Abstract
How cells maintain specific levels of each protein and whether that control is evolutionarily conserved are key questions. Here, we report proteome-wide steady-state protein turnover rate measurements for the evolutionarily distant, but ecologically similar yeasts, Saccharomyces cerevisiae and Schizosaccharomyces pombe. We find that the half-lives of most proteins is much longer than currently thought and determined to a large degree by proteins synthesis and dilution due to cell division. However, we detect a significant subset of proteins (~15%) in both yeasts that are turned over rapidly. In addition, the relative abundances of orthologous proteins between the two yeasts are highly conserved across the 400 million years of evolution. In contrast, their respective turnover rates differ considerably. Our data provide a high-confidence resource for studying protein degradation in common yeast model systems.
Keywords: mass spectrometry, proteomics, protein abundance, Saccharomyces cerevisiae, Schizosaccharomyces pombe, sterol synthesis regulation, regulation of mitochondria
Introduction
The levels of proteins often determine phenotypes. Protein abundance is a result of their synthesis and degradation rates. At steady state, these rates are equal and characteristic for each protein under a set of conditions. In S. cerevisiae, bulk measurements of protein mass turnover indicate that protein half-lives can range two orders of magnitude (Gancedo et al., 1982). Analyses of individual proteins have revealed different proteasomal or lysosomal pathways for their degradation.
Most measurements of individual proteins’ turnover rates were determined in experiments following a S35 pulse label in a specific protein isolated with specific antibodies during a chase. However, this approach is difficult to extend to the whole proteome. As an alternative, protein stability is assayed by blocking protein synthesis using cycloheximide and measuring the level of a protein of interest at different time points. However, in this approach, cells are not kept at steady state as blocking translation induces a stress response (MacGurn et al., 2011). In addition, performing such analyses systematically relies on tagging proteins, which may lead to changes in protein stability. Alternative approaches, such as imaging based approaches in combination with photobleaching (Eden et al., 2011), chemical modification (Bojkowska et al., 2011) or mass-spectrometry based proteomics studies (Boisvert et al., 2012; Cambridge et al., 2011; Helbig et al., 2011; Pratt et al., 2002; Schwanhausser et al., 2011; Toyama et al., 2013) have yielded important insights, but have not been extended to a system-wide characterization of protein turnover.
Here, we overcome these limitations of globally determining protein turnover by capitalizing on recent advances in mass spectrometry-based proteomic technology (Bensimon et al., 2012; Frohlich et al., 2013; Mann et al., 2013; Michalski et al., 2011; Zhang et al., 2013). We report protein turnover rates of nearly all proteins expressed under standard conditions for two model organisms, S. cerevisiae and S. pombe. We identify a subset of protein (~15%) that turn over rapidly and provide evidence that protein stability is less evolutionary conserved than protein abundance.
Results
Turnover Rate Measurements in S. cerevisiae and S. pombe by Pulse SILAC
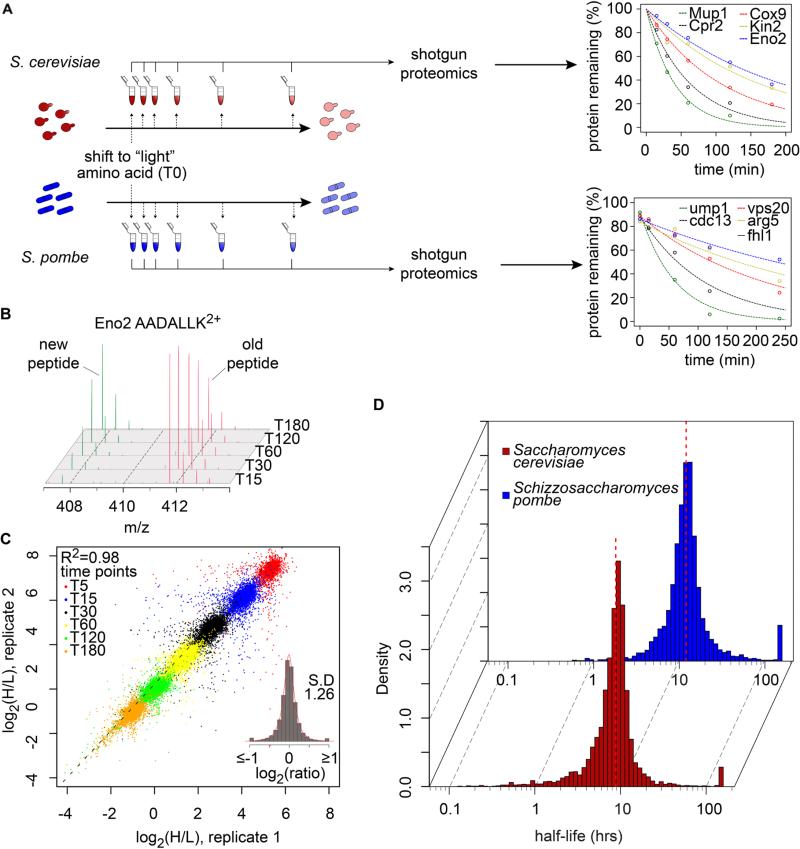
To determine protein turnover rates systematically, we metabolically labeled the yeasts with lysine containing heavy isotopes (“heavy” lysine, H) and diluted the cells in an excess of normal lysine (“light” lysine, L) at the beginning of the experiment (Fig.1A, S1A, B). We analyzed the decay of the “heavy” lysine signal in the proteome over time by high-resolution mass-spectrometry-based proteomics (Schwanhausser et al., 2011) (Fig. 1B). We identified 4,425 and 3,705 proteins with average sequence coverages of 11.2 and 8.9 peptides/protein in S. cerevisiae and S. pombe, respectively (Table 1). Heavy to light (H/L) ratios from independent biological replicates were highly reproducible in both yeasts (R2=0.98 and 0.96; Fig. 1C, Fig. S1C). The abundance of proteins did not change significantly during the experiment, as expected at steady state (90% and 89% of 3292 and 3108 proteins identified in at least three time points in S. cerevisiae and S. pombe, respectively, have ≤ 5% variation of summed peptide intensities) (Fig. S1D, E). Curve fitting and stringent filtering (Methods and Fig. S1F, G) yielded high-confidence half-life measurements for 3,676 (85% of identified proteins) and 3,025 (81% of identified proteins) proteins for the two yeasts, respectively (Tables S1, S2).
Fig. 1. Quantifying protein turnover in S. cerevisiae and S. pombe.
(A) Experimental design for turnover measurements in yeasts. Decay curves for the indicated proteins in S. cerevisiae and S. pombe are shown. (B) Mass spectra after labeling of a peptide from Eno2 (AADALLK2+) in S. cerevisiae. The old peptide (“heavy”, red) decays as the newly synthesized peptide (“light”, green) increases in intensity during the time course of the experiment. (C) Log2(H/L) intensities from 40,452 peptides measured in two independent biological replicates. The insert shows the histogram distribution of log2(H/L) (mean = −0.0018, SD = 1.26 expressed as a fold-change from the mean). (D) Histograms of protein half-lives in S. cerevisiae (red, dashed line indicates the median half-life = 8.8 hours) and S. pombe (blue, dashed line indicates the median half-life = 11.1 hours).
Table 1.
Description of mass-spectrometry data.
| Species | unique peptides identified | number of proteins identified | mean coverage (%) | peptides per protein | number of half-lives |
|---|---|---|---|---|---|
| S. cerevisiae | 48,448 | 4,425 | 30.45 | 11.24 | 3,748 |
| S. pombe | 32,980 | 3,705 | 29.9 | 8.9 | 3,016 |
Surprisingly, the vast majority of proteins in both yeasts are very long lived. The median half-lives in budding and fission yeast in the conditions of our experiments are 8.8 and 12.0 hours, respectively (Fig. 1D). Remarkably, and in agreement with previous measurements on the turnover of bulk protein mass (Gancedo et al., 1982), distribution of half-lives span two orders of magnitude and range from a few minutes to more than 100 hours in both species (Fig. 1D, Tables S1, S2). As observed in other model systems (Doherty et al., 2009), the distribution of half-lives in both species does not follow a normal distribution but is skewed with more short-lived proteins (Kolmogorov-Smirnov test, P < 2.2.10-16). Our results were further validated by comparing our dataset to known turnover rates determined by other experimental approaches, such as pulse-chase with radioactively labeled amino acids (Table S3). In addition, comparative analysis of each protein's turnover rate also highlighted problems with measuring the half-lives of tagged proteins during cycloheximide treatment with significant deviations from the rates measured by our proteomics approach. This might be due to altered cell physiology during protein synthesis inhibition and/or the modification of proteins with tags (Belle et al., 2006) (Fig. S2A, B).
Three Regimes of Protein Abundance Control
Our analyses on the overwhelming majority of yeast proteins revealed three classes, representing three distinct regimes of protein abundance control (Fig. 2A, Table S4). Two classes appear to mediate the rapid and competitive growth of the two yeasts. Class I contains a small fraction (~2% in S. cerevisiae and ~1% in S. pombe) of very short-lived proteins many driving the cell cycle (P-value = 4.4.10-3; Fig. 2B) for which the degradation rates are at least twice the dilution rate due to cell growth (t1/2<1.25 hrs in S. cerevisiae and t1/2<2 hrs in S. pombe). Their abundance is likely primarily determined by their rapid degradation. A second class of very stable proteins predominantly driving growth and mass accumulation (class III) is defined by degradation rates less than half the dilution rate (t1/2≥5 hrs and t1/2≥8 hrs in S. cerevisiae and S. pombe, respectively) and contains most proteins in both yeasts (86% and 84% of proteins). The abundance of these proteins is predominantly determined by different synthesis rates, but similar dilution rates due to cell division. Class II proteins show intermediate half-lives where abundance is determined by the interplay of protein synthesis, degradation and dilution (12.5% in S. cerevisiae and 15% in S. pombe). In S. cerevisiae, these proteins mediate many regulated processes, such as nutrient transport across the plasma membrane (Fig. 2B). Together, class I and II with significant contribution of degradation to half-live, contain 14.5% of the analyzed proteins. As we do not have information on 20% of the proteins not passing our very stringent filtering criteria, this class could contain some more proteins.
Fig. 2. Protein half-life differences in distinct sets of proteins.
(A) Contributions of degradation (Kdeg) and protein dilution due to cell growth (Kdil) in S. cerevisiae and S. pombe. Log2(Kdeg/Kdil) ratios define three classes of protein abundance regulation: class I (Kdeg ≥ 2xKdil), class II (0.5xKdil ≤ Kdeg ≥ 2xKdil) and class III (Kdeg ≤ 2xKdil). (B) Gene Ontology (GO) analysis of class I and class II proteins in S. cerevisiae. (C) Comparison of protein abundances with ribosome footprint data in S. cerevisiae for proteins of class I (purple), class II (orange) and class III (green). (D) Comparison of protein abundances between class I, II and III. (E) Sequence analysis of the 5′UTR (30-mer ahead of start codon) of the 10% most abundant proteins in S. cerevisiae.
The notion that the abundance of proteins in classes I and II is influenced by degradation is further supported by comparing the protein abundance in S. cerevisiae with available ribosome footprint data (Ingolia et al., 2009) (Fig. 2C). These data reflect the amount of actively translating ribosomes on a message and thus serve as a proxy for measuring the protein synthesis rate of each protein. In agreement with a dominant contribution of protein synthesis and dilution to overall protein abundance, ribosome footprint experiments accurately predict the abundance of class III proteins (R2=0.73 for class III). In contrast, ribosome footprint does not correlate as well with the abundance of class II proteins (R2= 0.60) and strikingly fails to predict the abundance of class I proteins (R2= 0.20). For the latter, degradation provides a significant additional contribution to determining protein abundance. Accordingly, the longer-lived proteins of class III are significantly more abundant than those of other classes, where protein degradation plays a significant role in determining overall levels (P < 1.10-15) (Fig. 2D). Remarkably, highly efficient translation to preferentially express large amounts of some proteins for cell growth and to outcompete bacteria and other yeasts appears to contribute to this. We detected a significantly overrepresented sequence motif in the 30-mer nucleotide localized in the 5′-untranslated region of mRNAs encoding the 10% most abundant proteins of class III. Interestingly, similar sequences, albeit shorter, have been selected in vitro as mediating highly efficient translation (Dvir et al., 2013).
Protein Abundances but not Turnover Rates Are Evolutionary Conserved
Abundance and turnover rate comparisons of 1,688 one-to-one orthologs between budding and fission yeast revealed surprising insights. Most importantly, the relative abundance of proteins was highly conserved and thus overall highly correlated between species (Pearson R2=0.64) (Fig. 3A). In contrast, the specific turnover rates of conserved proteins in both organisms are remarkably different (Pearson R2=0.16) (Fig. 3B, C). These findings suggest that relative protein abundances evolved under stronger evolutionary constraints across the 400 million years of evolution than the protein turnover rates that contribute to obtain these levels.
Fig. 3. Protein abundances, but not half-lives are evolutionarily conserved between S. cerevisiae and S. pombe.
(A) Scatter plot comparing homologous protein abundances. (B) Scatter plot comparing homologous protein half-lives. (C) Cumulative density distribution of protein half-lives. (D) Half-live comparison of the large (RLP, MRLP) and the small (RSP, MRSP) subunits of the cytosolic and mitochondrial ribosomes. (E) Half-live comparison of proteins involved in the arginine biosynthetic pathway.
Our dataset provides a rich resource for analysis of such divergent evolution. Proteins in S. pombe with half-lives at least twice as long as their counterparts in S. cerevisiae (329 proteins) are significantly enriched in factors for RNA processing (74 proteins, P=1.45.10-6), ribosome biogenesis proteins (71 proteins, P=2.5.10-13), and mitochondrial translation (27 proteins, P=3.66.10-5). For example, the relative abundance of ribosomal proteins is very similar in S. cerevisiae and S. pombe (R2=0.64, Fig. S3A, B). However, turnover rates of the same proteins are very different in S. cerevisiae (Fig. 3B). Protein turnover rates of the cytoplasmic ribosomal proteins fall within a very narrow range (Fig. 3D). In contrast, we observed great variation of ribosomal protein turnover in S. pombe (Fig. 3D). As ribosomal proteins in both organisms fall into class III of very-long-lived proteins, ribosomal protein synthesis rate, rather than assembly or degradation, is likely much more tightly coordinated in S. cerevisiae than in S. pombe. Interestingly, proteins with half-lives in S. cerevisiae twice as long as in S. pombe (64 proteins) are significantly enriched in factors playing a role in arginine metabolism (five proteins, P=6.6.10-4; Fig. 3B, E).
Evolutionary Divergence of Protein Degradation for Ergosterol Metabolic Enzymes
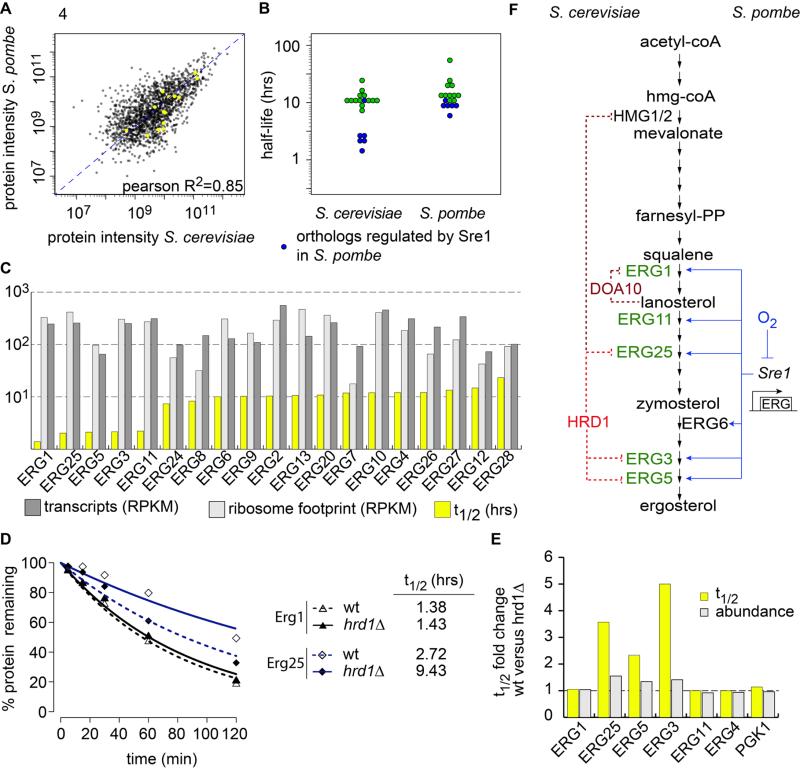
Divergence of gene expression regulation is thought to be important in evolution (2013). Using our datasets, we investigated whether evolution of protein turnover may also be important. We found that ergosterol synthetic enzymes provide examples for the evolution of protein levels governed either by gene expression or protein turnover in different species. Ergosterol synthesis enzymes have remarkably conserved relative abundances in both yeasts (R2=0.85, Fig 4A). In S. pombe, most of the ergosterol synthetic enzymes have similar turnover rates (Fig 4B). The long half-lives of these proteins suggest that, during exponential growth in S. pombe, protein synthesis governs their abundance. In contrast, we found that S. cerevisiae has two groups of Erg-enzymes with different turnover rates (Fig. 4B). Compared with most ergosterol metabolic enzymes the turnover of Erg1, Erg25, Erg5, Erg3 and Erg11 is much faster. Interstingly, their respective orthologs in S. pombe are anaerobically induced and regulated by the Sre1 transcription factor, which is absent in S. cerevisiae (Todd et al., 2006). This suggests that degradation of these enzymes, rather than their synthesis rate, has evolved to control protein levels in S. cerevisiae. Consistent with this notion, the mRNA abundance and ribosome footprint are similar for all Erg-genes in S. cerevisiae (Fig. 4C).
Fig. 4. Quantitative turnover analysis reveals the evolution of different strategies to control ergosterol metabolism enzymes.
(A) The abundance of ergosterol synthetic enzymes is conserved in S. cerevisiae and S. pombe (yellow). (B) The half-lives of ergosterol synthesis enzymes are similar in S. pombe whereas in S. cerevisiae, Erg1, Erg11, Erg3, Erg25 and Erg5 are short-lived proteins. In blue are anaerobically induced and Sre1 dependend gene in S. pombe and their counterpart in S. cerevisiae. (C) Plot of half-lives (yellow), transcripts abundances (light grey) and ribosome footprint (dark grey) for ergosterol synthesis enzymes. (D) Erg1 and Erg25 degradation followed by SILAC labeling decay in wild-type and hrd1Δ strains. (E) Half-live (yellow) and abundance (grey) fold changes of the indicated proteins in hrd1Δ compared with wild-type strains. (F) Representation of the regulation of the S. cerevisiae's short-lived ergosterol metabolic enzymes (green, left) and their orthologs in S. pombe (right). ERAD ubiquitin ligases are in red and previously characterized regulations are indicated in dashed dark red and new in dashed bright red. Previously described transcription factors regulation is indicated in blue.
Protein Turnover Profiling Identifies New Candidate Substrates for Hrd1-Dependant Pathway in S. cerevisiae
To test the applicability of our turnover approach to identify substrates of protein degradation pathways, we used proteome-wide turnover profiling in mutant yeast strain. We reasoned that substrates of a degradation pathway have a longer half-life when the pathway is compromised, thus potentially increasing their abundance. Endoplasmic reticulum–associated degradation (ERAD) regulates cholesterol metabolism by modulating turnover of the 3-hydroxy-3methylglutaryl-coenzyme A reductase (HMG-CoAR) and the squalene epoxidase (Erg1) in both mammals and budding yeast (Foresti et al., 2013; Ye and DeBose-Boyd, 2011). To test if ERAD also mediates the fast turnover of the newly identified short-lived Erg-enzymes, we quantitated changes in proteome turnover and abundance in hrd1Δ cells (Fig. S4). Hrd1 is an E3 ligase core component of one of the two ERAD machines in budding yeast (Thibault and Ng, 2012). The turnover of Erg3, Erg25 and, to a lesser extent, Erg5 were significantly slower in hrd1Δ cells, thus leading to moderate but reproducible increases of their protein level (Fig. 4D, E). In contrast, turnover and abundance of other Erg-enzymes, including the Doa10 E3–ubiquitin ligase substrate Erg1 (Foresti et al., 2013), Erg4 or Pgk1, was unchanged (Fig. 4E).
Discussion
In summary, we provide proteome-wide measurements of relative protein abundances and protein turnover for the two model systems S. cerevisiae and S. pombe. In comparison with other systematic approaches applied to investigate protein turnover in S. cerevisiae that rely on the perturbation of cells by inhibiting translation, our studies were performed at steady state with endogenous proteins using labeled amino acids. We find that the half-life of most proteins, especially those driving cell growth, is substantially longer than previously thought (Belle et al., 2006).
Complementary to ribosome profiling, which measures protein synthesis rates but does not predict the abundance of proteins that are degraded fast, our dataset reveals two classes of short-lived proteins. These classes are enriched in cellular regulators and likely constitute a good set of candidates for studying the different cellular protein degradation pathways. A comparison with available turnover data in mouse fibroblasts (Schwanhausser et al., 2011) shows that the number of proteins in these classes, with significant protein degradation (classes I and II), expands with organismal complexity (Fig. S2C, Table S4). Likely, this is explained by the presence of many more post-translationally regulated processes in mammalian cells.
Comparison of the protein abundance and turnover rates from S. cerevisiae with those from S. pombe, where no prior global dataset on protein turnover is available, highlights important commonalities and differences. Most strikingly, the relative abundance of one-to-one orthologous is remarkably conserved during roughly 400 Mio years of evolution. However, similar protein abundance is achieved differently for many orthologous proteins in both organisms.
Our data provides evidence for the evolution of different strategies governing protein abundance in the two yeasts. Specifically, ergosterol enzymes are regulated by protein synthesis in S. pombe and protein degradation in S. cerevisiae. Our approach further allowed us to identify candidate substrates for the Hrd1-dependent ERAD degradation pathway, such as Erg3, Erg25 and Erg5 (Table S5). Therefore, our findings have implications for understanding the evolution of phenotypic traits and the study of regulatory mechanisms of protein stability. The data provided here will also provide a reference resource, particularly for both yeast communities studying protein degradation pathways.
Experimental procedures
Strains and Cell Culture
All experiments with S. cerevisiae (wild-type) were performed with the BY4742 strain (Open Biosystems). Hrd1Δ were freshly prepared from sporulation of the heterozygous knock-out collection BY4743, selected and PCR confirmed to be isogenic to wild-type BY4742 genetic background (his3Δ1; leu2Δ; lys2Δ; ura3Δ). All experiments with S. pombe were performed with the lysine prototroph strain MKSP201 (ade6-M21; ura4Δ18; leu1-32).
S. cerevisiae strains were grown in synthetic medium containing 6.7 g/L yeast nitrogen base (YNB), 2 g/L drop-out mix (US Biological) containing all amino acids except lysine and 2% glucose. For heavy pre-labeling, “heavy” [13C6/15N2] L-lysine (Cambridge Isotope Labs) was added to a final concentration of 30 mg/L. Cells were pre-cultured in 5 mL medium containing heavy lysine overnight at 30°C and repeated twice.
S. pombe strains were grown in Edinburgh minimal medium (Sunrise Science Products) supplemented with (75 mg/L leucine, histidine, uracil and adenine). Native SILAC protocol was followed for heavy pre-labeling using heavy [13C6/15N2] L-lysine (Cambridge Isotope Labs) (Frohlich et al., 2013).
Pulse SILAC
After pre-culture or native SILAC labelling, cells are cultured in biological duplicates up to OD600=0.4 in 500 mL. After three washes at 4°C with cold SILAC medium without lysine, cells are transferred to SILAC medium containing light lysine. For S. cerevisiae, cells are harvested at 0, 5, 15, 30, 60, 120 and 180 minutes. S.pombe cells are harvest at 0, 15, 60, 120 and 240 minutes. MS analysis was run for each time point in technical duplicates. Also, peptides from time 5 minutes and 0 minute from S. cerevisiae and S. pombe pulse SILAC experiments, respectively, were fractionated in six fractions by strong anion chromatography (SAX).
Determination of protein half-lives
Protein half-lives were determined mainly following procedure described in (Schwanhausser et al., 2011) with slight modifications. For S. cerevisiae, which was fully labeled, a minimum of 2 time points was required to calculate decay rates (Kdeg). For S. pombe, a minimum of 3 time points was required. A script written in R language extracted the raw (H/L) ratios (r) and as described in (Schwanhausser et al., 2011), ln(r+1) were linearly fitted to get access to apparent degradation Kdeg rates. Then, Kdeg rates were further corrected for protein dilution due to cell growth rate (Kdil) using 2.5 and 4 hours as cells doubling time for S. cerevisiae and S. pombe, respectively, in the conditions of our experiments. Goodness-of-fit of linear regressions (R2) were calculated and protein with R2 ≥ 0.9 were kept for half-lives calculation. For subsequent comparison of half-lives between S. cerevisiae and S. pombe or S. cerevisiae wild-type and S. cerevisiae hrd1Δ, datasets were further filtered for degradation rates whose coefficient of variation of the slope analyzed by leave-one-out cross validation (Schwanhausser et al., 2011) was less than 10%.
Supplementary Material
Acknowledgments
We thank S. Waelde and M. King for kindly providing S. pombe strain. We would like to thank Drs. Robert Farese, Jr., Christopher Burd, Antonio Giraldez, Mark Hochstrasser, Julien Berro and members of the Walther laboratory for critical discussion and comments on the manuscript. This work was supported by grant R01GM095982 (to T.C.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information
Supplemental Information includes four figures, Supplemental Experimental Procedures, and five tables.
References and notes
- Belle A, Tanay A, Bitincka L, Shamir R, O'Shea EK. Quantification of protein half-lives in the budding yeast proteome. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon A, Heck AJ, Aebersold R. Mass spectrometry-based proteomics and network biology. Annual review of biochemistry. 2012;81:379–405. doi: 10.1146/annurev-biochem-072909-100424. [DOI] [PubMed] [Google Scholar]
- Boisvert FM, Ahmad Y, Gierlinski M, Charriere F, Lamont D, Scott M, Barton G, Lamond AI. A quantitative spatial proteomics analysis of proteome turnover in human cells. Molecular & cellular proteomics : MCP. 2012;11:M111 011429. doi: 10.1074/mcp.M111.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkowska K, Santoni de Sio F, Barde I, Offner S, Verp S, Heinis C, Johnsson K, Trono D. Measuring in vivo protein half-life. Chemistry & biology. 2011;18:805–815. doi: 10.1016/j.chembiol.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Cambridge SB, Gnad F, Nguyen C, Bermejo JL, Kruger M, Mann M. Systems-wide proteomic analysis in mammalian cells reveals conserved, functional protein turnover. Journal of proteome research. 2011;10:5275–5284. doi: 10.1021/pr101183k. [DOI] [PubMed] [Google Scholar]
- Doherty MK, Hammond DE, Clague MJ, Gaskell SJ, Beynon RJ. Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. Journal of proteome research. 2009;8:104–112. doi: 10.1021/pr800641v. [DOI] [PubMed] [Google Scholar]
- Dvir S, Velten L, Sharon E, Zeevi D, Carey LB, Weinberger A, Segal E. Deciphering the rules by which 5′-UTR sequences affect protein expression in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2792–2801. doi: 10.1073/pnas.1222534110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Geva-Zatorsky N, Issaeva I, Cohen A, Dekel E, Danon T, Cohen L, Mayo A, Alon U. Proteome half-life dynamics in living human cells. Science. 2011;331:764–768. doi: 10.1126/science.1199784. [DOI] [PubMed] [Google Scholar]
- Foresti O, Ruggiano A, Hannibal-Bach HK, Ejsing CS, Carvalho P. Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. eLife. 2013;2:e00953. doi: 10.7554/eLife.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, Christiano R, Walther TC. Native SILAC: metabolic labeling of proteins in prototroph microorganisms based on lysine synthesis regulation. Molecular & cellular proteomics : MCP. 2013;12:1995–2005. doi: 10.1074/mcp.M112.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo JM, Lopez S, Ballesteros F. Calculation of half-lives of proteins in vivo. Heterogeneity in the rate of degradation of yeast proteins. Molecular and cellular biochemistry. 1982;43:89–95. doi: 10.1007/BF00423096. [DOI] [PubMed] [Google Scholar]
- Helbig AO, Daran-Lapujade P, van Maris AJ, de Hulster EA, de Ridder D, Pronk JT, Heck AJ, Slijper M. The diversity of protein turnover and abundance under nitrogen-limited steady-state conditions in Saccharomyces cerevisiae. Molecular bioSystems. 2011;7:3316–3326. doi: 10.1039/c1mb05250k. [DOI] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGurn JA, Hsu PC, Smolka MB, Emr SD. TORC1 regulates endocytosis via Npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell. 2011;147:1104–1117. doi: 10.1016/j.cell.2011.09.054. [DOI] [PubMed] [Google Scholar]
- Mann M, Kulak NA, Nagaraj N, Cox J. The coming age of complete, accurate, and ubiquitous proteomes. Molecular cell. 2013;49:583–590. doi: 10.1016/j.molcel.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Michalski A, Damoc E, Hauschild JP, Lange O, Wieghaus A, Makarov A, Nagaraj N, Cox J, Mann M, Horning S. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Molecular & cellular proteomics : MCP. 2011;10:M111 011015. doi: 10.1074/mcp.M111.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JM, Petty J, Riba-Garcia I, Robertson DH, Gaskell SJ, Oliver SG, Beynon RJ. Dynamics of protein turnover, a missing dimension in proteomics. Molecular & cellular proteomics : MCP. 2002;1:579–591. doi: 10.1074/mcp.m200046-mcp200. [DOI] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Thibault G, Ng DT. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Roy S, Chan M, Styczynsky MP, Pfiffner J, French C, Socha A, Thielke A, Napolitano S, Muller P, et al. Evolutionary principles of modular gene regulation in yeasts. Elife (Cambridge) 2013;2:e00603. doi: 10.7554/eLife.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd BL, Stewart EV, Burg JS, Hughes AL, Espenshade PJ. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Molecular and cellular biology. 2006;26:2817–2831. doi: 10.1128/MCB.26.7.2817-2831.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR., 3rd Protein analysis by shotgun/bottom-up proteomics. Chemical reviews. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.