Abstract
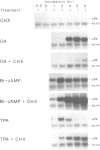
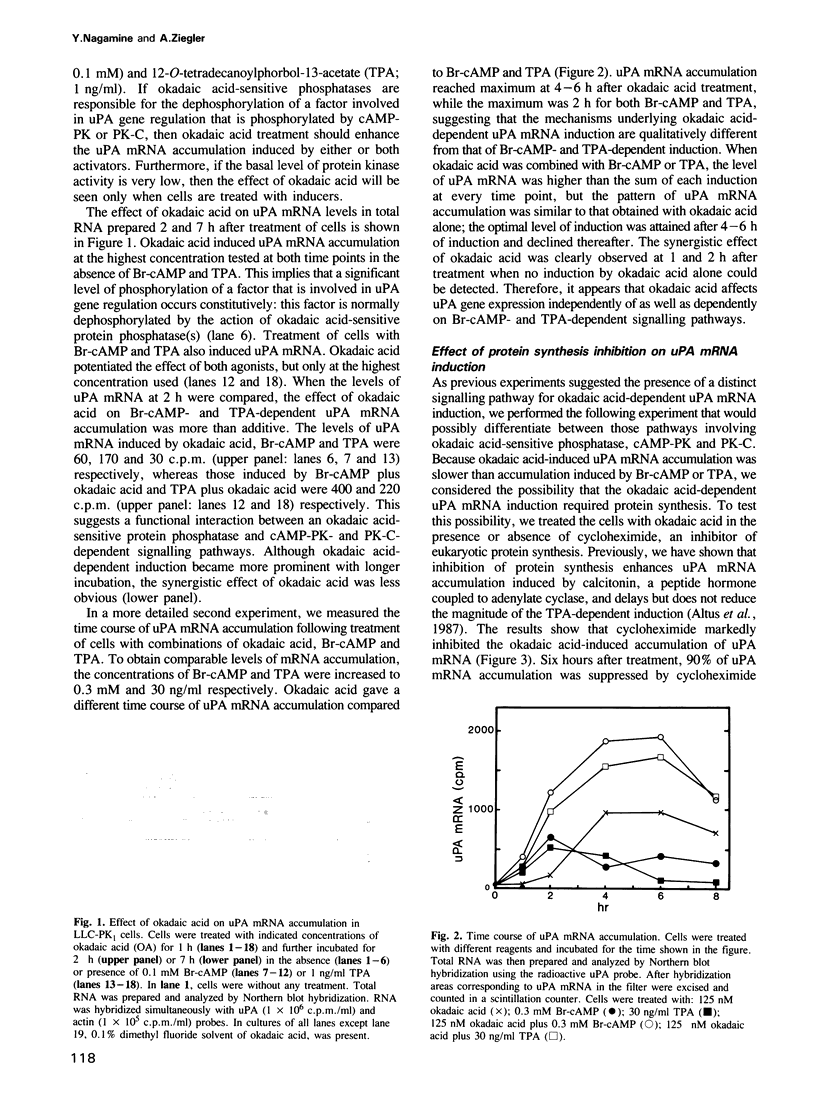
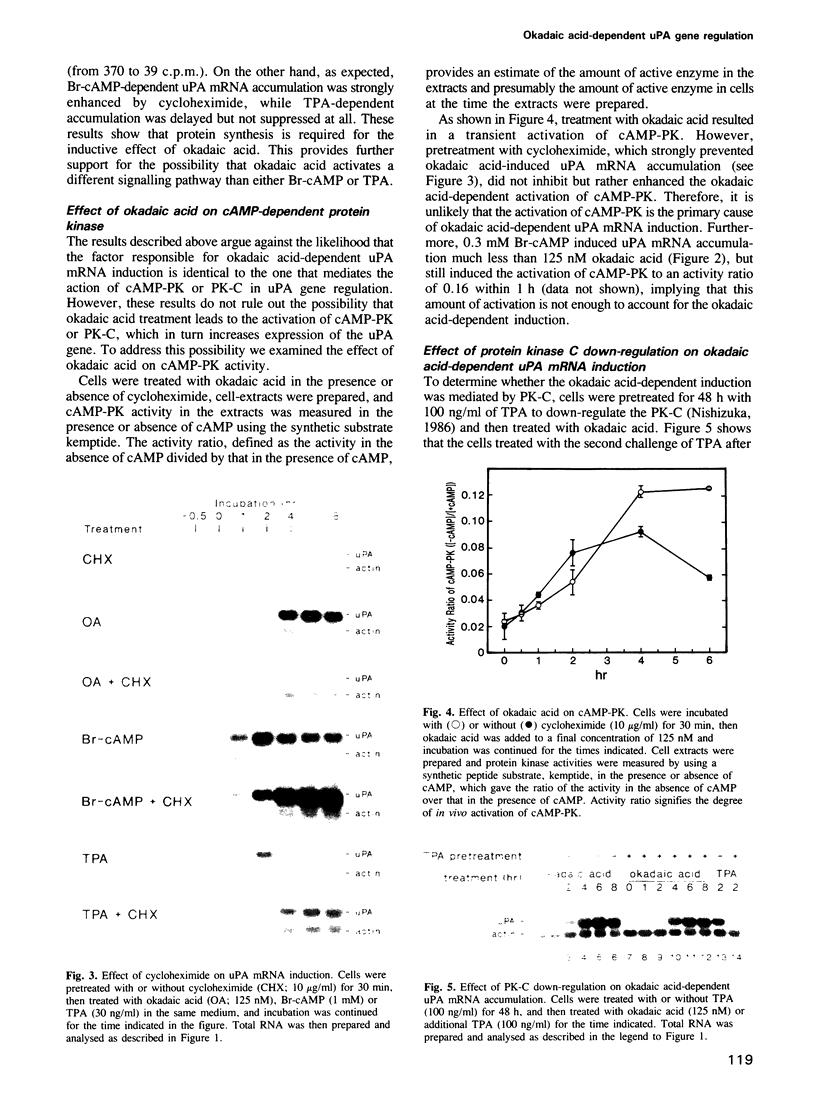
Urokinase-type plasminogen activator (uPA) gene expression in LLC-PK1 cells is induced by activation of cAMP-dependent protein kinase (cAMP-PK) or protein kinase C (PK-C). To determine whether protein phosphatases can also modulate uPA gene expression, we tested okadaic acid, a potent specific inhibitor of protein phosphatases 1 and 2A, in the presence and absence of cAMP-PK and PK-C activators. Okadaic acid by itself induced uPA mRNA accumulation. This induction was strongly attenuated by the inhibition of protein synthesis. In contrast, the inhibition of protein synthesis enhanced induction by 8-bromo-cAMP and only delayed induction by 12-O-tetradecanoylphorbol-13-acetate (TPA). In addition, down-regulation of PK-C by chronic treatment with TPA did not abrogate the okadaic acid-dependent induction. These results provide evidence for a novel signal transduction pathway leading to gene regulation that involves protein phosphorylation but is independent of both cAMP-PK and PK-C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altus M. S., Pearson D., Horiuchi A., Nagamine Y. Inhibition of protein synthesis in LLC-PK1 cells increases calcitonin-induced plasminogen-activator gene transcription and mRNA stability. Biochem J. 1987 Mar 1;242(2):387–392. doi: 10.1042/bj2420387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus L., Altus M. S., Pearson D., Grattan M., Nagamine Y. hsp70 mRNA accumulates in LLC-PK1 pig kidney cells treated with calcitonin but not with 8-bromo-cyclic AMP. J Biol Chem. 1988 May 5;263(13):6183–6187. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney C., Fink D., Schlichter D., Carr K., Wicks W. D. Direct evidence that the protein kinase catalytic subunit mediates the effects of cAMP on tyrosine aminotransferase synthesis. J Biol Chem. 1983 Apr 25;258(8):4911–4918. [PubMed] [Google Scholar]
- Botteri F. M., Ballmer-Hofer K., Rajput B., Nagamine Y. Disruption of cytoskeletal structures results in the induction of the urokinase-type plasminogen activator gene expression. J Biol Chem. 1990 Aug 5;265(22):13327–13334. [PubMed] [Google Scholar]
- Carmichael D. F., Geahlen R. L., Allen S. M., Krebs E. G. Type II regulatory subunit of cAMP-dependent protein kinase. Phosphorylation by casein kinase II at a site that is also phosphorylated in vivo. J Biol Chem. 1982 Sep 10;257(17):10440–10445. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. Protein phosphorylation and the control of glycogen metabolism in skeletal muscle. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):13–25. doi: 10.1098/rstb.1983.0034. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Estensen R. D., Nagamine Y., Reich E. Induction and desensitization of plasminogen activator gene expression by tumor promoters. J Biol Chem. 1985 Oct 15;260(23):12426–12433. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Price D. J., Goodman H. M., Avruch J. Recombinant fragment of protein kinase inhibitor blocks cyclic AMP-dependent gene transcription. Science. 1987 Oct 23;238(4826):530–533. doi: 10.1126/science.2821622. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A. cAMP mediated proteolysis of the catalytic subunit of cAMP-dependent protein kinase. FEBS Lett. 1986 Feb 3;196(1):126–130. doi: 10.1016/0014-5793(86)80226-5. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989 Apr;1(2):268–274. doi: 10.1016/0955-0674(89)90099-9. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Lüscher B., Kuenzel E. A., Krebs E. G., Eisenman R. N. Myc oncoproteins are phosphorylated by casein kinase II. EMBO J. 1989 Apr;8(4):1111–1119. doi: 10.1002/j.1460-2075.1989.tb03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Cohen P. Identification of high levels of type 1 and type 2A protein phosphatases in higher plants. Biochem J. 1989 Aug 15;262(1):335–339. doi: 10.1042/bj2620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine Y., Pearson D., Altus M. S., Reich E. cDNA and gene nucleotide sequence of porcine plasminogen activator. Nucleic Acids Res. 1984 Dec 21;12(24):9525–9541. doi: 10.1093/nar/12.24.9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., von der Ahe D., Pearson D., Hemmings B. A., Shibahara S., Nagamine Y. Transcriptional regulation of a plasminogen activator gene by cyclic AMP in a homologous cell-free system. Involvement of cyclic AMP-dependent protein kinase in transcriptional control. J Biol Chem. 1988 Feb 15;263(5):2460–2468. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Prywes R., Dutta A., Cromlish J. A., Roeder R. G. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-FOS enhancer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7206–7210. doi: 10.1073/pnas.85.19.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K. T., Fink J. S., Gilman M. Z., Walsh D. A., Goodman R. H., Feramisco J. R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988 Nov 3;336(6194):83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Titani K., Demaille J. G., Fischer E. H. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1979 Jul 25;254(14):6211–6214. [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W. Studies on the mechanism of hormone action. Science. 1972 Aug 4;177(4047):401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- Witters L. A. Protein phosphorylation and dephosphorylation. Curr Opin Cell Biol. 1990 Apr;2(2):212–220. doi: 10.1016/0955-0674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Pearson D., Nagamine Y. Macromolecular interaction on a cAMP responsive region in the urokinase-type plasminogen activator gene: a role of protein phosphorylation. Nucleic Acids Res. 1990 Apr 25;18(8):1991–1999. doi: 10.1093/nar/18.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]