Abstract
We investigated the autonomic innervation of the penis by using evoked cavernous activity (ECA). We recruited 7 males with thoracic spinal cord injury (SCI) and sexual dysfunction and 6 males who were scheduled to have pelvic surgery (PS), specifically non-nerve-sparing radical cystoprostatectomy. In the PS subjects, ECA was performed both pre- and postoperatively. The left median nerve was electrically stimulated and ECA was recorded with two concentric electromyography needles placed into the right and left cavernous bodies. We simultaneously recorded hand and foot sympathetic skin responses (SSRs) as controls. In the SCI group, all but one subject had reproducible hand SSRs. None of these subjects had ECA or foot SSRs. All the PS subjects had reproducible ECA and SSRs, both preoperatively and postoperatively. There was no difference in the latency and amplitude measurements of ECA and SSRs in the postoperative compared to the preoperative period (p>0.05). In conclusion, ECA is absent in men with SCI above the sympathetic outflow to the genitalia. In men following radical pelvic surgery, ECA is preserved, indicating the preservation of sympathetic fibers.
Keywords: Evoked cavernous activity, autonomic nerve, electrodiagnostic test, erectile dysfunction, penis
INTRODUCTION
Intact autonomic neural pathways are prerequisites for the vascular changes during erection and detumescence, with signals traveling through the spinal cord tracts, the pelvic and hypogastric plexuses, and the cavernous nerves. Only a few tests exist to assess the autonomic components of genital innervation. One autonomic testing technique called evoked cavernous activity (ECA) measures intrapenile electrical activity following a brief noxious stimulus. An applied noxious stimulus results in a generalized, sympathetic nervous system discharge that manifests throughout the body, including the corpus cavernosum.1,2 In healthy, potent men of varying ages, ECA is seen with consistent latency measurements.3 Although the sympathetic discharge mediates corporal vasoconstriction, and thus is anti-erectile, it is still a reflection of overall autonomic innervation. Furthermore, there are no direct measures of parasympathetic function for the penis.
In earlier studies we showed the absence of ECA in men with dysautonomias2 and in most men following non-nerve-sparing radical pelvic surgery4. The absence of ECA was interpreted as the disruption of sympathetic innervation to the corpora, which was presumed to impact on erectile function. We conducted this study to further investigate the autonomic innervation of the penis and the association to erectile function by using ECA to evaluate two groups of men with erectile dysfunction (ED): one cohort with ED from central nervous system disruption due to spinal cord injury (SCI), and one group with peripheral (cavernous) nerve disruption due to pelvic surgery (PS). Our hypothesis was that either peripheral or central injury to the autonomic innervation to the penis would result in loss of ECA. This result would be the basis from which ECA could be used as a diagnostic test for neurogenic erectile dysfunction.
METHODS
After study protocol approval by the Human Subjects Review Committee of our hospital we recruited 7 males with complete thoracic spinal cord injury (SCI) and sexual dysfunction and 6 males who were scheduled for pelvic surgery (PS), specifically non-nerve-sparing radical cystoprostatectomy. All men in the PS group were sexually active preoperatively. Exclusion criteria for all groups included moderate to severe cardiovascular disease, diabetes mellitus, untreated hypertension, current drug or alcohol abuse, major psychiatric disorder; and unwillingness or inability to complete the questionnaire. Except for spinal cord injury, there was no evidence of other neurologic disease.
Each subject underwent a history and physical examination, with a detailed urological and neurological examination of the lower extremities and genitalia.
All subjects completed the International Index of Erectile Function (IIEF) questionnaire5 prior to ECA testing. In the PS group, ECA was performed preoperatively and at the third postoperative month visit.
ECA testing
The electrodiagnostic technique for ECA was previously described.2-4 Briefly, a noxious stimulus was delivered to the median nerve on the left wrist from a Viking IV electrophysiology machine (Viasys Healthcare, Madison, WI) with the subject in the supine position. The stimulus amplitude was 5-7 milliamperes (mA), duration 0.5 msec, and delivered at irregular intervals of at least 60 seconds in order to avoid habituation. ECA was recorded from both corpora cavernosa through concentric 28 gauge needle electrodes (20mm long, 0.4 mm diameter, gold plated needle, Nicolet Biomedical Inc., WI) placed into the corpora at the lateral aspects at the base of the penis, with the tip of the electrodes positioned in the center of each corporal body. The band-pass filters were set to 0.5-100 Hz. A ground plate was affixed to the skin overlying the right anterior/superior iliac crest.
If ECA was present following the first 6 stimuli, then no further stimuli were given. If ECA was not readily identifiable following all of the first 6 stimuli, then more stimuli were delivered, up to a total of 12. The latency was measured at the first deflection from baseline following the stimulus delivery, and amplitude measurements were made peak to peak. Three responses were averaged for final latency and amplitude values. Sympathetic skin responses (SSR) were measured simultaneously from the hand and foot contralateral to the median nerve stimulus. SSRs are slow wave recordings that are temporally related to ECA and share similar waveforms (Figure 1). These responses were recorded as a control for the presence of a generalized sympathetic discharge. Latency and amplitude measurements of the SSRs from the hand and foot were measured in standard fashion.6
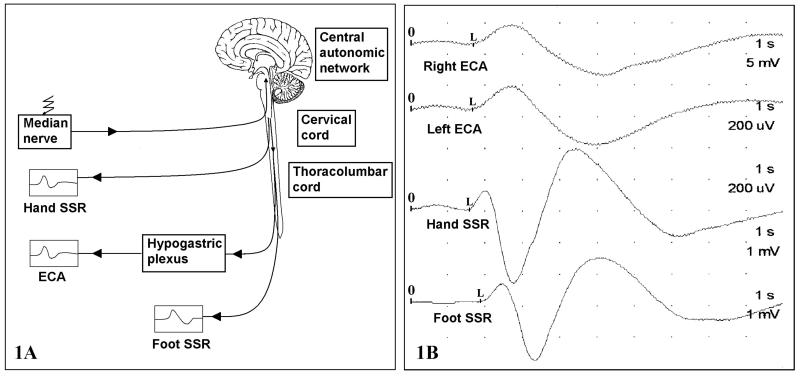
Figure 1.
(1A) Diagram of ECA reflex. Afferent signals travel through the median nerve to the CNS. The resultant sympathetic discharge is manifested throughout the body, including the erectile tissue. (1B) waveforms of ECA and SSRs of a PS subject in the preoperative period. The stimulus is delivered at time 0 and the latency is measured between 0 and L while the amplitudes are measured from the peak of the first deflection to the peak of the following (opposite) deflection. The time-base is 1 second per division, and the entire trace measures 10 seconds.
Statistical analyses
The groups were compared using the Kruskal-Wallis and Mann-Whitney U tests. In the PS group, the parameters were also compared between the preoperative and postoperative periods using the Wilcoxon test.
RESULTS
All subjects tolerated the procedure well. Average age of the SCI group was younger than the PS group (34.7±7.6 years vs 59.1±5.6 years, p<0.001).
IIEF
The results of the Erectile Domain of the IIEF were tabulated (Table 1). Postoperative PS IIEF and SCI IIEF scores were not statistically different from each other (p=0.268).
Table 1.
Mean ECA amplitude and latency measurements (SCI: Spinal cord injury, PS: pelvic surgery group)
| PS group (n=6) | SCI group (n=7) | ||
|---|---|---|---|
| Preoperative | Postoperative | ||
| Latency (msec) | Mean (SD) | Mean (SD) | Mean (SD) |
| Right ECA | 1 841 (405) | 2 212.2 (1 100) | No response |
| Left ECA | 1 842 (437) | 1 725 (185) | No response |
| Hand SSR | 1 692 (184) | 1 675 (360) | 1 716 (170) |
| Foot SSR | 2 071 (98) | 2 577 (1 383) | No response |
| Amplitude (μV) | |||
| Right ECA | 295 (140) | 493 (592) | No response |
| Left ECA | 288 (238) | 451 (577) | No response |
| Hand SSR | 2 670 (2 366) | 1 763 (854) | 2 793 (2 187) |
| Foot SSR | 1 658 (1 136) | 1 870 (1 298) | No response |
|
IIEF, erectile domain (total possible score = 30) |
16.3 (5.6) | 5.6 (6.5) | 10.7 (10.3) |
ECA
All the PS subjects had reproducible ECA and SSRs, both preoperatively and postoperatively (Figure 1). There was no difference in the latency and amplitude measurements of ECA and SSRs in the postoperative compared to the preoperative period (Table 1, p>0.05). Both preoperative and postoperative values were within the normal limits.3 Despite the reported loss of erectile function in the post surgery group, there was not a corresponding change in ECA parameters, indicating no consistent association between ECA and erectile function.
In the SCI group, all but one subject had reproducible hand SSRs. This one subject had very dry skin, and thus ECA was not recordable. None of the SCI subjects had foot SSRs or ECA (Figure 2).
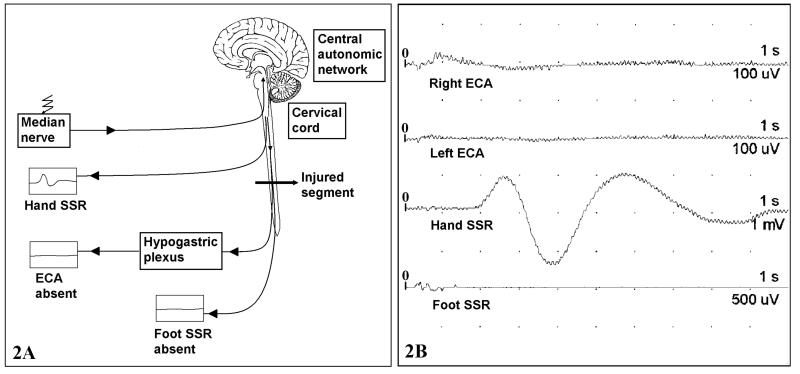
Figure 2.
ECA in SCI patient. (2A) Diagram of disrupted ECA reflex and (2B) ECA waveforms. Note the absence of activity in both corpora cavernosa as well as foot SSR. Deflections off the baseline prior to 1 second in the right ECA and foot SSR trace are artefact. Stimulus is delivered at time 0. The time-base is 1 second per division, and the entire trace measures 10 seconds. Amplitude sensitivities are set higher than those in Figure 1.
DISCUSSION
ECA is a measure of sympathetic (autonomic) discharge in the erectile tissue of the penis. In this study, we examined the measurements in men with ED due to central nervous system disruption from spinal cord injury, and men with ED due to peripheral (cavernous) nerve disruption following radical pelvic surgery. The rationale of selecting these groups was to explore the pathways involved in the transmission of autonomic signals to the corpora cavernosa, and the possible association with erectile function. In men with spinal injury above the level of the sympathetic outflow to the penis (T10-L2), there was no ECA response, and in men with peripheral nerve injury following surgery, there was a persistence of ECA. These findings can be used to further define autonomic penile innervation.
The absence of ECA and foot SSR in men with SCI between T1-T9 is what would be expected: the sympathetic outflow to the penis and lower extremities is derived from the T10-L2 spinal segments, and any injury to the spinal cord proximal to these levels precludes normal autonomic outflow to these structures. Although the noxious stimulus is able to enter the CNS through intact somatic pathways via the median nerve (C5-C7), the sympathetic outflow of the reflex is disrupted proximal to the nerve roots to the penis and lower extremities (Figure 2). Thus, central disruption of the autonomic pathways results in no recordable ECA or SSR.
Peripheral injury to the autonomic fibers presents a different scenario. In the present study, each subject in the surgical cohort served as his own control, and we found that ECA is reproducibly present with no changes in the event latency, following non-nerve-sparing pelvic surgery that should have ablated the path of the autonomic fibers to the penile erectile tissue. In our previous study, 8 of 11 subjects following non-nerve-sparing prostatectomy did not have measurable ECA,4 which we interpreted as denervation following surgery. However, the average age of these men was significantly older than the nerve-sparing cohort, and it may have been that senescent changes to the erectile tissue was the primary determinant for the loss of ECA, rather than the fact that the men had undergone non-nerve-sparing radical prostatectomy.
The persistence of ECA following the radical pelvic surgery can possibly be explained by the highly variable course of the parasympathetic and sympathetic fibers through the pelvic plexus to the erectile tissue. One study showed the distribution of autonomic fibers to be wider than expected, extending posterolaterally on the rectal wall, separate from the neurovascular bundle immediately adjacent to the prostate.7 It was also suggested that the cavernous nerve had interindividual variations in its course near the apex of the prostate and the surgically defined neurovascular bundle is often likely to differ from the actual axial course of the cavernous nerve fibers passing through the pararectal space and the rectourethral muscle. Therefore, an axial course of the cavernous nerve fibers lateral to the surgical field seemed to provide “unexpected nerve-sparing” after non-nerve-sparing radical prostatectomy, because the fibers are likely to be located outside the surgical margin.8 Terada et al reported that of 16 instances in which the neurovascular bundle was macroanatomically resected, a positive intracavernous pressure increase after intraoperative electrical stimulation was detected in 5 cases (31%).9 We think the persistence of ECA in the subjects in our study also suggests a lateral location of the sympathetic nerves to the penis, away from the main surgical site, thereby preserving sympathetically mediated neural activity (Figure 3). The autonomic fibers in the neurovascular bundle immediately adjacent to the prostate are likely primarily parasympathetic fibers. This finding of differentially located autonomic fibers may also explain erectile dysfunction with shortening of penile length following non-nerve-sparing radical prostatectomy procedures.10 Injury of parasympathetic fibers but preservation of more laterally located sympathetic fibers can cause an autonomic imbalance within the penis, resulting in the dominance of sympathetically-mediated smooth muscle contraction of the corpus cavernosum, and subsequent erectile dysfunction and penile shortening. All these findings further support the concept that the cavernous nerve is not a singular structure within a neurovascular bundle, but a “net” or “layer” of fibers extending from the anterior surface of the prostate, and extending posterolaterally to the lateral rectal wall.11 Another explanation for the presence of ECA can be based on an alternate route of penile sympathetic innervation, such as through the dorsal nerve of the penis.12
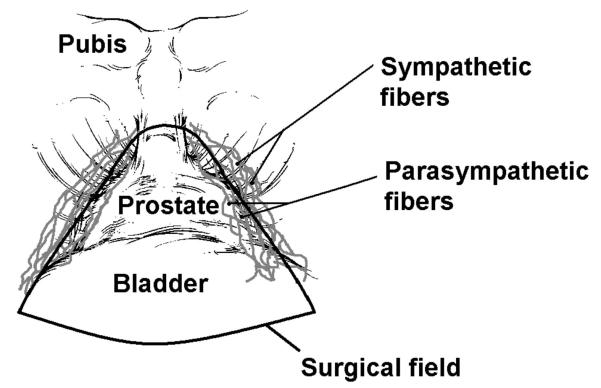
Figure 3.
The surgical field of a radical cystoprostatectomy is schematically presented. Autonomic fibers innervating the penis are known to travel on the postero-lateral aspects of the prostate gland. Based on the ECA responses, we posit that the sympathetic fibers lie lateral to the area of resection, and thus are spared, as compared to the more medial parasympathetic fibers, which are resected.
Because of the persistence of ECA in this cohort of men following radical pelvic surgery, it is not likely that ECA is going to be able to diagnose men with ED due to iatrogenic peripheral nerve injury, and cannot be used to prognosticate on the return of erectile function, as we previously believed. Our original assumption was incorrect that ECA, which is a measure of sympathetic activity, was a surrogate measure for the parasympathetic function of the autonomic fibers, because we believed that the parasympathetic and sympathetic fibers ran together along the cavernous nerve neurovascular bundle alongside the prostate gland. However, ECA still may be useful in diagnosing denervation due to neurologic disease.2
CONCLUSION
ECA is not present in men with SCI above the sympathetic outflow to the genitalia, indicating that it is a centrally generated signal. In men following radical pelvic surgery, ECA is preserved, indicating that at least sympathetic fibers are preserved, which warrants further studies to delineate periprostatic autonomic fibers relative to the sympathetic/parasympathetic components. ECA does not appear to be clinically useful for diagnosing peripheral penile autonomic disruption.
ACKNOWLEDGEMENTS
Supported by NIH NIDDK 1 R21 DK069315
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Yarnitsky D, Sprecher E, Barilan Y, Vardi Y. Corpus cavernosum electromyogram: spontaneous and evoked electrical activities. J Urol. 1995;153(3 Pt 1):653–654. [PubMed] [Google Scholar]
- 2.Yilmaz U, Soylu A, Ozcan C, Kutlu R, Gunes A. Evoked cavernous activity. J Urol. 2002;167(1):188–191. doi: 10.1016/s0022-5347(05)65409-2. [DOI] [PubMed] [Google Scholar]
- 3.Yang CC, Yilmaz U, Vicars BG. Evoked cavernous activity: normal values. J Urol. 2008;179(6):2312–2316. doi: 10.1016/j.juro.2008.01.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmaz U, Ellis W, Lange P, Yang C. Evoked cavernous activity: measuring penile autonomic innervation following pelvic surgery. Int J Impot Res. 2006;18(3):296–301. doi: 10.1038/sj.ijir.3901407. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 6.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13(4):256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 7.Takenaka A, Tewari A, Hara R, Leung RA, Kurokawa K, Murakami G, et al. Pelvic autonomic nerve mapping around the prostate by intraoperative electrical stimulation with simultaneous measurement of intracavernous and intraurethral pressure. J Urol. 2007;177(1):225–229. doi: 10.1016/j.juro.2006.08.104. [DOI] [PubMed] [Google Scholar]
- 8.Takenaka A, Murakami G, Matsubara A, Han SH, Fujisawa M. Variation in course of cavernous nerve with special reference to details of topographic relationships near prostatic apex: histologic study using male cadavers. Urology. 2005;65(1):136–142. doi: 10.1016/j.urology.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Terada N, Arai Y, Kurokawa K, Ohara H, Ichioka K, Matui Y, et al. Intraoperative electrical stimulation of cavernous nerves with monitoring of intracorporeal pressure to confirm nerve-sparing during radical prostatectomy: Early clinical results. Int J Urol. 2003;10(5):251–256. doi: 10.1046/j.1442-2042.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- 10.Savoie M, Kim SS, Soloway MS. A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J Urol. 2003;169(4):1462–1464. doi: 10.1097/01.ju.0000053720.93303.33. [DOI] [PubMed] [Google Scholar]
- 11.Sievert KD, Hennenlotter J, Laible I, Amend B, Schilling D, Anastasiadis A, et al. The periprostatic autonomic nerves--bundle or layer? Eur Urol. 2008;54(5):1109–1116. doi: 10.1016/j.eururo.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano F, Rampin O, Jardin A, Rousseau JP. Electrophysiological study of relations between the dorsal nerve of the penis and the lumbar sympathetic chain in the rat. J Urol. 1993;150(6):1960–1964. doi: 10.1016/s0022-5347(17)35946-3. [DOI] [PubMed] [Google Scholar]