Abstract
Thermal ablation to destroy tumor tissue may help activate tumor-specific T cells by elevating the presentation of tumor antigens to the immune system. However, the antitumor activity of these T cells may be restrained by their expression of the inhibitory T cell co-receptor CTLA-4, the target of the recently FDA-approved antibody drug ipilumimab. By relieving this restraint, CTLA-4 blocking antibodies like ipilumimab can promote tumor rejection, but the full scope of their most suitable applications has yet to be fully determined. In this study, we offer a preclinical proof of concept in the TRAMP-C2 mouse model of prostate cancer that CTLA-4 blockade cooperates with cryoablation of a primary tumor to prevent the outgrowth of secondary tumors seeded by challenge at a distant site. While growth of secondary tumors was unaffected by cryoablation alone, the combination treatment was sufficient to slow growth or trigger rejection. Additionally, secondary tumors were highly infiltrated by CD4+ T cells and CD8+ T cells and there was a significant increase in the ratio of intratumoral T effector cells to CD4+FoxP3+ T regulatory cells, compared to monotherapy. These findings documented for the first time an effect of this immunotherapeutic intervention on the intratumoral accumulation and systemic expansion of CD8+ T cells specific for the TRAMP C2-specific antigen, SPAS-1. Although cryoablation is currently used to treat a targeted tumor nodule, our results suggest that combination therapy with CTLA-4 blockade will augment anti-tumor immunity and rejection of tumor metastases in this setting.
Introduction
Thermal ablation treatments such as cryoablation have emerged as alternatives to surgical resection, to treat many types of inoperable tumors including prostate, kidney, liver, bone, adrenal, and lung. Cryoablation involves the insertion of a probe into a tumor nodule in order to administer tissue ablative freezing temperatures (1). Its mechanism of action has been attributed to the mechanical forces of crystallization, the osmotic changes due to crystallization, and the ischemic effects of microvascular injury (2). Further, as an image-guided, needle based technique, it can be administered percutaneously making it less invasive than traditional surgery (3, 4). As a result, it is associated with decreased morbidity and mortality and is more cost effective when compared to conventional therapies such as surgical resection (5).
Following ablation, the necrotic tumor lesion remains within the body, and it has been hypothesized that the release of tumor antigens by dying cells could activate a tumor-specific immune response through antigen presentation by antigen-presenting cells (APCs) to T cells. This antigen release is potentially significant because, while ablative procedures are very effective in eradicating the targeted tumor nodule, a tumor-specific immune response may facilitate elimination of distant metastases and prevent recurrent disease. Although a few cases of spontaneous remission of metastases following cryoablation have been reported (6), studies in patients and animal models have revealed weak or absent immune responses after ablation (7), despite the massive release of proteins resulting from tumor cell death observed in animal models (8). It has, therefore, been proposed that the immune response could be augmented if cryoablation is combined with immunotherapies that target APCs or modulate T cell function. A number of tumor studies combining immunomodulation, such as injection of toll-like receptor agonists, with cryoablation have demonstrated a synergistic effect on tumor rejection and this was attributed to enhanced activation of APC function (9, 10). Here, we investigate how immunotherapies that target the inhibitory pathways in T cells can potentially synergize with cryoablation to generate systemic anti-tumor immunity.
Monoclonal antibodies that block the function of CTLA-4, a transmembrane protein expressed by activated T cells, are a promising new therapy to treat cancer. CTLA-4 inhibits the activation of self-reactive T cells, and it was proposed many years ago that blockade of this pathway, could enhance T cell responses to tumors. Indeed, in preclinical studies, CTLA-4 blockade led to rejection of immunogenic tumors such as 51Blim10 colon carcinoma and SA/1N fibrosarcoma (11). In additional animal studies, rejection of less immunogenic tumors was achieved when CTLA-4 blockade was combined with a cellular vaccine, or radiation therapy, which likely increase the efficiency of antigen presentation (12-15). Studies in mouse models of prostate cancer have demonstrated decreased metastatic lesions and a reduction of primary tumor incidence when CTLA-4 blockade was combined with surgical resection or a GM-CSF secreting tumor vaccine, respectively (16, 17). In addition, CTLA-4 blockade was demonstrated to synergize with thermal ablation in protection of B16 melanoma tumor growth in a prophylactic setting (8, 18).
Clinical trials to validate the efficacy of anti-CTLA-4 monoclonal antibody (anti-CTLA-4) therapy in humans have been completed or are currently underway for the treatment of various cancers including melanoma, prostate and renal. Clinical trials in prostate cancer patients have shown improved results when CTLA-4 blockade was combined with a GM-CSF secreting tumor vaccine (GVAX) (19, 20). Furthermore, a Phase 3 trial of unresectable stage III and IV melanoma patients showed anti-CTLA-4 therapy (Ipilimumab, Bristol Meyers-Squibb) to improve the median survival time to 10 months compared to 6.4 months in the control group (21), and this work led to the recent approval of this therapy by the FDA. This was the first drug of any type to show survival benefit in metastatic melanoma in a blinded, randomized Phase 3 trial. Notably, approximately 25% of the patients had durable responses lasting 2 years and more. Thus, this therapy holds promise for metastatic melanoma patients who have failed the first line therapies and for whom there is currently no other approved treatment. While these results were obtained using anti-CTLA-4 as monotherapy, the current hypothesis is that an even more substantial extension of patient survival in a larger fraction of patients may be achieved when this checkpoint blockade is combined with other treatment modalities that promote tumor destruction and subsequent antigen presentation.
In this report, we test the synergistic effect of CTLA-4 blockade on preventing the development of a secondary tumor when administered in combination with cryoablation of a large primary tumor using the transplantable TRAMP C2 tumor model of metastatic prostate cancer. We report that, in contrast to cryoablation or CTLA-4 blockade alone, the combination therapy slowed or prevented growth of a majority of secondary tumors. Rejection of the second tumor was associated with infiltration or expansion of CD4+ and CD8+ effector T cells as well as an increase in the T effector (Teff) to T regulatory cell (Treg) ratio within the secondary tumor. Importantly, endogenous CD8+ T cells specific for the TRAMP tumor antigen SPAS-1 (22) were enriched in both secondary tumors and spleens of combination-treated mice compared to controls. Elimination of CD8+ T cells using depleting antibodies demonstrated that CD8+ T cells are important for rejection implying that SPAS-1 specific T cells contribute to the rejection of TRAMP C2 tumors during immunotherapy. These results suggest that cryoablation, while not effective on its own, can mediate the rejection of metastatic lesions and prevent recurrent disease when combined with CTLA-4 blockade.
Materials and Methods
Cell culture, media and mice
TRAMP C2 cells were obtained from N. M. Greenburg (Baylor College of Medicine, Houston, Texas) in 1999 (23). The cells were periodically authenticated by morphological inspection and tested negative in 2004 for microbial contamination using the mouse antibody production (MAP) test. The cells tested negative for mycoplasma contamination by PCR test in 2008. TRAMP C2 cells were grown in DMEM high glucose supplemented with 5% FCS (Mediatech, Inc.), 5% Nu Serum IV (BD Biosciences) HEPES, 2-ME, pen/strep, L-glut, 5 μg/ml insulin (Sigma), and 10 nM DHT (Sigma). 7-9 week old male C57BL/6 mice were purchased from Taconic Farms. Mice were used for experiments no earlier than one week after arrival. Mice were bred, housed, and treated according to the approved institutional animal protocols. Mice were injected with 200 μg anti-CTLA-4 (clone 9H10 BioXCell) or hamster IgG isotype control antibodies (BioXCell) in 0.2 ml PBS intraperitoneally on day 1 and 100 μg on days 4, 7, and 10 (Fig 1A). For CD8+ T cell depletion 0.5 mg of anti-CD8 (clone 2.43, BioXCell) was injected i.p. 4 days prior to tumor injection and 4 and 14 days after tumor injection.
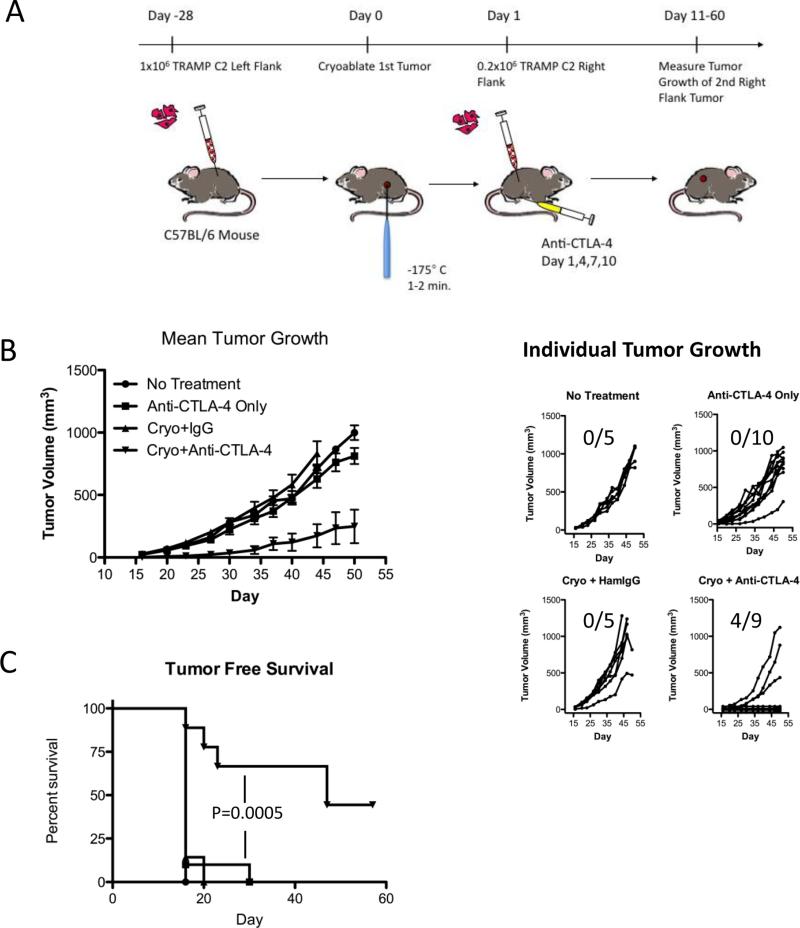
Figure 1. Cryoablation and anti-CTLA-4 combination therapy synergize to mediate rejection of a second TRAMP C2 tumor challenge.
A. Scheme to study the immune response to cryoablation and anti-CTLA-4 combination therapy. C57BL/6 male mice were challenged with TRAMP C2 prostate tumor cells on the left flank. Cryoablation was administered when the tumors reached about 5-8 mm in diameter. Mice were challenged the following day after cryoablation with a second injection of TRAMP C2 on the right flank and some of the mice were also treated with CTLA-4 blocking antibodies (clone 9H10) or hamster IgG isotype control antibodies. Calipers were used to monitor the growth of the second tumor. T cell infiltrates into the second tumor were analyzed on day 15. B. Mean and individual tumor growth. Error bars represent standard error of the mean. C. Tumor free survival. Data shown is representative of at least 3 independent experiments. Tumor free survival was plotted as a Kaplan-Meier curve and the log-rank test was used for statistical analysis. Number of mice that rejected the right flank tumor challenge: No Treatment: 0/5; Anti-CTLA-4 only 0/10; Cryoablation+IgG: 0/5; Cryo+Anti-CTLA-4: 4/9. One mouse from the Cryo+Anti-CTLA-4 group was omitted from the analysis due to partial ablation of the primary tumor.
Antibodies, tetramer and flow cytometry
Cells were blocked with 50 μg/ml 24.G2 (MSKCC monoclonal antibody core) and stained with surface antibodies or tetramer for 60 minutes on ice. Antibodies were used at a 1:100 dilution, while the tetramer was used at at a final concentration of 1.1 μg/ml. Antibodies detecting the following surface proteins were used: CD4 PerCP (clone RM4-5, BD Pharmingen), CD4 APC (Clone GK1, eBioscience) CD8 pacific blue or PE (clone 53-6.7, eBioscience), CD44 APC (clone IM7, eBioscience). The SPAS-1 H-2 Db tetramer PE was generated by the MSKCC tetramer core facility. The FoxP3 staining set (eBioscience) was used according to the manufacturer's instructions using anti-FoxP3 APC (clone FJK-16s, eBioscience) 1:100 in perm/wash buffer for 60 minutes on ice. The cells were analyzed on an LSR II (BD Biosciences) flow cytometer. Flow cytometry data were further analyzed with FlowJo (Treestar, Inc.)
ELISPOT
IFNγ ELISPOT kits (BD Biosciences) containing plates, coating antibody, detection antibody, and streptavidin-HRP enzyme were used according to the manufacturer's protocol using 200,000 lymphocytes and 1 μM SNC9H8 peptide or media. Plates were incubated for 20 hours at 37°C, 5% CO2. Spots were developed using the AEC substrate set (BD Biosciences). Spots were counted by Zellnet Consulting on an automated ELISPOT reader system (Carl Zeiss) with KS ELISPOT Software 4.9. The number of spots in response to H8 peptide minus the spots with no peptide are plotted.
Tumor inoculation and measurement of growth
TRAMP C2 cells were grown to about 90% confluency in 15 cm plates and harvested with trypsin. After washing three times with HBSS, cell suspensions were counted and finally resuspended in the appropriate volume of PBS. Mice were briefly anesthetized by isoflurane inhalation. For tumor measurement experiments, 0.2-0.5×106 cells were injected and for tumor harvest experiments, 1×106 cells were injected. For tumor implantation, mice were injected with cells in 0.1 ml PBS intradermally on day 0. Tumors were measured every 3-4 days using Vernier calipers (Fisher Scientific) and tumor volume was calculated as the product of length, width and height.
Immunofluorescence and confocal microscopy
Tumors were dissected from the mice and snap frozen in optimal cutting medium (O.C.T. TissueTek). 7 μM sections were cut using a cryotome, and mounted on slides. Sections were fixed in ice-cold acetone for 10 minutes prior to rehydration with PBS. After blocking in a solution of 50 μg/ml 24.G2 with 5% normal goat, rabbit and rat serum and 3% FCS, sections were stained with primary antibodies overnight at 4°C. The following directly conjugated primary antibodies at a dilution of 1:50 in blocking solution were used: anti-CD4 Alexa Flour 488 (Clone GK1, eBioscience), anti-CD8 pacific blue (clone 53-6.7, eBioscience), and anti-CD31 APC (clone 390, eBioscience). Slides were mounted in SlowFade Gold anti-fade reagent (Invitrogen) and analyzed on an inverted confocal microscope (Leica) with a 20× water immersion objective.
Preparation of single cell suspensions of tumor infiltrates
Mice were sacrificed by CO2 inhalation and the inside of the skin containing the tumor was exposed. Tumors were removed using forceps and surgical scissors and weighed. Tumors from each group were pooled and minced with scissors prior to incubation with 1.67 Wünsch U/ml Liberase and 0.2 mg/ml DNase for 30 minutes at 37°C. Tumors were homogenized by repeated pipetting and filtered through a 70 μM nylon filter. Remaining tumor pieces were pushed through the filter using the end of a 1 ml syringe. Cell suspensions were washed once with complete RPMI and ficolled. Live cells at the interface were collected and counted for further analysis.
Cryoablation
Mice were inoculated with 1×106 TRAMP C2 cells on the left flank as described. Tumors were cryoablated when they reached 5-8 mm in diameter, about 28 days after tumor inoculation. The mice were anesthetized with a mixture of 100 mg/kg ketamine and 10mg/kg xylazine. The mice were surgically prepped by shaving the targeted area and cleansing it with alternating povidone-iodine scrub and 70% alcohol rinse. A cryoablation probe (Endocare 1.7mm PerCryo Cryoprobe) was inserted into the tumor and freezing was administered for 1-2 minutes to less than −100°C at the needle hub. After the ablation, the site was monitored for bleeding or any other post procedural complications. Standard recovery procedures were implemented.
Statistical Analysis
Statistical significance was calculated using Prism 5.0 (GraphPad Software, Inc.).
Results
Cryoablation and CTLA-4 blockade synergize to mediate the rejection of a subsequent TRAMP C2 tumor challenge
We developed a model system to test the capacity of cryoablation and anti-CTLA-4 combination therapy to generate sufficient immunity to slow or reject a second tumor challenge. To this end, we injected TRAMP C2 tumor cells on the left flank of male B6 mice and administered cryoablation when it reached 5-8 mm in diameter, about 28 days after injection (Fig 1A). To model recurrent disease due to the outgrowth of a micrometastatic lesion, the following day, mice were challenged with a second injection of TRAMP C2 on the opposite flank. We monitored the growth of secondary tumors in mice treated with cryoablation and anti-CTLA-4 combination therapy (Cryo+Anti-CTLA-4), mice treated with cryoablation plus hamster IgG isotype control antibodies (Cryo+IgG), mice that received only the secondary tumor implant and anti-CTLA-4 monotherapy (Anti-CTLA-4 Only), and mice that received only the secondary tumor implant with no further treatment (No Treatment). Mice treated with only anti-CTLA-4 or cryoablation plus IgG grew secondary tumors at a similar rate compared to untreated controls (Fig 1B). In contrast, cryoablation and anti-CTLA-4 combination therapy led to slower outgrowth or rejection of the second tumor (Fig 1B). Similarly, while 100% of the untreated, anti-CTLA-4 only and cryoablation plus IgG treated mice succumbed to progressive growth of the second tumor, combination treatment led to rejection by 44% of the mice (Fig 1C). Our results indicate that, while cryoablation alone does not influence the growth of a second tumor challenge, when it is combined with CTLA-4 blockade, induction of sufficient immunity leads to tumor rejection.
Secondary tumors from cryoablation/anti-CTLA-4 combination treated mice are infiltrated by CD8+ and CD4+ T cells
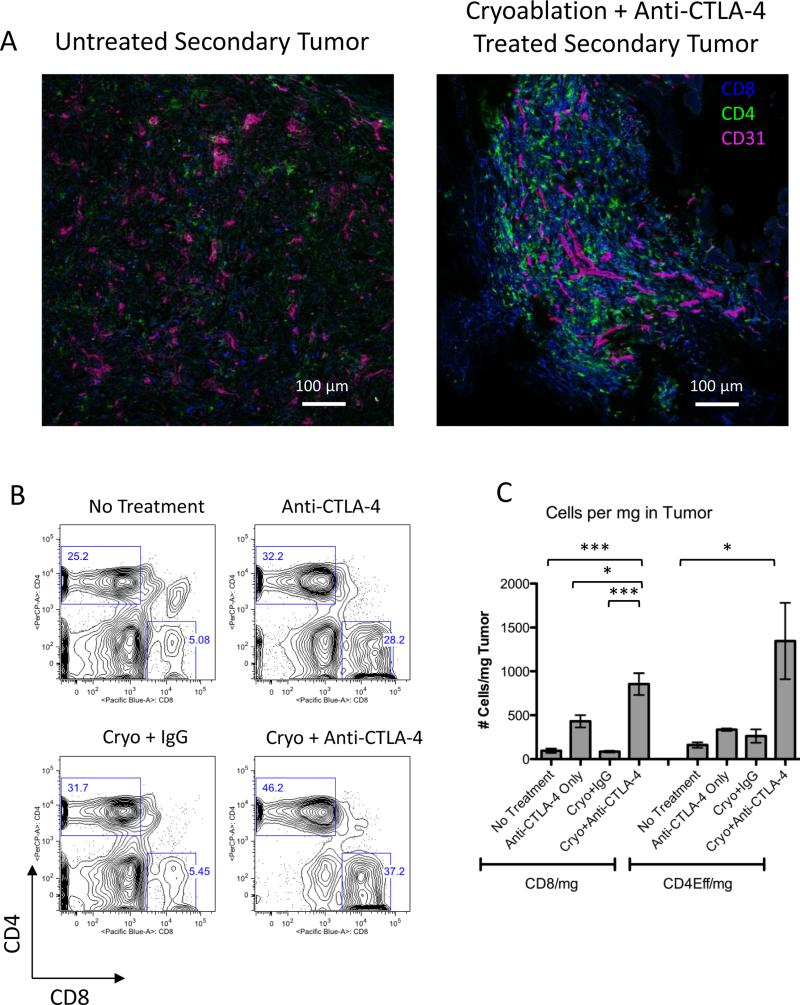
To identify the T cell types that mediate rejection of the secondary tumors in our system, we isolated the tumors and assayed them for T cell infiltration by immunofluorescence staining and flow cytometry. T cell infiltration into contralateral tumors was assessed 15 days after implantation of TRAMP C2 tumors into naïve or cryoablation-treated mice. Cryo-sections of secondary tumors were stained with anti-CD4 and anti-CD8 antibodies to determine the types of T cells that infiltrated the tumor. In addition, anti-CD31, a marker of endothelial cells was used to stain the tumor vasculature. Immunofluorescence staining of untreated tumors revealed limited T cell infiltration (Fig 2A left panel). In contrast, secondary tumors from combination-treated mice were highly infiltrated by both CD8+ and CD4+ T cells (Fig 2A right panel).
Figure 2. CD4+ T cells and CD8+ T cells infiltrate the secondary tumors of combination treated mice.
A. Immunofluorescence, B. Representative plots of flow cytometric analysis and C. Quantification of T cells in secondary tumors from untreated, anti-CTLA-4, cryoablation+IgG and cryoablation+anti-CTLA-4 combination treated mice. Right flank tumors were pooled from 6 mice for each experimental group. C. Shows pooled data from 3 independent experiments. Statistical significance was calculated by one-way ANOVA using the Bonferroni post test with the following notations for P value: * P = 0.01-0.05, and *** P < 0.001. Error bars represent standard error of the mean.
To better quantify the T cells that we observed by immunofluorescence, we evaluated T cell infiltration by flow cytometry. On day 15, TRAMP C2 tumors are very small due to the slow growth of this tumor model. Therefore, to obtain enough tumor material to analyze by flow cytometry, we increased the tumor inoculum to 1×106 TRAMP C2 cells and pooled 6 tumors from each group. Tumors from untreated, anti-CTLA-4 only, or cryoablation plus IgG-treated mice contained smaller percentages of CD4+ T cells, compared to combination treated tumors (Fig 2B). Anti-CTLA-4 treatment alone, or when combined with cryoablation resulted in an increased percentage CD8+ T cells within the secondary tumor (Fig 2B). When absolute cell numbers and the mass of the pooled tumors were accounted for in the analysis, the total CD8+ T cells per mg of tumor in combination-treated mice increased two-fold over anti-CTLA-4 alone and ten-fold over no treatment and cryoablation plus IgG (Fig 2C). Likewise, CD4+FoxP3- T cells were increased in combination treated tumors eight-fold and four-fold compared to both untreated and cryoablation plus IgG treated tumors and anti-CTLA-4 treated tumors, respectively (Fig 2C). The increased T cell infiltration into tumors observed in the combination treated mice correlates with tumor rejection and suggests that these T cell types are important for tumor eradication.
CD8+ T cells are important for tumor rejection and combination treatment produces an immune response to the immunodominant, CD8+ TRAMP C2 antigen, SPAS-1
Having demonstrated the efficacy of combination treatment in mediating tumor infiltration by T cells, we next focused on the specificities of these infiltrates. The data from Figure 2 suggested that CD8+ T cells are important for tumor rejection since we observed a correlation between CD8+ T cell infiltration and tumor rejection. To determine the necessity of CD8+ T cells in controlling tumor growth after combination treatment, we depleted CD8+ T cells prior to therapy by in vivo administration of CD8 depleting antibodies. The antibody was successful in depleting most of the CD8+ T cells, as assessed by staining of peripheral blood leukocytes prior to cryotherapy (Fig S1). A significant difference in survival between combination treated mice with and without depletion was observed, indicating that CD8+ T cells contribute to tumor eradication in our system (Fig 3).
Figure 3. Depletion of CD8+ T cells diminishes the therapeutic effect.
Kaplan-Meier curve showing tumor free survival of mice with no treatment and cryoablation/anti-CTLA-4 combination treated mice with and without depletion of CD8+ T cells. Data were pooled from 3 independent experiments. The log-rank test was used for statistical analysis.
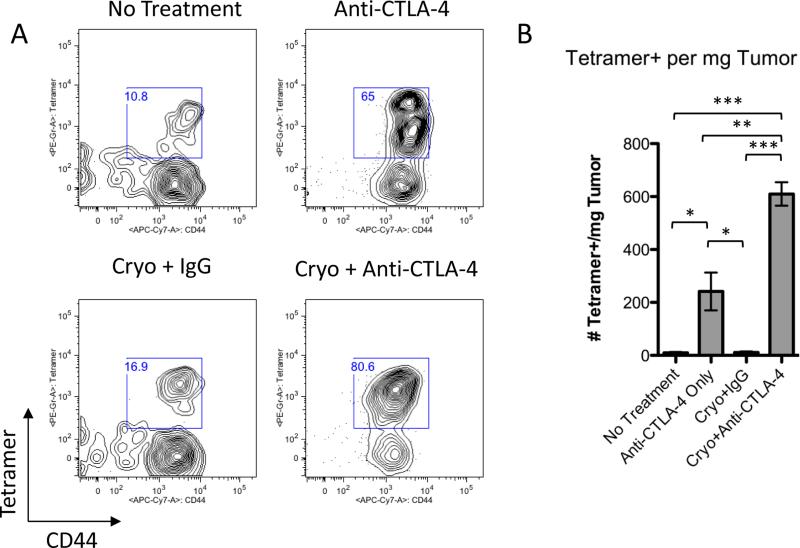
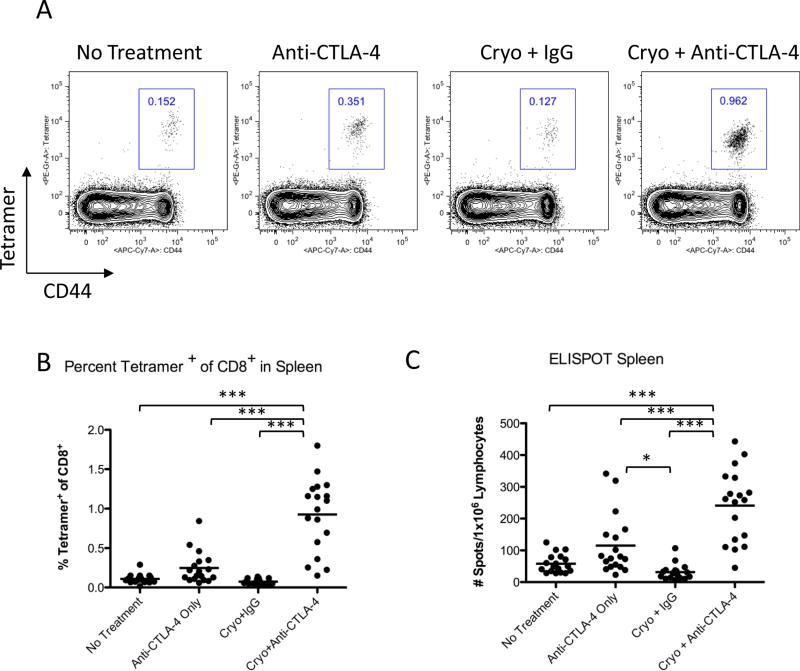
In order to demonstrate that these CD8+ T cells were specific for the TRAMP tumor, we stained both tumors and spleens with SPAS-1/MHC class I tetramers. We have previously shown that the SPAS-1 TRAMP tumor antigen is an immunodominant CD8+ T cell target on TRAMP cells (22). Of the CD8+ T cells infiltrating TRAMP C2 tumors, 10.8%, 65%, and 16.9% co-stained for both tetramer and the activation marker CD44 in untreated, anti-CTLA-4 only, and cryoablation plus IgG treated tumors, respectively (Fig 4A). Interestingly, the percentage of CD44+ SPAS-1-specific CD8+ T cells increased to 80.6% in combination therapy-treated tumors (Fig 4A). This increase in percentage translated to a 58-fold increase in total SPAS-1-specific T cells per mg of tumor over no treatment and cryoablation plus IgG and a 2.5-fold increase over anti-CTLA-4 monotherapy treatment, indicating that tumors from treated mice are highly infiltrated by activated tumor-specific CD8+ T cells (Fig 4B). Finally, we determined the frequency of SPAS-1 specific T cells in the spleen. We found that CD44+ SPAS-1 specific CD8+ T cells were significantly increased in the spleens of combination treated mice compared to controls suggesting that the combination treatment induced systemic expansion of tumor specific CD8+ T cells (Fig 5A and B).
Figure 4. Secondary tumors from combination treated mice are infiltrated by TRAMP-C2 specific CD8+ T cells.
A. Flow cytometry of tumors after staining with SPAS-1 peptide-loaded MHC class I tetramers. Plots shown are gated on CD3+CD8+ cells. B. Quantification of tetramer positive cells in tumors. Tumors were harvested 15 days after injection of the right flank tumor. Right flank tumors were pooled from 6 mice for each experimental group and data were pooled from 3 independent experiments. Experimental data were analyzed by one-way ANOVA using the Bonferroni post test with the following notations for P value: * P = 0.01-0.05, ** P = 0.001-0.01, and *** P < 0.001. Error bars represent standard error of the mean.
Figure 5. Cryoablation+Anti-CTLA-4 combination treatment induces SPAS-1 specific CD8+ T cells to accumulate in spleens.
A. Percentage of CD8+ T cells that are tetramer+ in spleens. Representative plots (gated on CD8+ cells) are shown. B. Percentage of CD8+tetramer+ T cells in the spleen. Data were pooled from 3 independent experiments. C. Detection of IFNγ secretion by splenocytes in response to SPAS-1 peptide (SNC9H8) by ELISPOT. Data were pooled from 3 independent experiments. Experimental data were analyzed by one-way ANOVA using the Bonferroni post test with the following notations for P value: * P = 0.01-0.05 and *** P < 0.001. Error bars represent standard error of the mean.
In order to determine if the SPAS-1 specific T cells detected by tetramer staining were capable of exerting effector function, we performed ELISPOT assays for IFNγ secretion. Splenocytes were incubated with SPAS-1 peptide (SNC9H8) for 20 hours to induce IFNγ secretion by SPAS-1-reactive cells. Consistent with the increased numbers of tetramer positive cells observed in spleens, we detected a significant increase in IFNγ-positive cells in spleens from cryoablation/anti-CTLA-4 treated mice compared to controls (Fig 5C). Taken together, these data indicate that cryoablation and CTLA-4 blockade synergize to induce an antigen-specific CD8+ infiltrate capable of producing an effector response within a secondary tumor. Since CD8+ T cells contribute to tumor rejection in our model (Fig 3) and based on our previous data showing that SPAS-1 is the major target in the anti-TRAMP tumor response (22) it is likely that these SPAS-1 specific T cells are playing a major role in the immune response induced by combination therapy.
Cryoablation/CTLA-4 blockade combination therapy increases the intratumoral effector to Treg ratio
CD4+FoxP3+ T regulatory cells have been shown to play a role in dampening the immune response against tumors (24). Several studies from our lab using the B16 melanoma tumor model reported that the efficiency of different immunotherapeutic regimens in combination with CTLA-4 blockade (GVAX, Treg depletion, adoptive cell therapy, and FLT3 ligand expressing vaccine) directly correlates with a shift in the intratumoral balance from Tregs to Teffs (25-28). To assess the role of Tregs in cryoablation/anti-CTLA-4 therapy, we evaluated secondary tumors 15 days after cryoablation for the presence of CD4+FoxP3+ Tregs by flow cytometry. 29-44% of the CD4+ T cells inside untreated, anti-CTLA-4 and cryoablation plus IgG treated tumors were FoxP3+ (Fig 6A). In contrast, a much lower 6.22% of the intratumoral CD4+ T cells from the combination-treated tumors stained for FoxP3 indicating that a relatively suppressive environment exists within progressively growing TRAMP C2 tumors (Fig 6A). This decrease in percentage of Tregs was not due to fewer Tregs infiltrating the combination treated tumors, as the total number of CD4+FoxP3+ Tregs per mg of tumor was similar among the four groups (Fig 6B). Rather, the decrease was due to the elevated expansion or infiltration of CD4+FoxP3- T cells, which are likely to be CD4+ effector cells in treated tumors. This result is consistent with the relative increase in the CD4+ Teff to Treg ratio compared to control tumors (Fig 6C). In addition, an increase in the intratumoral CD8+ T cell to Treg ratio was observed in the tumor reflecting the increased numbers of CD8+ T cells observed in the treated tumors depicted in Fig 2C (Fig 6C). Thus, consistent with results obtained previously in our lab in the Gvax/anti-CTLA-4 B16 system (27), cryoablation and CTLA-4 blockade therapy dramatically shifts the intratumoral balance, increasing the ratio of Teff to Tregs, and this shift correlates with tumor immunity.
Figure 6. Combination treated tumors have an increased Teff to Treg ratio compared to controls.
A. Representative flow cytometry plots showing percentage (gated on CD4+ cells) of CD4+FoxP3+ T cells in pooled tumors. B. Number of FoxP3+ Cells per mg of tumor. C. Effector to Treg ratio in pooled tumors. Right flank tumors were pooled from 6 mice for each experimental group and data were pooled from 3 independent experiments. Experimental data were analyzed by one-way ANOVA using the Bonferroni post test with the following notations for P value: * P = 0.01-0.05, ** P = 0.001-0.01, and *** P < 0.001. Error bars represent standard error of the mean.
Discussion
We have shown here that cryoablation and anti-CTLA-4 combination therapy produces systemic immunity and tumor rejection in the TRAMP C2 mouse prostate model. Our model system involved the cryoablation of a large primary tumor followed one day later by anti-CTLA-4 administration and a secondary tumor challenge on the opposite flank. We injected the second tumor the day following cryoablation in order to model the outgrowth of micrometastases that become established after cryoablation and lead to recurrent disease. A study in the B16 model showed that proteins are released from the tumor into the draining lymph nodes and presented on dendritic cells (DC) up to three days following cryoablation (18). Thus, the injection of a second tumor one day after cryoablation in our model represents a nascent micrometastasis that develops during this antigen release.
We tracked the antigen-specific CD8+ T cell response to TRAMP C2 cells using tetramer and ELISPOT assays to detect the recently identified, immunodominant TRAMP C2 antigen SPAS-1 (22). We report that, after combination therapy, SPAS-1 reactive T cells made up over 80% of the intratumoral CD8+ T cells and increased in absolute numbers in these treated mice, demonstrating the therapy's ability to enhance the tumor-specific CD8 response. The combination therapy also resulted in an enhanced frequency of SPAS-1 reactive CD8+ T cells in the spleen, which is evidence of a systemic response with the potential to eradicate disseminated disease. Depleting CD8+ T cells diminished the therapeutic effect and since most of the CD8+ T cells in the tumors of treated mice were specific for SPAS-1, it is likely that SPAS-1 specific T cells are important for tumor rejection.
TRAMP C2 cells were derived from a primary prostate tumor of a TRAMP mouse, which develop tumors due to prostate-specific expression of SV40 T antigen (29). The mutation in SPAS-1 likely arose in the prostate tumor of the mouse from which it was derived, and therefore, is only present in the cell line making it unique to the TRAMP C2 cells. The antigenic epitope of SPAS-1 was found to be highly immunogenic compared to its non-mutated counterpart, and peptide-pulsed DCs conferred protection against a TRAMP C2 tumor challenge (22). In human tumors, mutated tumor antigens may be important targets of the immune response generated by immunotherapies. A study by Segal et al. identified mutations in breast and colorectal tumors that resulted in neo-epitopes that could bind HLA-A*0201 (30). They found that breast tumors contained an average of 10 neo-epitopes while colorectal tumors had an average of 7 neo-epitopes. The extent by which these neo-antigens elicit immune responses is currently unknown, but because of their proposed higher immunogenicity relative to non-mutated shared antigens, they are likely to play an important role in immunity to tumors and thus represent potential targets in therapies such as cryoablation, that increase antigen presentation. Since patient tumors are likely to contain immunogenic, mutated self-antigens as a result of genomic instability (30), we believe that following the SPAS-1 response is a suitable readout of the immune response to a unique tumor antigen, the type likely to be targeted in patients undergoing this combination therapy. Furthermore, in pre-clinical studies of novel immunotherapies using the transplantable TRAMP tumor model, the tetramer assays for SPAS-1 described for the first time herein will likely be useful tools to demonstrate a favorable immunologic response.
We addressed the cellular mechanisms mediating tumor rejection in our system by assessing both the effector and regulatory T cell responses. We showed that both CD8+ and CD4+FoxP3- T cells are increased in tumors as a result of cryoablation/anti-CTLA-4 combination therapy. Importantly, the intratumor ratio of Teff to Treg was also increased as a result of our treatment, which has been correlated with tumor rejection in the B16 Gvax/anti-CTLA-4 model (27). This has also been observed in clinical studies of ovarian cancer, where a high Teff to Treg ratio in tumors was prognostic of improved survival (31) and associated with increased tumor necrosis in patients treated with anti-CTLA-4 (32). These studies further support the use of this ratio as a marker of a favorable immunologic response to immunotherapy. Moreover, tumor immunity was enhanced when Treg depletion, through anti-CD25 depleting antibodies or cyclophosphamide treatment was combined with cryoablation (8, 33), indicating that Tregs are important modulators of tumor immunity in response to cryoablation.
Since the regression of metastases after cryoablation was first observed in patients, investigators have examined the immune response in animal models in hopes of demonstrating a “cryoimmunologic” effect. The results of these studies are mixed, preventing a consensus regarding the effect of cryoablation as a monotherapy to treat metastatic disease. Numerous studies report immunologic benefit resulting in the rejection of secondary tumor challenges following cryoablation (34-37). However, others have reported no effect (38) or an enhancement of secondary tumor growth after cryoablation (39-41). The results of our cryoablation study indicate that in our system, cryoablation alone had no effect on secondary tumor growth or T cell infiltration into secondary tumors. Despite the lack of a response from cryoablation alone, we observed a synergistic effect when cryoablation was combined with anti-CTLA-4. While the immune response to cryoablation alone may vary with each patient, combination with CTLA-4 blockade has the potential to create a robust anti-tumor immune response that controls the growth of metastases and prevents the recurrence of disease. This work has led to the generation of proposals for clinical trials in which cryoablation will be combined with CTLA-4 blockade to treat cancer.
Supplementary Material
Precis.
One use of the recently approved immune activating drug ipilumimab (Yervoy) may be to enhance the benefits of tumor cryoablation, a simple older treatment strategy being explored anew in breast and prostate cancers where it might be very effectively combined with immunotherapy to enhance cure rates in patients with localized tumors.
Acknowledgements
We thank J. Geddes, W. Montalvo, J. Lu, and J. Wei for technical assistance and S. A. Quezada, C. Ariyan, and R. Kendle for critical reading of the manuscript. J. P. Allison holds the David H. Koch chair in immunological studies at the Memorial Sloan-Kettering Cancer Center and is an investigator of the Howard Hughes Medical Institute.
Financial Support: Howard Hughes Medical Institute; National Institutes of Health
Footnotes
Conflict of Interest: JPA is the inventor of intellectual property licensed by Bristol Myers Squibb. RW disclosed no potential conflicts of interest.
References
- 1.Weld KJ, Landman J. Comparison of cryoablation, radiofrequency ablation and high-intensity focused ultrasound for treating small renal tumours. BJU Int. 2005;96:1224–9. doi: 10.1111/j.1464-410X.2005.05848.x. [DOI] [PubMed] [Google Scholar]
- 2.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 3.Link RE, Permpongkosol S, Gupta A, Jarrett TW, Solomon SB, Kavoussi LR. Cost analysis of open, laparoscopic, and percutaneous treatment options for nephron-sparing surgery. J Endourol. 2006;20:782–9. doi: 10.1089/end.2006.20.782. [DOI] [PubMed] [Google Scholar]
- 4.Maybody M, Solomon SB. Image-guided percutaneous cryoablation of renal tumors. Tech Vasc Interv Radiol. 2007;10:140–8. doi: 10.1053/j.tvir.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60:3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 6.Soanes WA, Ablin RJ, Gonder MJ. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J Urol. 1970;104:154–9. doi: 10.1016/s0022-5347(17)61690-2. [DOI] [PubMed] [Google Scholar]
- 7.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 8.den Brok MHMGM, Sutmuller RPM, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 9.den Brok MHMGM, Sutmuller RPM, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–92. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 10.Redondo P, del Olmo J, López-Diaz de Cerio A, et al. Imiquimod enhances the systemic immunity attained by local cryosurgery destruction of melanoma lesions. J Invest Dermatol. 2007;127:1673–80. doi: 10.1038/sj.jid.5700777. [DOI] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95:10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 16.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 17.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–9. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Brok MHMGM, Sutmuller RPM, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong L, Kwek S, O'brien S, et al. Potentiating Endogenous Antitumor Immunity to Prostate Cancer through Combination Immunotherapy with CTLA4 Blockade and GM-CSF. Cancer Res. 2009;69:609. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–91. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, O'Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. The New England Journal of Medicine. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fassò M, Waitz R, Hou Y, et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci USA. 2008;105:3509–14. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 25.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–55. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peggs K, Quezada S, Chambers C, Korman A, Allison J. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009 doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–38. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 31.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy MY, Sidana A, Chowdhury WH, et al. Cyclophosphamide unmasks an antimetastatic effect of local tumor cryoablation. J Pharmacol Exp Ther. 2009;330:596–601. doi: 10.1124/jpet.109.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joosten JJ, Muijen GN, Wobbes T, Ruers TJ. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49–58. doi: 10.1006/cryo.2001.2302. [DOI] [PubMed] [Google Scholar]
- 35.Sabel MS, Arora A, Su G, Chang AE. Adoptive immunotherapy of breast cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology. 2006;53:360–6. doi: 10.1016/j.cryobiol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JLM, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90:97–104. doi: 10.1007/s10549-004-3289-1. [DOI] [PubMed] [Google Scholar]
- 37.Urano M, Tanaka C, Sugiyama Y, Miya K, Saji S. Antitumor effects of residual tumor after cryoablation: the combined effect of residual tumor and a protein-bound polysaccharide on multiple liver metastases in a murine model. Cryobiology. 2003;46:238–45. doi: 10.1016/s0011-2240(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann NE, Coad JE, Huot CS, Swanlund DJ, Bischof JC. Investigation of the mechanism and the effect of cryoimmunology in the Copenhagen rat. Cryobiology. 2001;42:59–68. doi: 10.1006/cryo.2001.2305. [DOI] [PubMed] [Google Scholar]
- 39.Friedman EJ, Orth CR, Brewton KA, Ponniah S, Alexander RB. Cryosurgical ablation of the normal ventral prostate plus adjuvant does not protect Copenhagen rats from Dunning prostatic adenocarcinoma challenge. J Urol. 1997;158:1585–8. [PubMed] [Google Scholar]
- 40.Hayakawa K, Yamashita T, Suzuki K, et al. Comparative immunological studies in rats following cryosurgery and surgical excision of 3-methylcholanthrene-induced primary autochthonous tumors. Gann. 1982;73:462–9. [PubMed] [Google Scholar]
- 41.Yamashita T, Hayakawa K, Hosokawa M, et al. Enhanced tumor metastases in rats following cryosurgery of primary tumor. Gann. 1982;73:222–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.