [PubMed] [Google Scholar]
- 39.Naswa N, Sharma P, Kumar A, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am. J. Roentgenol. 2011;197:1221–1228. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 40.Fanti S, Ambrosini V, Tomassetti P, et al. Evaluation of unusual neuroendocrine tumours by means of 68Ga-DOTA-NOC PET. Biomed. Pharmacother. 2008;62(10):667–671. doi: 10.1016/j.biopha.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2010;37(1):67–77. doi: 10.1007/s00259-009-1205-y. [DOI] [PubMed] [Google Scholar]
- 42.Ambrosini V, Campana D, Nanni C, et al. Is (6)(8)Ga-DOTA-NOC PET/CT indicated in patients with clinical, biochemical or radiological suspicion of neuroendocrine tumour? Eur. J. Nucl. Med. Mol. Imaging. 2012;39(8):1278–1283. doi: 10.1007/s00259-012-2146-4. [DOI] [PubMed] [Google Scholar]
- 43.Kroiss A, Putzer D, Decristoforo C, et al. 68Ga-DOTATOC uptake in neuroendocrine tumour and healthy tissue: differentiation of physiological uptake and pathological processes in PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(4):514–523. doi: 10.1007/s00259-012-2309-3. [DOI] [PubMed] [Google Scholar]
- 44.Oksuz MO, Winter L, Pfannenberg C, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90) Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn. Interv. Imaging. 2014;95(3):289–300. doi: 10.1016/j.diii.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Gabriel M, Oberauer A, Dobrozemsky G, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J. Nucl. Med. 2009;50(9):1427–1434. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 46.Giesel FL, Stefanova M, Schwartz LH, et al. Impact of pepide receptor radionuclide therapy on the 68Ga-DOTATOC-PET/CT uptake in normal tissue. Q. J. Nucl. Med. Mol. Imaging. 2013;57:171–176. [PubMed] [Google Scholar]
- 47.Vallabhajosula S, Nikolopoulou A. Radioiodinated metaiodobenzylguanidine (MIBG): radiochemistry, biology, and pharmacology. Semin. Nucl. Med. 2011;41(5):324–333. doi: 10.1053/j.semnuclmed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Raja A, Leung K, Stamm M, Girgis S, Low G. Multimodality imaging findings of pheochromocytoma with associated clinical and biochemical features in 53 patients with histologically confirmed tumors. AJR Am. J. Roentgenol. 2013;201(4):825–833. doi: 10.2214/AJR.12.9576. [DOI] [PubMed] [Google Scholar]
- 49.Lumachi F, Tregnaghi A, Zucchetta P, et al. Sensitivity and positive predictive value of CT, MRI and 123I-MIBG scintigraphy in localizing pheochromocytomas: a prospective study. Nucl. Med. Commun. 2006;27:583–587. doi: 10.1097/00006231-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Ezziddin S, Logvinski T, Yong-Hing C, et al. Factors Predicting tracer uptake in somatostatin receptor and MIBG scintigraphy of metastatic gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2006;47:223–233. [PubMed] [Google Scholar]
- 51.Hicks RJ. Use of molecular targeted agents for the diagnosis, staging and therapy of neuroendocrine malignancy. Cancer Imaging. 2010;10(1A):S83–S91. doi: 10.1102/1470-7330.2010.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Sherman SK, Carr JC, Wang D, O’dorisio MS, O’dorisio TM, Howe JR. Gastric inhibitory polypeptide receptor (GIPR) is a promising target for imaging and therapy in neuroendocrine tumors. Surgery. 2013;154(6):1206–1213. doi: 10.1016/j.surg.2013.04.052. discussion 1214. An interesting study that may shed light on the direction of future research in NET imaging and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]