Abstract
In Niemann-Pick type C (NPC) disease, loss of function mutations in either NPC1 or NPC2 result in progressive accumulation of unesterified cholesterol (UC) and glycosphingolipids in all organs leading to neurodegeneration, pulmonary dysfunction and sometimes liver failure. There is no cure for this disorder. Studies using primarily NPC mouse models have shown that systemic administration of 2-hydroxypropyl-β-cyclodextrin (2HPβCD), starting in early neonatal life, diminishes UC accumulation in most organs, slows disease progression, and extends lifespan. The key question now is whether delaying the start of 2HPβCD treatment until early adulthood when the amount of entrapped UC throughout the body is markedly elevated has any of the benefits found when treatment begins at 7 days of age. Npc1−/− and Npc1+/+ mice were given saline or 2HPβCD subcutaneously at 49, 56, 63, and 70 days of age, and used for tissue cholesterol and other measurements at 77 days. In the Npc1−/− mice, treatment with 2HPβCD from 49 days reduced whole-liver cholesterol content at 77 days from 33.0±1.0 to 9.1±0.05 mg/organ. Comparable improvements were seen in other organs, such as spleen, and in the animal as a whole. There was a transient increase in biliary cholesterol concentration in the Npc1−/− mice after 2HPβCD. Plasma ALT and AST activities in the 77-day-old 2HPβCD-treated Npc1−/− mice were reduced. The lifespan of Npc1−/− mice given 2HPβCD marginally exceeded that of their saline-only controls (99±1.1 vs 94±1.4 days, P<0.05). Thus 2HPβCD is effective in mobilizing entrapped cholesterol in late-stage NPC disease leading to improved liver function.
Keywords: biliary cholesterol, brain, hepatosplenomegaly, lifespan, lung, lysosomal storage disease, relative organ weight
INTRODUCTION
Niemann-Pick type C disease (NPC) is a multisystemic disorder affecting the central nervous system as well as the peripheral organs. It is a result of mutations in either the NPC1 or NPC2 genes.1 These mutations cause the accumulation of unesterified cholesterol (UC), along with glycosphingolipids, within the late endosomal/lysosomal compartment of every cell. Although different classes of lipids accumulate, it is the sequestration of UC that is the fundamental cause of NPC disease.2, 3 Organ damage from cell dysfunction and death result in significant disease in multiple peripheral organs including the liver, spleen and lungs, as well as the central nervous system leading to progressive neurodegeneration and early death.
Several classes of agents have been, or are being evaluated as potential disease-specific therapy for NPC deficiency, including 2-hydroxypropyl-β-cyclodextrin (2HPβCD), which is a cyclic oligosaccharide that is used to increase the solubility of lipophilic drugs in aqueous solutions.4–7 Early studies in a mouse model for NPC1 disease showed that a single subcutaneous (sc) injection into 7-day-old Npc1−/− pups resulted in a decisive reduction in tissue cholesterol content and a significant prolongation of life.8, 9 Subsequent investigations demonstrated that chronic administration of 2HPβCD to Npc1−/− mice starting at 7 days of age and then continuing on a weekly basis thereafter, significantly slowed neurodegeneration, improved peripheral disease (especially liver disease), and prolonged life while dramatically reducing cholesterol accumulation in the entire animal.10 Lifespan was nearly doubled with chronic 2HPβCD therapy in Npc1−/− mice but progressive neurological and pulmonary disease continued. While direct delivery of 2HPβCD into the intracerebroventricular space in Npc1−/− mice showed promising results with a single injection11, continuous direct intracerebroventricular delivery via implantable osmotic minipumps posed surgical complications that made these studies difficult to complete.
Despite these technical advances, one key unanswered question about the impact of 2HPβCD treatment on peripheral organ disease in NPC deficiency is whether delaying the start of treatment until such time that symptoms of disease are clearly evident would result in a marked or even complete loss of efficacy. This question is important because typically in children with NPC1 or NPC2 deficiency a firm diagnosis is not made until after they have become symptomatic. Extensive published data show that 49-day old Npc1−/− mice are not only visually symptomatic, but also manifest a 2.4-fold expansion of their whole-body cholesterol content.8, 9 This reflects an increase in UC content of nearly every organ, in particular the liver.10, 12 Before the potency of 2HPβCD in reducing cholesterol accumulation in the Npc1 mouse model was firmly established, we had tested the impact of feeding Npc1−/− mice the cholesterol absorption inhibitor, ezetimibe, starting at different stages of disease progression.13 We found that if start of treatment was delayed until 35 days of age when liver cholesterol content was already elevated about 10-fold, the ezetimibe prevented further expansion of hepatic cholesterol levels but overall there was limited impact on disease progression in the animal as a whole. While 2HPβCD is a very different agent than ezetimibe, and is given sc, and at far higher doses, it was not certain how delaying the start of 2HPβCD treatment in Npc1−/− mice until they were approaching early adulthood might alter their level of tissue cholesterol entrapment. Thus, the primary objectives of the present studies were to determine tissue cholesterol accretion rates, liver histology, and lifespan in NPC1-deficient mice given 2HPβCD weekly starting at 49 days of age.
RESULTS
Systemic administration of 2HPβCD to 49 day-old, symptomatic Npc1−/− mice reversed hepatosplenomegaly and the accumulation of cholesterol in the spleen and kidneys, and markedly reduced hepatic cholesterol content
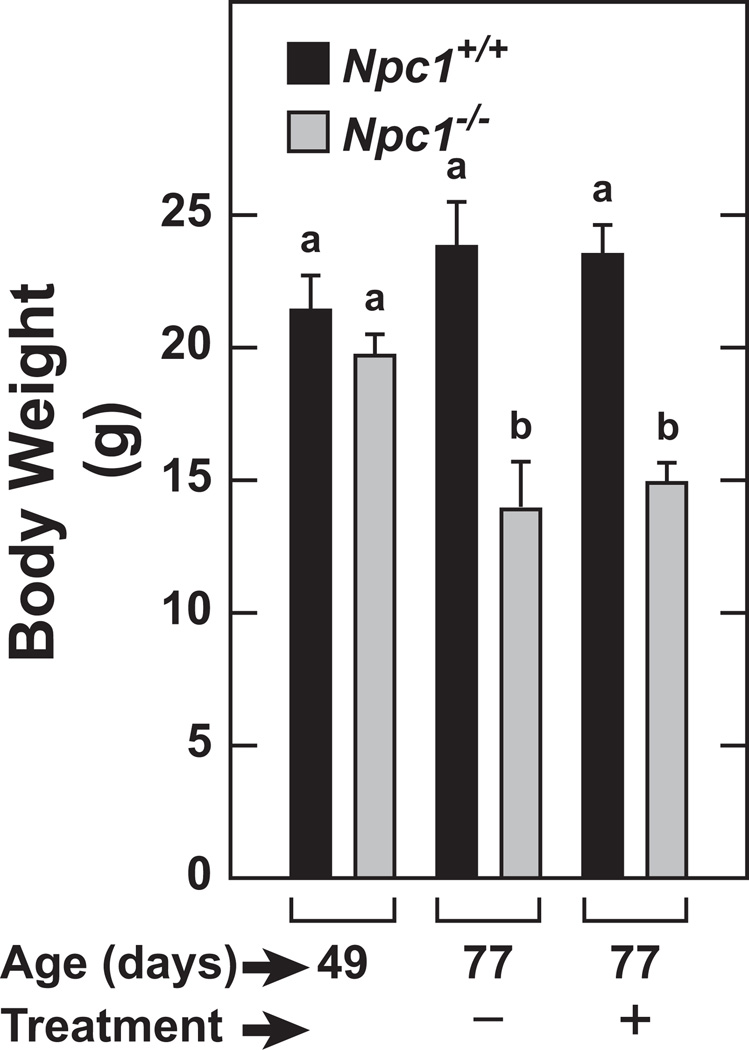
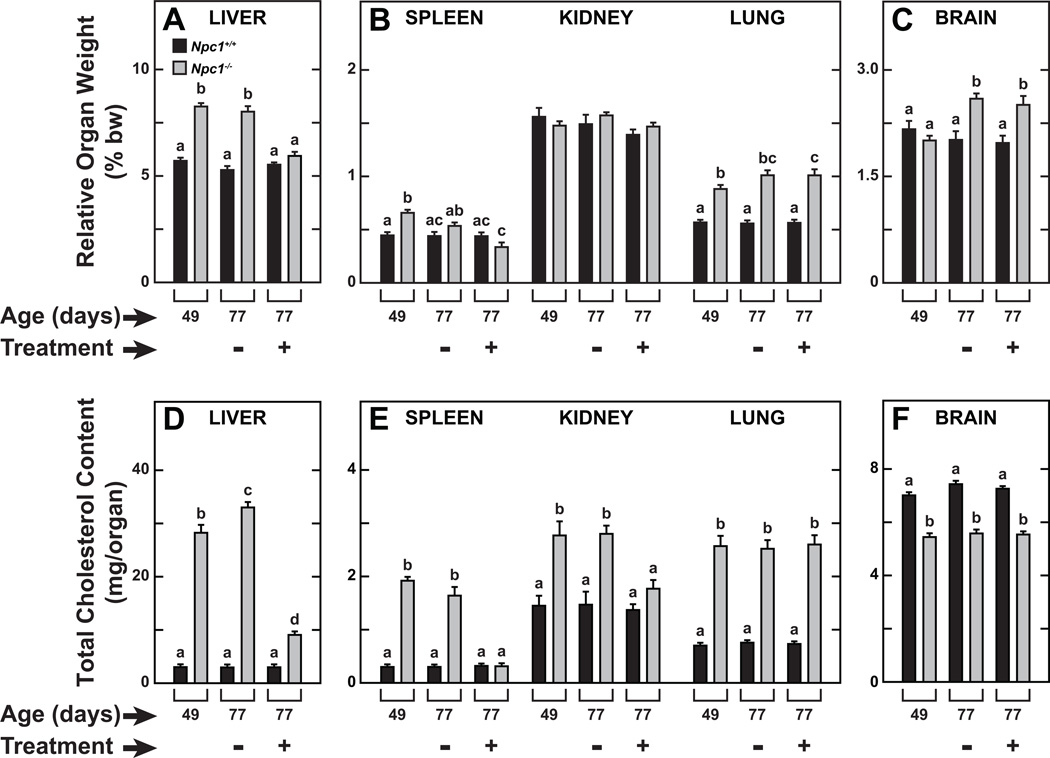
Npc1−/− mice and their Npc1+/+ controls at 49 days of age were given four weekly subcutaneous injections of either saline or 2HPβCD (4000mg/kg bw) and studied at 77 days. In addition, two other cohorts of untreated mice were studied at 49 days to obtain baseline values for all parameters. The average body weights of the baseline 49-day old Npc1+/+ and Npc1−/− mice were 21.4±1.3 and 19.7±0.8 g, respectively. The trend toward a lower body weight in the Npc1 mutants at 49 days of age became considerably more pronounced over the ensuing 28 days (Figure 1). When compared to their Npc1+/+ controls at 49 days of age, NPC1-deficient mice exhibited the prototypical organomegaly of the liver, spleen, and lung (Figures 2A and 2B, respectively). The enlargement of the liver seen in the 49 day-old Npc1−/− mice persisted in the 77 day-old mutants given saline but was substantially diminished in their counterparts receiving 2HPβCD. The changes in relative spleen weight paralleled those of the liver, whereas relative kidney weights did not change with 2HPβCD treatment (Figure 2B). In the case of the lung, relative weights were consistently greater in the Npc1−/− mice but otherwise did not change as a function of age or treatment. The relative brain weight data (Figure 2C) showed a different pattern of change compared to that for the other organs. Although the data are not presented, the baseline absolute brain weight in the Npc1−/− mice was 0.393±0.003 g vs 0.454±0.005 g (P<0.05) in their age-matched Npc1+/+ controls. In the 77 day-old Npc1−/− mice given saline only, the brain mass contracted further to 0.358±0.007 g whereas in their 77-day old wildtype controls brain weight increased marginally to 0.467±0.005 g. Thus, when these data are viewed together with the body weight data in these mice (Figure 1), it becomes clear why the relative brain weight in the 77 day-old mutants given saline was significantly increased compared to the baseline brain weight for mutants. This same pattern prevailed in the 77 day-old mutants that had been given 2HPβCD (Figure 2C). In 49 day-old Npc1−/− mice, the cholesterol content of the liver was 28.25±1.49 mg/organ. Over the ensuing 28 days this content (mg/organ) increased to 33.01±1.02 in the mutants given saline, but decreased by 68% to 9.06±0.52 in the matching group given 2HPβCD (Figure 2D). The cholesterol content of the spleen and kidney in the Npc1−/− mice given saline did not change between 49 and 77 days of age, but treatment with 2HPβCD completely normalized cholesterol content in both organs (Figure 2E). In contrast, 2HPβCD had no effect on cholesterol content of the lungs or brain (Figure 2E and 2F, respectively).
Fig. 1.
Final body weights of Npc1+/+ and Npc1−/− mice studied at baseline (49 days) or after 4 weeks of treatment with 2HPβCD. This experiment was carried out as described in “study design” in Methods. One set of mice was studied at 49 days without any earlier treatment whereas two other sets received subcutaneous injections of either normal saline only (−) or 2HPβCD in saline (+) starting at 49 days of age and then again at 56, 63, and 70 days before study at 77 days. All values are the mean±1 SEM of data from 7 mice in each group. Different letters (a or b) denote statistically significant differences between values (P<0.05) as determined by two-way ANOVA with genotype, treatment, and age as variables.
Fig. 2.
Relative weight (A–C) and total cholesterol content (D–F) of multiple organs in 77-day old Npc1+/+ and Npc1−/− mice treated weekly with either saline only (−) or 2HPβCD (+) from 49 days of age. Values are the mean±1 SEM of data from 7 animals in each group. Different letters (a–d) denote statistically significant differences between values (P<0.05) as determined by two-way ANOVA with genotype, treatment, and age as variables.
2HPβCD treatment resulted in a significant contraction of the whole-body cholesterol content in symptomatic NPC1-deficient mice
At baseline, the Npc1+/+ control mice had a whole-body cholesterol pool of 2240±51 mg/kg bw and this value did not change significantly with age or in response to the 2HPβCD treatment (Table 1). For the Npc1−/− mice at 49 days, the whole-animal cholesterol pool was 2.2-fold greater than their age-matched Npc1+/+ controls. Over the ensuing four weeks, the NPC1-deficient mice given saline only accumulated an additional ~1850 mg of cholesterol per kg bw, which represented a 3.1-fold greater content than was found in their Npc1+/+ littermates. In marked contrast, in the 2HPβCD-treated Npc1−/− mice, the whole-animal cholesterol pool was 3977±213 mg/kg bw. Thus, not only did the 2HPβCD prevent further expansion of the cholesterol pool that existed in the mutants at 49 days, but it actually reduced the size of this pool as the animal aged. The reduction was due mostly to a lowering of cholesterol levels in the liver and to a lesser degree the levels in the spleen and kidneys (Figures 2D and 2E). Together, these accounted for about two thirds of the total reduction seen, with the remainder presumably occurring in the gastrointestinal tract and the bulk tissues of the residual carcass such as skin and muscle.
Table 1.
Whole-animal cholesterol content in Npc1+/+ and Npc1−/− mice started on 2-hydroxypropyl-β-cyclodextrin treatment in early adulthood
| Genotype | Treatment starting at 49 days of age |
Age at time of study (days) |
Whole-animal cholesterol content (mg/kg bw) |
|---|---|---|---|
| Npc1+/+ | —— | 49 | 2240±51a |
| Npc1−/− | —— | 49 | 5016±137b |
| Npc1+/+ | Saline | 77 | 2212±45a |
| Npc1−/− | Saline | 77 | 6865±161c |
| Npc1+/+ | 2HPβCD | 77 | 2229±48a |
| Npc1−/− | 2HPβCD | 77 | 3977±213d |
Values are mean±1 SEM of data from 7 animals in each group (3 males and 4 females in every case). The 77-day old mice received four successive subcutaneous injections of either saline or 2HPβCD weekly, starting at 49 days of age. These data are from the same mice used for whole-organ cholesterol content measurements and the plasma ALT and AST activity (Figures 2 and 4, respectively). Different letters (a–d) denote statistically different values (P<0.05) as determined by 2-way ANOVA with genotype, treatment and age as variables.
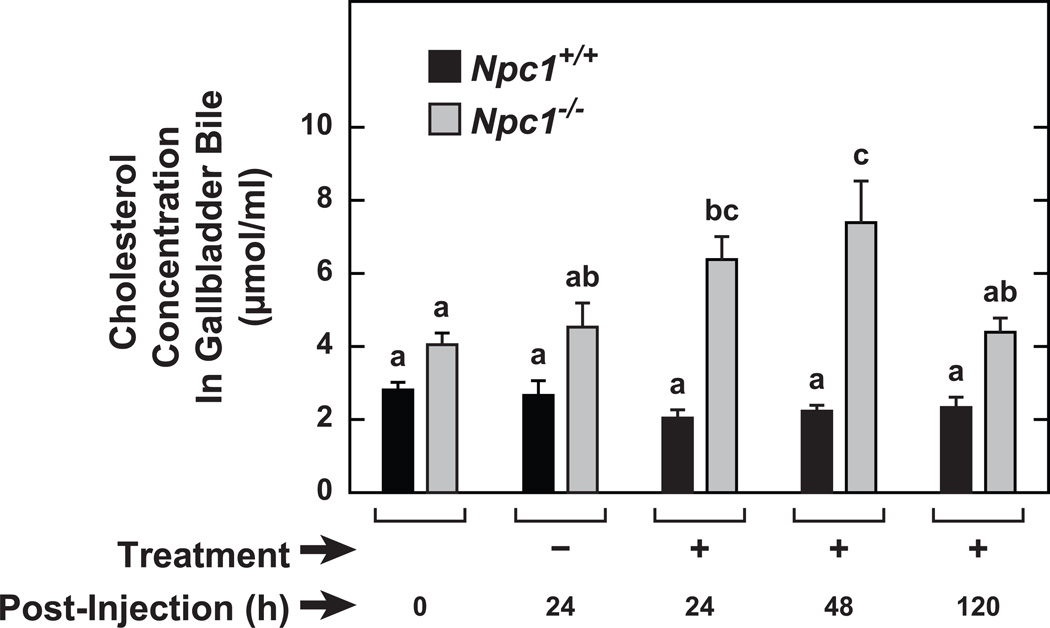
2HPβCD treatment caused a transient increase in biliary cholesterol content in the Npc1−/− mice but not in their Npc1+/+ controls
Although previous studies involving a single dose of 2HPβCD to 49-day old Npc1−/− mice had identified the major metabolic changes that ensued, including a marked suppression of cholesterol synthesis along with a decisive increase in the esterified cholesterol concentration in several organs, particularly in the liver, it remained unknown whether biliary cholesterol levels also changed acutely. The data in Figure 3 show that in the Npc1−/−, but not the Npc1+/+ mice, the cholesterol content of gallbladder bile was higher at 24 and 48 h following 2HPβCD treatment. However, after 120 h this increase was no longer evident.
Fig. 3.
Cholesterol concentration in the gallbladder bile of Npc1+/+ and Npc1−/− mice at varying times following a single treatment with either saline or 2HPBCD. Groups of Npc1+/+ and Npc1−/− mice, all in the age range of 49 to 53 days, either remained untreated, or received a single sc injection of either saline (−) or 2HPBCD (+). At 24, 48 and 120 h later, gallbladder bile was obtained from several mice in each group for the measurement of cholesterol concentration. Values represent the mean±1 SEM of measurements in 5 to 10 mice in each group. Different letters (a–c) denote statistically significant differences between values (P<0.05) as determined by two-way ANOVA, with genotype, treatment, and time after treatment as variables.
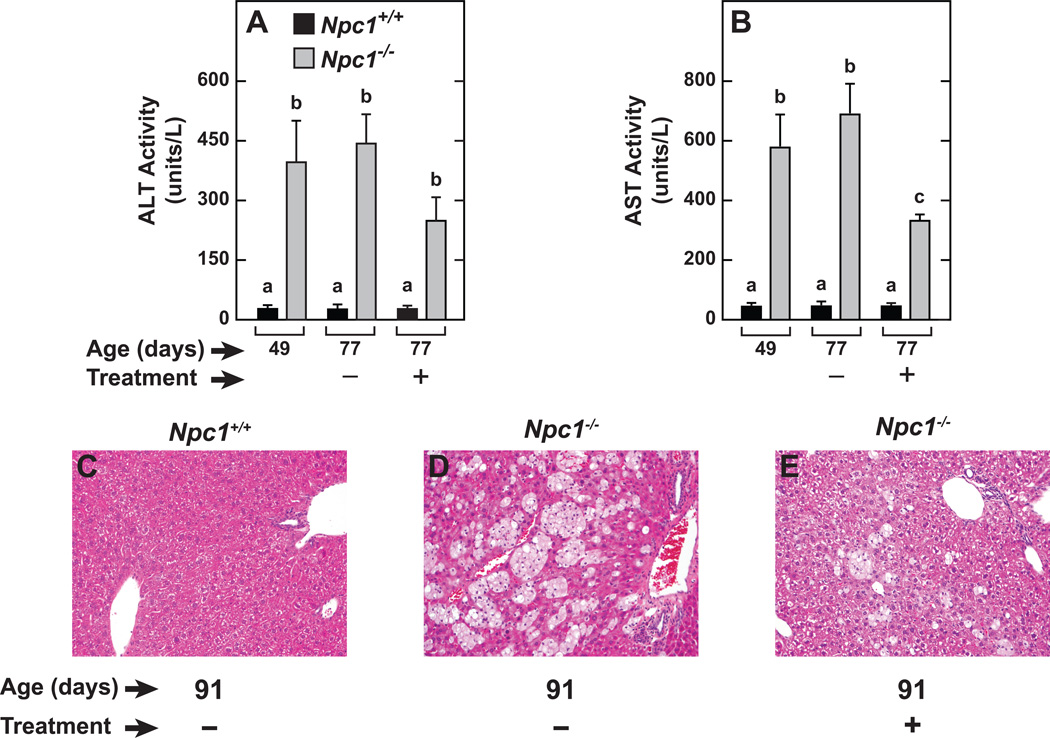
2HPβCD treatment reduced liver transaminase activity in the Npc1−/− mice
Both the ALT and AST activities in NPC1-deficient mice were significantly greater than in their age-matched Npc1+/+ littermates at 49 days, and this observation persisted at 77 days of age in Npc1−/− mice given saline (Figures 4A and 4B). The 2HPβCD-treated Npc1−/− mice exhibited lower ALT and AST activities compared to their counterparts receiving saline only. The liver histology for the 91-day old mice is fully consistent with the plasma transaminase activity data described in the 77-day old mice. Thus, in the livers of the Npc1−/− mice given saline compared to those from their Npc1+/+ littermates also given saline, large numbers of foamy lipid-laden macrophages are readily discernable (Figure 4D vs Figure 4C). In contrast, in the 91-day old mice that had been given 2HPβCD, a marked reduction in the presence of these macrophages is clearly evident (Figure 4C).
Fig. 4.
Plasma transaminase activities and representative liver histology in Npc1+/+ and Npc1−/− mice given either saline or 2HPBCD at weekly intervals starting at 49 days of age. In one experiment, plasma ALT (A) and AST (B) activities were determined in 77-day old Npc1+/+ and Npc1−/− mice that had received either saline (−) or 2HPBCD (+) injections at 49, 56, 63 and 70 days of age. Liver histology, by hematoxylin and eosin staining, (C, D and E) was carried out in a smaller number of 91-day old mice that had received treatment at 49, 56, 63 70, 77, and 84 days of age. In all panels photos are at the same magnification (×200). The values for plasma ALT and AST activity are the mean±1 SEM of data from 4 to 7 animals in each group. Different letters (a–c) denote statistically significant differences between values (P<0.05) as determined by two-way ANOVA, with genotype, age and treatment as variables.
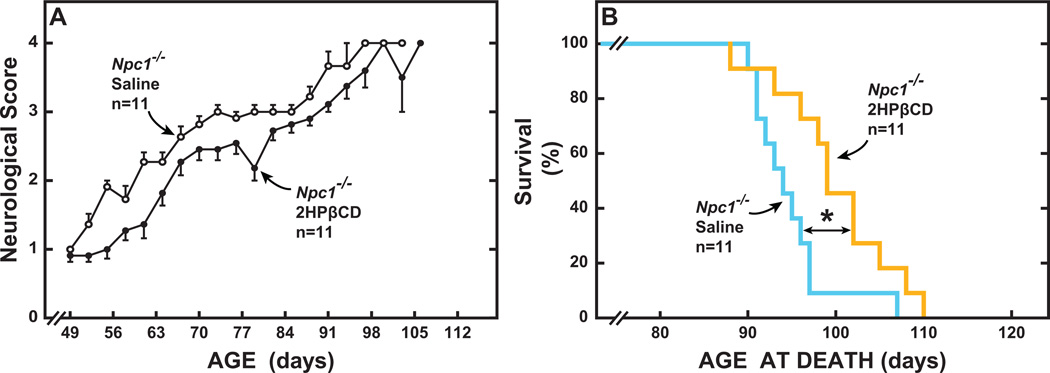
2HPβCD treatment resulted in a marginal increase in the lifespan of the Npc1−/− mice
The data for the neurological score in the males and females overlapped considerably and therefore were combined (Figure 5A). All mutants had a score of 1.0 at 49 days of age when treatment commenced. On that day, and throughout the course of the study, the score for all the Npc1+/+ mice was zero. Hence, the values for only the Npc1−/− mice appear in Figure 5A. Clearly, the score was almost always better in the 2HPβCD-treated mutants than in their counterparts given saline only. On average, the lifespan of the Npc1−/− mice given 2HPβCD exceeded that of their controls receiving saline by 5 days (Figure 5B). The longevity data for the Npc1+/+ mice are not shown because, at the time the last mutant mouse had died, all the Npc1+/+ mice were alive.
Fig. 5.
Lifespan of Npc1−/− mice treated systemically with either saline or 2HPβCD at weekly intervals starting at 49 days of age until death. This study utilized 22 Npc1−/− mice (10 males, 12 females) and 12 matching Npc1+/+ controls (6 males and 6 females). In each case, half of the males and females were given 2HPβCD and the remaining mice received only saline. Neurological scores were used to determine when euthanasia of mice was warranted. There were no gender related differences in the neurological scores so the values for the male and female mutants within each treatment group were combined (A). This score, which broadly measures neurological function, rises as the animal’s condition deteriorates. All of the Npc1−/− mice used for these measurements were symptomatic at 49 days and were assigned a score of 1.0 at that time. For the Npc1+/+ controls, this score remained at 0 throughout the study and so are not shown (A). Values in A are the mean±1 SEM of data from the specified number of animals, up until some of the Npc1−/− mice given saline or 2HPβCD reached the end of their lifespan. Nearly all of the Npc1−/− mice given saline only had died by 98 days whereas the majority of those receiving 2HPβCD died in the week following (B). The statistical analyses for the lifespan data were performed using the Gehan-Breslow-Wilcoxon test and log-rank analyses. The asterisk indicates a statistically significant difference (P<0.05) in age at death.
DISCUSSION
In contrast to the marked increase in tissue UC levels seen in NPC1 and NPC2 deficiency, loss of function mutations in the gene (LIPA) for lysosomal acid lipase (LAL), the enzyme responsible for cholesteryl ester and triacylglycerol hydrolysis, lead to dramatic increases in tissue esterified cholesterol (EC) and triacylglycerol content, especially in the liver.14, 15 Unlike NPC disease, LAL deficiency, while affecting multiple organ systems, does not result in neurodegeneration. An enzyme replacement therapy for treatment of LAL deficiency has been developed.16 In a landmark, newly published study, Sun et al. showed that this treatment, when given to Lal−/− mice with advanced disease, resulted in reversal of many of its features and an increase in lifespan.17 The present studies represent the first attempt to determine the efficacy of 2HPβCD treatment in countering the effects of NPC1 deficiency in mice with established disease. This was designed in line with earlier investigations in which 2HPβCD was given to Npc1−/− mice sc at a dose of 4000 mg/kg bw at weekly intervals starting at 7 days of age.10 For the current project, 49 days was chosen as the starting point for treatment largely because of the plethora of published data defining the Npc1 mouse model at this age.
In evaluating the present findings, a comment on the high dose of 2HPβCD that was employed is warranted. Although the dose of 2HPβCD tested in mouse models for NPC disease has varied over an enormous range depending partly on the route of delivery and the objectives of the studies, the most widely used protocol has involved sc administration at a dose of 4000 mg/kg bw. Published data pertaining to the tolerability of humans and animal models to treatment with high doses of 2HPβCD are limited and difficult to compare to what was observed in the present studies. Nevertheless, several reported findings are noteworthy. In both normal cats and those with NPC disease, the weekly sc administration of 4000 mg/kg bw resulted in an increase in hearing threshold.18 Similar effects in normal mice of the FVB strain have since been described.19 The intravenous administration of extremely high doses of 2HPβCD to an Npc1 patient20, and also to a pig model resulted in what were interpreted as toxic effects on the lungs.21 An earlier review article noted transient histopathological changes in the lungs, liver and kidneys in rats given 2HPβCD intravenously.22 Oral administration of 2HPβCD above certain doses could potentially have significant gastrointestinal side effects.23 For the question being addressed by the present studies, we concluded that sc administration, and a dose of 4000 mg/kg bw, together represented the most appropriate strategy despite the possibility of an adverse effect on hearing.
While there are substantial data attesting the efficacy of 2HPβCD in preventing expansion of tissue cholesterol levels in multiple peripheral organs when treatment starts in very young Npc1−/− mice, the present studies are the first to explore whether 2HPβCD has a discernible impact if treatment does not start until the animals’ whole-body cholesterol pool has expanded to the point where disease is clearly manifest. The data shown here demonstrate unequivocally that there is. The efficacy of 2HPβCD in reversing to a major degree the buildup of UC in the 49-day old mutants was remarkable, particularly considering that this was achieved with just four successive weekly injections of 2HPβCD. It was less surprising that this reversal of cholesterol sequestration was articulated mainly at the level of the liver, spleen and kidneys because these organs showed most response to early start cyclodextrin treatment.9, 10 The lack of impact on the cholesterol content of the lungs and brain also was not unexpected given the findings of numerous earlier studies in mice started on treatment before they were symptomatic.9, 10, 24
By one or more mechanisms, 2HPβCD penetrates the late endosomal/lysosomal compartment and facilitates the exit of the sequestered UC into the cytosol25, 26 where it can be processed and ultimately eliminated.9, 27 The release of this UC leads to a rapid compensatory inhibition of cholesterol synthesis and a marked transient rise in the cellular content of esterified cholesterol11, 12, 27, as well as in biliary cholesterol concentration (Figure 3). A comment on the significance of the increase in biliary cholesterol content is warranted. Although it cannot be determined from biliary cholesterol concentration data what fraction of all the UC that is released after each 2HPβCD injection leaves the liver as unmetabolized cholesterol in bile, this is likely to be relatively small otherwise there would be an ensuing rise in the amount of neutral sterol appearing in the stools. Our previous studies showed a clear increase in fecal bile acid, but not fecal neutral sterol excretion rates.9 Added to this is the likelihood that, as the pool of excess UC in all the tissues, particularly the liver, contracts with each successive 2HPβCD treatment the mature Npc1−/− mouse receives, the magnitude of the transient rise in biliary cholesterol would likely decrease.
In earlier studies we measured the ED50 of 2HPβCD for suppression of cholesterol synthesis over 24 h in various organs of 49-day-old Npc1−/− mice given a single sc injection of 2HPβCD.12 It was found that the ED50 for the brain, and lungs in particular, was orders of magnitude greater than it was for other organs such as the liver, spleen and kidneys.12 However, we subsequently showed that when the blood brain barrier was circumvented by delivering 2HPβCD directly into the brains of Npc1−/− mice, a marked and sustained inhibition of cholesterol synthesis was achieved at doses that were just a fraction of those given sc.11 In the lungs there was no discernable inhibition of synthesis at doses of 2HPβCD as high as 8000 mg/kg bw.12 If this non response is caused by an inability of 2HPβCD to traverse the pulmonary epithelial cells into the type II pneumocytes and alveolar macrophages where there is accumulation of cholesterol, then delivery through inhalation might be required.28, 29 Thus far, an effective technique for this has not become available. Here it should be noted that in a mouse model for NPC2 disease, enzyme replacement therapy given twice weekly starting at 21 days until 87 days of age, there was a marked reduction in the tissue cholesterol concentration not only in the liver and spleen but in the lungs as well.30 Importantly, this shows that there is nothing intrinsically different in the way that UC is sequestered in the lungs compared to other organs that might cause 2HPβCD to be ineffective.
Taken together, the present data show that 2HPβCD can appreciably lower the level of cholesterol sequestration in much of the periphery of symptomatic NPC1-deficient mice with a clear diminution in liver disease. As is the case with the lungs, the challenge will be in establishing a route of administration and a dose that can potentially achieve a favorable outcome in the other peripheral organs of humans with NPC disease. These challenges are additional to the need for a proven strategy for halting Purkinje cell loss. In this regard the findings of an NIH trial currently testing the efficacy of intrathecal 2HPβCD delivery using implantable osmotic minipumps in young NPC patients will be critical. As pointed out by Vance and Karten in their recently published review6, the management of NPC disease using 2HPβCD could ultimately require separate concurrent strategies for the brain, lung, and other peripheral organs. This goal might potentially be more attainable if a polymeric form of 2HPβCD31, 32 can be developed that requires much lower doses of compound which act for extended periods of time.
METHODS
Animals and treatment with 2HPβCD
The NPC1 mice (Npc1nih/nih) we used originated from a colony at the National Institutes of Health. Control (Npc1+/+) and homozygous (Npc1−/−) mice were generated from heterozygous (Npc1+/−) breeding stock on a pure BALB/c background. Pups were weaned and genotyped at 19 to 21 days of age. After weaning, all mice were fed a basal low-cholesterol rodent chow diet ad libitum as described.29 The mouse room had alternating 12-h periods of dark and light. Npc1−/− and Npc1+/+ mice that had not received any prior treatments were administered weekly a subcutaneous injection at the scruff of the neck of either normal saline or a 20% (w/v) solution (in saline) of 2HPβCD (Sigma-Aldrich Corp; product H107) starting when they were 49-days old. This provided an approximate dose of 4000 mg/kg bw.12 As described below, the treatment period varied according to the types of measurements to be made. Mice were studied in the fed state in all experiments except the one involving bile collection when they were fasted for 4 h beforehand. All experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern Medical Center.
Study design
Broadly, three separate sets of experiments were carried out. In the first set, matching groups of 49-day old Npc1−/− and Npc1+/+ mice received their respective treatments (saline vs 2HPβCD) at 49, 56, 63, and 70 days of age. At 77 days of age these mice were anesthetized, exsanguinated, and multiple organs were taken. Whole-organ total cholesterol content for liver, spleen, kidney, lung, brain and residual carcass was measured, along with plasma ALT and AST levels. Such measurements were also made concurrently in Npc1−/− and Npc1+/+ mice at 49 days of age to obtain baseline data for all these parameters. In the next group of experiments, large numbers of Npc1−/− and Npc1+/+ mice in the age range of 49 to 53 days, either remained untreated, or were given saline or 2HPβCD sc, once only, and then used for the harvesting of gallbladder bile at various time intervals up to 120 h after treatment. The third set of experiments measured the lifespan of groups of Npc1−/− and their Npc1+/+ controls that began receiving their respective treatments at 49 days of age and at weekly intervals thereafter for the remainder of their lives. For liver histology, pairs of Npc1−/− and Npc1+/+ mice that were not part of the lifespan study received weekly saline or 2HPβCD treatment from 49 to 84 days of age. Livers were taken at 91 days. In all experimental groups there were approximately equal numbers of male and female mice.
Relative organ weights and cholesterol content
The liver, spleen, kidneys, lungs, and brain were carefully excised, rinsed in saline, blotted and weighed, as was the residual carcass. Organ weights were expressed relative to body weight (% bw). The total cholesterol concentration (mg/g tissue) in all these organs was quantitated by gas-chromatography.33 Total concentration means that both the unesterified and esterified cholesterol fractions were measured. To obtain whole-organ cholesterol content, the tissue total cholesterol concentration was multiplied by the respective organ weight (mg/organ). For whole-animal cholesterol measurement, the organ cholesterol contents were summed and normalized per kg bw (mg/kg bw).
Gallbladder bile cholesterol content
Following exsanguination of the mice under isoflurane anesthesia, bile was aspirated directly from the gallbladder. The volume of bile (averaging about 12 µl) was recorded. The bile was extracted in 1.0 ml of methanol and this was used for quantitating cholesterol content (µmol/ml bile).33
Liver function tests
Npc1−/− and Npc1+/+ mice, all 77 days old, that had been given 2HPβCD or saline at 49, 56, 63 and 70 days of age, along with 49-day old mice of both genotypes that had not received any treatment, were exsanguinated via the inferior vena cava and plasma alanine transaminase (ALT) and aspartate transaminase (AST) activities were measured.10
Liver histology
Matching pairs of Npc1−/− and Npc1+/+ mice that had received injections of either saline or 2HPβCD at 49, 56, 63, 70, 77 and 84 days were studied at 91 days. Pieces of liver were fixed in 10% (v/v) buffered formalin and embedded in paraffin. These blocks were then sectioned (5 µm thick) and stained with hematoxylin and eosin.
Survival study
This study involved 22 Npc1−/− mice (10 males, 12 females) and 12 Npc1+/+ mice (6 males, 6 females). Half of the males and females received saline only, whereas the other half were given 2HPβCD. From day 49 until death, the body weight of the mice was recorded at frequent intervals, as was their neurological score. To gauge changes in the neurological function of the mice from 49 days onwards we employed the five-point system developed for a mouse model of amyotrophic lateral sclerosis (ALS). Scores ranged from zero (normal) to a maximum of 4.0. The highest score was assigned when a mouse was unable to right itself from either side within 30 seconds.34 At that point the mice were euthanized and the age at death was recorded. Thus all the mice used for scoring were also used to generate the lifespan data.
Analysis of data
Values are presented as mean±1 SEM of data from the specified number of animals. Differences between means were tested for statistical significance (P<0.05) using two-way ANOVA, followed by the Newman-Keuls multiple comparison test (GraphPad 6 Software, Inc.; San Diego, CA). Significant differences between groups are designated with letters. Statistical differences among Kaplan-Meier survival curves were determined utilizing the Gehan-Breslow-Wilcoxon test and Log-rank analyses.
ACKNOWLEDGEMENTS
This work was supported by US Public Health Service Grant R01HL009610. We thank Dennis K Burns, M.D. for help with liver histology.
Footnotes
DISCLOSURE
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie X, Brown MS, Shelton JM, Richardson JA, Goldstein JL, Liang G. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc Natl Acad Sci USA. 2011;108:15330–15335. doi: 10.1073/pnas.1112751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson RP. Current controversies in Niemann-Pick C1 disease: steroids or gangliosides; neurons or neurons and glia. J Appl Genet. 2013;54:215–224. doi: 10.1007/s13353-012-0130-0. [DOI] [PubMed] [Google Scholar]
- 4.Helquist P, Wiest O. Current status of drug therapy development for Niemann-Pick type C disease. Drugs Future. 2009;34:315–331. [Google Scholar]
- 5.Liu B. Therapeutic potential of cyclodextrins in the treatment of Niemann–Pick type C disease. Clin Lipidol. 2012;7:289–301. doi: 10.2217/clp.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vance JE, Karten B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J Lipid Res. 2014 doi: 10.1194/jlr.R047837. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci USA. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez CM, Liu B, Taylor AM, et al. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr Res. 2010;68:309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aqul A, Liu B, Ramirez CM, et al. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J Neurosci. 2011;31:9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez CM, Liu B, Aqul A, et al. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J Lipid Res. 2011;52:688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltroy EP, Liu B, Dietschy JM, Turley SD. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J Lipid Res. 2007;48:869–881. doi: 10.1194/jlr.M600488-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama M, Yoshida H, Suzuki M, Fujiyama J, Igata A. Lysosomal acid lipase deficiency in rats: lipid analyses and lipase activities in liver and spleen. J Lipid Res. 1990;31:1605–1612. [PubMed] [Google Scholar]
- 15.Du H, Heur M, Duanmu M, et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 16.Balwani M, Breen C, Enns GM, et al. Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology. 2013;58:950–957. doi: 10.1002/hep.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Xu YH, Du H, et al. Reversal of advanced disease in lysosomal acid lipase deficient mice: A model for lysosomal acid lipase deficiency disease. Mol Genet Metab. 2014 doi: 10.1016/j.ymgme.2014.04.006. (In press). [DOI] [PubMed] [Google Scholar]
- 18.Ward S, O'Donnell P, Fernandez S, Vite CH. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr Res. 2010;68:52–56. doi: 10.1203/PDR.0b013e3181df4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crumling MA, Liu L, Thomas PV, et al. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-beta-cyclodextrin. PLoS One. 2012;7:e53280. doi: 10.1371/journal.pone.0053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuo M, Togawa M, Hirabaru K, et al. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab. 2013;108:76–81. doi: 10.1016/j.ymgme.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Chien YH, Shieh YD, Yang CY, Lee NC, Hwu WL. Lung toxicity of hydroxypropyl-β-cyclodextrin infusion. Mol Genet Metab. 2013;109:231–232. doi: 10.1016/j.ymgme.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol. 2005;43:1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 24.Muralidhar A, Borbon IA, Esharif DM, et al. Pulmonary function and pathology in hydroxypropyl-beta-cyclodextin-treated and untreated Npc1−/− mice. Mol Genet Metab. 2011;103:142–147. doi: 10.1016/j.ymgme.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci USA. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plazzo AP, Hofer CT, Jicsinszky L, et al. Uptake of a fluorescent methyl-beta-cyclodextrin via clathrin-dependent endocytosis. Chem Phys Lipids. 2012;165:505–511. doi: 10.1016/j.chemphyslip.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Taylor AM, Liu B, Mari Y, Liu B, Repa JJ. Cyclodextrin mediates rapid changes in lipid balance in Npc1−/− mice without carrying cholesterol through the bloodstream. J Lipid Res. 2012;53:2331–2342. doi: 10.1194/jlr.M028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roszell BR, Tao JQ, Yu KJ, et al. Pulmonary abnormalities in animal models due to Niemann-Pick type C1 (NPC1) or C2 (NPC2) disease. PLoS One. 2013;8:e67084. doi: 10.1371/journal.pone.0067084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez CM, Lopez AM, Le LQ, Posey KS, Weinberg AG, Turley SD. Ontogenic changes in lung cholesterol metabolism, lipid content, and histology in mice with Niemann-Pick type C disease. Biochim Biophys Acta. 2014;1841:54–61. doi: 10.1016/j.bbalip.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen GK, Dagnaes-Hansen F, Holm IE, et al. Protein replacement therapy partially corrects the cholesterol-storage phenotype in a mouse model of Niemann-Pick type C2 disease. PLoS One. 2011;6:e27287. doi: 10.1371/journal.pone.0027287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins CJ, McCauliff LA, Hyun SH, et al. Synthesis, characterization, and evaluation of pluronic-based beta-cyclodextrin polyrotaxanes for mobilization of accumulated cholesterol from Niemann-Pick type C fibroblasts. Biochemistry. 2013;52:3242–3253. doi: 10.1021/bi3010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura A, Yui N. Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating Niemann-Pick type C disease. Scientific reports. 2014;4:4356. doi: 10.1038/srep04356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 34.Tesla R, Wolf HP, Xu P, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2012;109:17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]