Abstract
Objectives
To evaluate the effect of a novel divided attention task—walking under auditory constraints—on gait performance in older adults and to determine whether this effect was moderated by cognitive status.
Design
Validation cohort.
Setting
General community.
Participants
Ambulatory older adults without dementia (N=104).
Interventions
Not applicable.
Main Outcome Measures
In this pilot study, we evaluated walking under auditory constraints in 104 older adults who completed 3 pairs of walking trials on a gait mat under 1 of 3 randomly assigned conditions: 1 pair without auditory stimulation and 2 pairs with emotionally charged auditory stimulation with happy or sad sounds.
Results
The mean age of subjects was 80.6±4.9 years, and 63% (n=66) were women. The mean velocity during normal walking was 97.9±20.6cm/s, and the mean cadence was 105.1±9.9 steps/min. The effect of walking under auditory constraints on gait characteristics was analyzed using a 2-factorial analysis of variance with a 1-between factor (cognitively intact and minimal cognitive impairment groups) and a 1-within factor (type of auditory stimuli). In both happy and sad auditory stimulation trials, cognitively intact older adults (n=96) showed an average increase of 2.68cm/s in gait velocity (F1.86,191.71=3.99; P=.02) and an average increase of 2.41 steps/min in cadence (F1.75,180.42=10.12; P<.001) as compared with trials without auditory stimulation. In contrast, older adults with minimal cognitive impairment (Blessed test score, 5–10; n=8) showed an average reduction of 5.45cm/s in gait velocity (F1.87,190.83=5.62; P=.005) and an average reduction of 3.88 steps/min in cadence (F1.79,183.10=8.21; P=.001) under both auditory stimulation conditions. Neither baseline fall history nor performance of activities of daily living accounted for these differences.
Conclusions
Our results provide preliminary evidence of the differentiating effect of emotionally charged auditory stimuli on gait performance in older individuals with minimal cognitive impairment compared with those without minimal cognitive impairment. A divided attention task using emotionally charged auditory stimuli might be able to elicit compensatory improvement in gait performance in cognitively intact older individuals, but lead to decompensation in those with minimal cognitive impairment. Further investigation is needed to compare gait performance under this task to gait on other dual-task paradigms and to separately examine the effect of physiological aging versus cognitive impairment on gait during walking under auditory constraints.
Keywords: Attention, Cognition, Gait, Rehabilitation
Walking is the most commonly reported physical activity in adults.1–5 Even in young healthy individuals, walking is a complex motor task, similar to catching a moving object, and has been shown to require higher-level cognitive resources, such as executive function.6 Performing gait analysis during a simultaneous cognitive challenge represents an opportunity to screen elderly individuals who may be at a higher risk of falls and may facilitate their identification before a sentinel event.7–16
The walking while talking task is a divided attention task that involves both cognitive and motor components and has been shown to be a reliable and valid test to identify older adults at high risk of multiple adverse outcomes such as falls,9 frailty, disability, and death.17,18 Walking while talking replicates what many would consider a “typical” experience for elderly individuals because many individuals may engage in intermittent conversation during ambulatory activity. However, walking during listening to background auditory stimuli is an even more ubiquitous occurrence.19–24 Walking while listening to background auditory stimuli may be more ecologically valid because it has been shown that older adults have greater difficulty focusing on tasks when distracted by irrelevant information.25 Acoustically novel stimuli demand attention and produce a distracting effect that can persist for a period of time. In fact, the degree of distraction and its effect on cognitive tasks have been shown to increase with age.26 The increased distractibility may be partially due to an age-related reduction in processing speed and attention.27
However, all auditory stimuli are not the same. Some types of auditory stimuli have been shown to increase function. For example, rhythmic auditory stimulation has been shown to increase gait velocity and stride length via motor entrainment.28–34 Affective nonverbal vocalizations—laughing and crying—have also been shown to activate the human amygdala regardless of attentive state,35–39 suggesting that we might be more sensitive to emotionally salient background auditory stimuli. Emotional states have also been shown to have specific effects on gait40–43: for example, depressive states decrease ground reaction forces, pleasant emotional states facilitate initiation of forward gait, and anxiety states increase the attentional demand for locomotion.44 Given the sensitivity of gait parameters to different emotional states, an emotionally salient listening task may be an ideal interference task to challenge the cognitive reserve for mobility and assess fall risk in elderly individuals.
The objective of this preliminary study was to evaluate the effect of a novel divided attention task—walking under auditory constraints—in older adults. A second objective was to determine whether this effect was moderated by cognitive status. We hypothesized that emotionally charged auditory stimuli would elicit compensation (improvement in gait performance) in cognitively intact older adults, but decompensation (decline in gait performance) in older adults with minimal cognitive impairment.
Methods
Subjects
One hundred and five community-residing older adults who were participants in the Latent Mobility Abnormality Study45,46 were included for the walking under auditory constraints protocol. Informed consent was obtained as per the study protocol approved by the local institutional review board (Division of Cognitive and Motor Aging, Saul R. Korey Department of Neurology, Albert Einstein College of Medicine, Bronx, NY). Inclusion criteria were age ≥70 years and ambulatory status. Participants who were ambulatory but used walking aids were excluded from this sub-study. Exclusion criteria included severe audiovisual loss (unable to follow questions asked in a loud voice or corrected vision <20/200), dementia (as diagnosed by a consensus case conference), and being bed bound or institutionalized.
A detailed neuropsychological test battery was administered, consisting of the Blessed Information-Memory-Concentration Test for general cognition,47 the Free and Cued Selective Reminding Test48 for memory, the digit symbol substitution test for cognitive processing,49 the letter fluency test50 for executive function, and the digit span test49 for attention. For the purposes of this study, the Blessed-Information-Memory Concentration Test47 and the Geriatric Depression Scale51 were used to test associations between gait and cognitive status.52 Dementia diagnosis was assigned at consensus case conferences using the Diagnostic and Statistical Manual, Fourth Edition, criteria53 and subtyped using established criteria.45,46 The consensus case conference included a neurologist with geriatric expertise, a psychologist, and a social worker54 who reviewed all available clinical history, examination, and neuropsychological test findings, as previously described.55,56 The literature57,58 supports a high correlation between consensus diagnoses and pathological findings. We excluded 1 subject who met the study criteria for dementia. Of the 105 subjects, 104 (99%) were eligible for this analysis.
At the baseline study visit, all subjects were assessed on their ability to perform 7 activities of daily living (ADL): bathing, dressing, grooming, feeding, toileting, walking around the home, and getting up from a chair. For each task, participants were asked, “At the present time, are you unable to or do you need help from another person to complete the task?” If the response was “yes,” the task was scored as 2. If the response was “no,” participants were asked a follow-up question: “Do you have difficulty in completing the task?” The task was scored as 1 for the response of “yes” and 0 for the response of “no.” The disability score was calculated as a sum of the scores from 7 ADLs, with a maximum disability score of 14 (requiring help for all 7 ADLs) and a minimum of 0. Disability was defined as inability or requiring personal assistance in any of the 7 ADLs.59 The interviewer also assessed fall history over the last 12 months or 1 calendar year during the same baseline study visit. A fall was defined as an event which results in a person coming to rest inadvertently on the ground or other lower level and other than as a consequence of the following: sustaining a violent blow; loss of consciousness; sudden onset of paralysis, as in a stroke; or an epileptic seizure.60
Quantitative gait
Gait parameters were obtained using a computerized mat (457.2×90.17×0.63cm) embedded with pressure sensors. Start and stop points were marked by white lines on the floor and included 3ft each for initial acceleration and terminal deceleration for a total length of 6.4m. Subjects were instructed to walk on the mat at their “normal pace” for 2 trials in a quiet, well-lit hallway, wearing comfortable footwear. They were asked to resume walking as soon as they could in case they stopped walking for any reason during the trial. Trial administrators did not advise or encourage subjects during the trials and intervened only in situations in which subject safety was an issue. A trial was not repeated if it was interrupted for any reason. Monitoring devices were not attached to the participants during the test.
The GAITRite walkway systema computed quantitative gait parameters on the basis of footfalls recorded. Each trial was 1 walkway in length, and values analyzed were the mean of 2 trials computed automatically by the software. The following variables were obtained from the gait mat: step length, the distance between heel points of the current footfall and previous footfall on the opposite foot; cadence, the number of steps taken in a minute; stride length, the distance between the heel points of 2 consecutive footfalls of the same foot; gait velocity, the distance covered on 2 trials divided by ambulation time; and gait variability, the SD of stride length. Measurements made using this equipment have shown excellent reliability and validity.46,61
Auditory trials
Subjects were asked to walk on a computerized walkway twice for each of the 3 randomly assigned conditions: without auditory stimulation, with positively valenced auditory stimulation (happy sounds, the sound of a baby’s laughter), and with negatively valenced auditory stimulation (sad sounds, the sound of a woman wailing). Each sound track was approximately 10 seconds in length, lacked any specific rhythm, was begun before the start of walking, and was looped continuously for the duration of the walking trial. At the end of each trial, subjects were asked to rate the emotional valence of the happy or sad sounds on a scale of 1 (no emotion) to 10 (highest emotion) and their feelings in relation to just having heard the happy or sad sounds on a scale of 1 (no feeling) to 10 (highest feeling).
Statistical analysis
Two-way factorial analysis of variance with a 1-between factor (cognitively intact and minimal cognitive impairment groups) and a 1-within factor (type of auditory stimuli) (mixed factorial design) was used on each of the gait parameters of interest to determine the effect of walking under auditory constraints. We included the Blessed test score that indicated cognitive impairment as a moderating variable to determine the effect of cognitive status on walking under auditory constraints. Subjects were dichotomized as cognitively intact for a Blessed test score of <5 and as having minimal cognitive impairment for a Blessed test score between 5 and 9.62 Continuous variables were reported with means and SDs, whereas categorical variables were reported as percentiles.
Because of the sample size imbalance between cognitively intact and minimal cognitive impairment groups, equality of variance assumptions were occasionally violated in the subsequent tests. To account for these discrepancies, statistical corrections were made where appropriate. A Greenhouse-Geisser correction for the effective degrees of freedom was implemented when Mauchly’s test of sphericity was violated, and the subsequent statistics were reported. A Python extension for SPSS version 20b called Fuzzy was used to search for 8 cognitively intact subjects matched to the group with minimal cognitive impairment on emotional ratings of both auditory stimuli, and the mixed factorial analysis of variance was performed again on the matched groups. In addition, fall history and performance of ADLs were compared between the 2 groups to further validate the results. SPSS for Mac version 20 was used for all analyses.
Results
The mean age of the 104 subjects was 80.6±4.9 years. Emotionally charged auditory stimulation had a significant effect on gait velocity (F1.86,191.7=3.9; P=.02) and cadence (F1.75,180.4=10.1; P<.001) for the entire sample.
Subjects were then separated into cognitively intact (n=96) and minimal cognitive impairment (n=8) groups on the basis of their Blessed test scores (1 subject met amnestic mild cognitive impairment syndrome criteria and 7 did not). Demographic information for both groups of subjects is given in table 1. Adding cognitive status as a between-subjects factor showed significant interaction effects with emotionally charged stimuli for both cadence and velocity, but not for walking time, stride length, swing time, or gait variability (table 2). Post hoc pairwise comparisons showed that in both happy and sad auditory stimulation trials, cognitively intact older adults (n=96) showed an average increase of 2.68cm/s in gait velocity (F1.86, 191.71=3.99; P=.02) (fig 1A) and an average increase of 2.41 steps/min in cadence (F1.75,180.42=10.12; P<.001) (fig 1B) as compared with trials without auditory stimulation. In contrast, older adults with minimal cognitive impairment (Blessed test score, 5–10; n=8) showed a reduction of 5.45cm/s in gait velocity (F1.87,190.83=5.62; P=.005) and a reduction of 3.88 steps/min in cadence (F1.79,183.10=8.21; P=.001) under both auditory stimulation conditions (see fig 1; for additional statistics, see table 3). Additional post hoc tests showed that velocity was significantly different between the cognitively intact and minimal cognitive impairment groups under the sad condition (P=.043). There were no significant differences between groups at baseline or under the happy condition.
Table 1.
Demographic statistics for both groups of subjects
| Statistic | No Cognitive Impairment (n=96) | Minimal Cognitive Impairment (n=8) |
|---|---|---|
| Height (cm) | 161.2±9.2 | 163.4±10.5 |
| Weight (kg) | 73.4±14.5 | 76.3±16 |
| Education (y) | 14.9±3 | 13.3±4.6 |
| Age (y) | 80.6±5.1 | 80.9±4 |
| Geriatric Depression Scale score (range, 0–15) | 2.3±2.5 | 2.3±2.8 |
| Blessed score | 1.2±1.3 | 6.4±1.2 |
| Sex: female | 60 (62.5) | 6 (75) |
NOTE. Values are mean ±SD or as n (%).
Table 2.
Gait parameters for both groups of subjects
| Gait Variables | No Cognitive Impairment (n=96) | Minimal Cognitive Impairment (n=8) |
|---|---|---|
| Velocity | ||
| Neutral | 98.3±2.1 | 93.8±7.29 |
| Happy | 101±2.07 | 91±7.19 |
| Sad | 100.8±2.05 | 85.6±7.1 |
| Cadence | ||
| Neutral | 104.8±1 | 108.1±3.5 |
| Happy | 107.5±1 | 105.8±3.5 |
| Sad | 107±1.1 | 102.7±3.7 |
NOTE. Values are mean ±SE.
Fig 1.
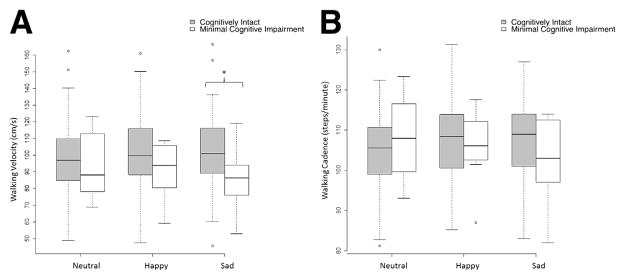
Effect of walking under auditory constraints on (A) gait velocity and (B) cadence compared between cognitively intact subjects (gray boxplots) and subjects with minimal cognitive impairment (white boxplots). There was a significant interaction effect betw6een the 2 groups (tables 2 and 3). *P<.05.
Table 3.
Gait variables and their interaction with cognitive status
| Gait Variable | Interaction Effect |
|---|---|
| Time | F1.43,145.59=.69; P=.46 |
| Velocity | F1.87,190.8=5.6; P=.005 |
| Cadence | F1.79,183.1=8.21; P=.001 |
| Stride length | F2,204=2.21; P=.11 |
| Swing time | F1.87,191.13=1.18; P=.31 |
| Gait variability (stride length SD) | F2,204=.61; P=.55 |
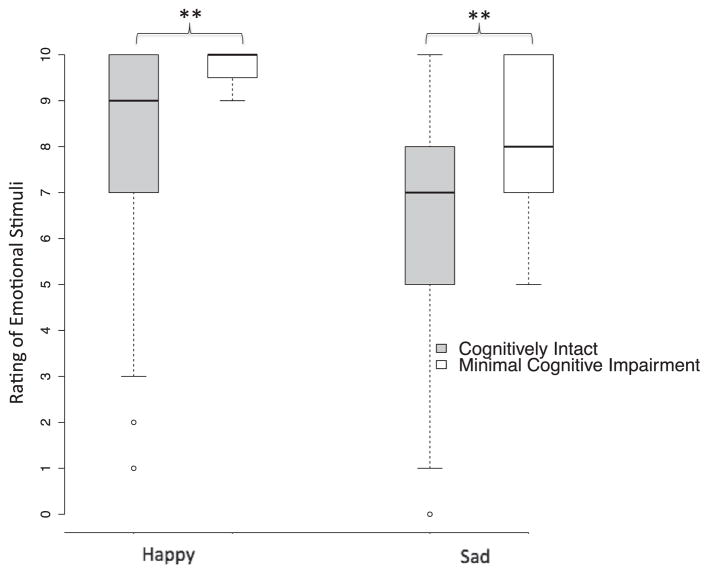
The mean rating scores of emotional valence for happy and sad sounds in subjects with minimal cognitive impairment were significantly higher than those in cognitively intact subjects (happy ratings: t54.5=5.97; P<.01; sad ratings: t102=2.18; P=.03) (fig 2). Subjective feeling scores were also compared between the 2 groups for happy and sad sounds. The mean feeling scores of happy and sad sounds in cognitively intact subjects were 6.7±3.2 and 3.7±3.1, respectively. In contrast, the rating scores in subjects with minimal cognitive impairment were 8.8±2.8 and 7.3±2.6, respectively. The t tests showed a near-significant between-group difference in happy feelings (t102=1.8; P=.07) and a significant between-group difference in sad feelings (t102=3.1; P=.02).
Fig 2.
Rating of emotional stimuli in cognitively intact subjects (gray boxplots) and subjects with minimal cognitive impairment (white boxplots). *P<.01.
To further validate the main findings in light of group differences in stimuli ratings, subjects with minimal cognitive impairment were matched to an equal number of subjects without cognitive impairment. Congruent with previous analyses (see figs 1 and 2), subjects with minimal cognitive impairment showed contrasting effects compared with the 8 cognitively intact subjects on gait velocity (F2,28=3.65; P=.039) and cadence (F2,28=4.56; P=.019), which were reduced with happy and sad sounds.
To assess the validity of differentiation by cognitive status, the 2 groups were also compared for fall history and performance of ADLs. We found no significant differences between the cognitively intact (mean score ±SD, 1.5±0.5) and minimal cognitive impairment (mean score ±SD, 1.6±0.5) groups in falls history over a period of 1 year before the study visit (t102=.54; P=.57). There were also no differences between the cognitively intact (1.6±1.6) and minimal cognitive impairment (1.7±1.9) groups in ADL performance (t101=.19; P=.84).
Discussion
To our knowledge, this is the first study to characterize gait performance under emotionally charged auditory stimulation conditions in an elderly population. As hypothesized, our findings reveal that gait performance improved in individuals who were cognitively intact when they walked amid a background of emotionally charged auditory sounds. In contrast, gait performance declined under the same conditions in individuals with minimal cognitive impairment.
Gait performance improves under emotionally charged auditory conditions in cognitively intact elderly individuals
Walking parameters have been shown to be influenced by time-evolving auditory stimuli, including television sounds.30 The effect of rhythmic auditory stimuli on ambulation is also well documented29,31,33,34,63 and is often ascribed to an “entraining” effect.34 In our study, older adults with intact cognition were able to improve gait performance (faster velocity and cadence), suggesting effective recruitment of their cognitive resources when challenged. In our study, auditory stimulation represents a distraction. Previous literature64 supports the idea that older adults compensate for increased distractibility by focusing more strongly on task-relevant stimuli.
Neuroimaging evidence suggests that neural activity associated with cognitive aging is characterized by both age-related increases and decreases in brain activity in specific regions. Failure to activate brain regions typically recruited by younger adults during cognitive tasks usually suggests neurocognitive decline. However, additional neural recruitment during task performance beyond that seen in younger adults is thought to indicate neural compensation.65–67 Functional imaging studies have revealed that neural compensation results in increased brain activation in the contralateral prefrontal cortex, also known as hemispheric asymmetry reduction in old adults.68 Thus, low-performing older adults appear to use a similar strategy as do younger adults, but use it inefficiently. In contrast, high-performing older adults appear to counteract age-related neural decline through a plastic reorganization of neurocognitive networks by symmetrical brain activation in functionally relevant neural networks,66,68 thereby increasing the availability of cognitive resources69 for task performance.
Gait performance declines under emotionally charged auditory conditions in elderly individuals with minimal cognitive impairment
We found that individuals with minimal cognitive impairment, as suggested by their Blessed test scores,47,62,70 decreased their gait velocity and cadence when walking under auditory constraints. These findings may be explained by a reduced ability to allocate additional cognitive resources to maintain gait performance when distracted. These findings, therefore, support the decline-compensation hypothesis,65–67 where an inability to compensate neurally or functionally when challenged uncovers a picture of rapid decline. Even rhythmic auditory stimulation at a comfortable tempo has been shown to produce deleterious effects on gait in those with Alzheimer dementia.71 Our findings extend this phenomenon to individuals at predementia stages.
It is known that divided attention markedly impairs the ability to regulate gait in individuals with Alzheimer disease.10,11,14 It appears that performance deficits in aging are due to higher distractibility, in combination with deficits in orienting-reorienting mechanisms.66 The literature72 suggests strong associations between age and speed reduction and between cognitive status and speed reduction under dual-task conditions. Neuroimaging reveals a common prefrontoparietal neural network for performing 2 tasks simultaneously or successively.73,74 It has been suggested that smaller prefrontal area volume may contribute to a slower gait through reduced information processing speeds.75 Frontal and temporoparietal metabolic impairment on positron emission tomographic scans has been shown to be closely related to the progression of Alzheimer disease.76 These networks are also thought to explain age-related differences in processing of auditory information. Thus, auditory distraction represents an ecologically valid approach to evaluate cognitive reserve for mobility. The decline in gait velocity during walking under auditory constraints may be an early indication of reduced cognitive reserve.
Significance of using emotionally salient auditory backgrounds
When asked to rate the emotional significance of auditory stimuli, subjects with minimal cognitive impairment consistently rated both happy and sad conditions higher than did subjects without cognitive impairment. This may explain the difference in gait metrics between the 2 groups. The valence scores also provide qualitative support for the effect of auditory emotional stimuli on gait during dual-task conditions. Emotionally charged auditory stimuli activate the amygdala and auditory cortices,35–38 and affect regulation using cognitive control may be achieved through active cortical suppression of the amygdalar structures.39 Subjects with minimal cognitive impairment may be unable to suppress amygdalar activation as seen in patients with early dementia,77–79 resulting in higher emotional ratings. However, when a subsample of cognitively intact subjects with identical emotional ratings was matched to subjects with minimal cognitive impairment in this study, the effect on gait velocity and cadence remained. This suggests that the underlying cognitive impairment, rather than the emotional rating alone, accounts for the effect on gait velocity and cadence.
Study limitations
The main limitation of this study was the small number of subjects with minimal cognitive impairment. A second limitation was that auditory stimuli were not matched against more conventional dual-task paradigms, such as serial 3s or verbal fluency, to compare task effects. Furthermore, although the effects of auditory stimulation on gait metrics were statistically significant, they were not large. In the future, we will incorporate minimal clinical difference analyses and use larger cohorts in longitudinal studies to determine the clinical relevance of the effects of auditory stimulation on gait. In addition, we found differences in velocity and cadence metrics and not in the other measured gait variables. In a recent study, principal component analysis was used to cluster the variables derived from quantitative gait analyses into statistically independent gait domains: rhythm, pace, and variability.17 Velocity and step length belong to the pace domain; abnormalities in this domain have been shown to predict falls.17 Cadence, swing time, and step time belong to the variability domain, and abnormalities in this domain have been shown to predict dementia.17 Thus, the walking under auditory constraints task may differentially affect the control of pacing and variability during gait, and velocity and cadence may be the dominant variables in these domains. However, discrete effects may also be due to the small sample size.
Conclusions
The distinct performance responses to the walking under auditory constraints task in cognitively intact older individuals versus individuals with minimal cognitive impairment provide insights into the nature of cognitive reserve utilization under real-world conditions. Our findings should be replicated in larger samples with a wider spectrum of cognitive impairment and compared with accepted dual-task paradigms. Further exploration is needed to separately examine the effects of physiological aging and cognitive impairment on the relation between auditory stimulation and gait performance.
Acknowledgments
Supported by the National Institute on Aging (grant no. RO1 AG025119).
List of abbreviations
- ADL
activities of daily living
Footnotes
GAITRite; CIR Systems Inc./GAITRite.
SPSS version 20; IBM Corp.
Disclosures: none.
References
- 1.Centers for Disease Control and Prevention (CDC) Vital signs: walking among adults—United States, 2005 and 2010. MMWR Morb Mortal Wkly Rep. 2012;61:595–601. [PubMed] [Google Scholar]
- 2.Yang Y, Diez-Roux AV. Walking distance by trip purpose and population subgroups. Am J Prev Med. 2012;43:11–9. doi: 10.1016/j.amepre.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen N, Humpel N, Leslie E, Bauman A, Sallis JF. Understanding environmental influences on walking: review and research agenda. Am J Prev Med. 2004;27:67–76. doi: 10.1016/j.amepre.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Simpson ME, Serdula M, Galuska DA, Gillespie C, Donehoo R, Macera C, Mack K. Walking trends among U.S. adults: the Behavioral Risk Factor Surveillance System, 1987–2000. Am J Prev Med. 2003;25:95–100. doi: 10.1016/s0749-3797(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 5.Rafferty AP, Reeves MJ, McGee HB, Pivarnik JM. Physical activity patterns among walkers and compliance with public health recommendations. Med Sci Sports Exerc. 2002;34:1255–61. doi: 10.1097/00005768-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–8. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 7.Beauchet O, Dubost V, Allali G, Gonthier R, Hermann FR, Kressig RW. ‘Faster counting while walking’ as a predictor of falls in older adults. Age Ageing. 2007;36:418–23. doi: 10.1093/ageing/afm011. [DOI] [PubMed] [Google Scholar]
- 8.Szturm T, Maharjan P, Marotta JJ, Shay B, Shrestha S, Sakhalkar V. The interacting effect of cognitive and motor task demands on performance of gait, balance and cognition in young adults. Gait Posture. 2013;38:596–602. doi: 10.1016/j.gaitpost.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–6. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 10.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology. 1997;48:955–8. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- 11.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people [letter] Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- 12.de Hoon EW, Allum JH, Carpenter MG, et al. Quantitative assessment of the stops walking while talking test in the elderly. Arch Phys Med Rehabil. 2003;84:838–42. doi: 10.1016/s0003-9993(02)04951-1. [DOI] [PubMed] [Google Scholar]
- 13.Hauer K, Pfisterer M, Weber C, Wezler N, Kliegel M, Oster P. Cognitive impairment decreases postural control during dual tasks in geriatric patients with a history of severe falls. J Am Geriatr Soc. 2003;51:1638–44. doi: 10.1046/j.1532-5415.2003.51517.x. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–7. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 15.Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? J Mot Behav. 2005;37:259–64. [PubMed] [Google Scholar]
- 16.Beauchet O, Annweiler C, Dubost V, et al. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–95. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 17.Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology. 2014;60:108–13. doi: 10.1159/000355119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60:1901–5. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitney SL, Marchetti GF, Schade A, Wrisley DM. The sensitivity and specificity of the Timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J Vestib Res. 2004;14:397–409. [PubMed] [Google Scholar]
- 20.Jenkins ME, Johnson AM, Holmes JD, Stephenson FF, Spaulding SJ. Predictive validity of the UPDRS postural stability score and the Functional Reach Test, when compared with ecologically valid reaching tasks. Parkinsonism Relat Disord. 2010;16:409–11. doi: 10.1016/j.parkreldis.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Sterke CS, Huisman SL, van Beeck EF, Looman CW, van der Cammen TJ. Is the Tinetti Performance Oriented Mobility Assessment (POMA) a feasible and valid predictor of short-term fall risk in nursing home residents with dementia? Int Psychogeriatr. 2010;22:254–63. doi: 10.1017/S1041610209991347. [DOI] [PubMed] [Google Scholar]
- 22.Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90:761–73. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]
- 23.Foreman KB, Addison O, Kim HS, Dibble LE. Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord. 2011;17:166–71. doi: 10.1016/j.parkreldis.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan RP, Earhart GM. Four square step test performance in people with Parkinson disease. J Neurol Phys Ther. 2013;37:2–8. doi: 10.1097/NPT.0b013e31827f0d7a. [DOI] [PubMed] [Google Scholar]
- 25.Smith PA. Aging, Auditory Distraction and Grammaticality Judgment. Aphasiology. 2010;24:1342–53. [Google Scholar]
- 26.Parmentier FB, Andres P. The involuntary capture of attention by sound: novelty and postnovelty distraction in young and older adults. Exp Psychol. 2010;57:68–76. doi: 10.1027/1618-3169/a000009. [DOI] [PubMed] [Google Scholar]
- 27.Harris KC, Eckert MA, Ahlstrom JB, Dubno JR. Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear Res. 2010;264:21–9. doi: 10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak EE. Effect of rhythmic auditory stimulation on gait performance in children with spastic cerebral palsy. J Music Ther. 2007;44:198–216. doi: 10.1093/jmt/44.3.198. [DOI] [PubMed] [Google Scholar]
- 29.Wittwer JE, Webster KE, Hill K. Rhythmic auditory cueing to improve walking in patients with neurological conditions other than Parkinson’s disease—what is the evidence? Disabil Rehabil. 2013;35:164–76. doi: 10.3109/09638288.2012.690495. [DOI] [PubMed] [Google Scholar]
- 30.Sejdić E, Jeffery R, Vanden Kroonenberg A, Chau T. An investigation of stride interval stationarity while listening to music or viewing television. Hum Mov Sci. 2012;31:695–706. doi: 10.1016/j.humov.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Kwak EE, Park ES, Cho SR. Differential effects of rhythmic auditory stimulation and neurodevelopmental treatment/Bobath on gait patterns in adults with cerebral palsy: a randomized controlled trial. Clin Rehabil. 2012;26:904–14. doi: 10.1177/0269215511434648. [DOI] [PubMed] [Google Scholar]
- 32.Arias P, Cudeiro J. Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS One. 2010;5:e9675. doi: 10.1371/journal.pone.0009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thaut MH, Leins AK, Rice RR, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21:455–9. doi: 10.1177/1545968307300523. [DOI] [PubMed] [Google Scholar]
- 34.Styns F, van Noorden L, Moelants D, Leman M. Walking on music. Hum Mov Sci. 2007;26:769–85. doi: 10.1016/j.humov.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12:181–98. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- 36.Sander K, Brechmann A, Scheich H. Audition of laughing and crying leads to right amygdala activation in a low-noise fMRI setting. Brain Res Brain Res Protoc. 2003;11:81–91. doi: 10.1016/s1385-299x(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 37.Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci. 2005;17:1519–31. doi: 10.1162/089892905774597227. [DOI] [PubMed] [Google Scholar]
- 38.Sander K, Frome Y, Scheich H. FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum Brain Mapp. 2007;28:1007–22. doi: 10.1002/hbm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschacher W, Schildt M, Sander K. Brain connectivity in listening to affective stimuli: a functional magnetic resonance imaging (fMRI) study and implications for psychotherapy. Psychother Res. 2010;20:576–88. doi: 10.1080/10503307.2010.493538. [DOI] [PubMed] [Google Scholar]
- 40.Sloman L, Pierrynowski M, Berridge M, Tupling S, Flowers J. Mood, depressive illness and gait patterns. Can J Psychiatry. 1987;32:190–3. doi: 10.1177/070674378703200306. [DOI] [PubMed] [Google Scholar]
- 41.Naugle KM, Joyner J, Hass CJ, Janelle CM. Emotional influences on locomotor behavior. J Biomech. 2010;43:3099–103. doi: 10.1016/j.jbiomech.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Naugle KM, Hass CJ, Joyner J, Coombes SA, Janelle CM. Emotional state affects the initiation of forward gait. Emotion. 2011;11:267–77. doi: 10.1037/a0022577. [DOI] [PubMed] [Google Scholar]
- 43.Naugle KM, Hass CJ, Bowers D, Janelle CM. Emotional state affects gait initiation in individuals with Parkinson’s disease. Cogn Affect Behav Neurosci. 2012;12:207–19. doi: 10.3758/s13415-011-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gage WH, Sleik RJ, Polych MA, McKenzie NC, Brown LA. The allocation of attention during locomotion is altered by anxiety. Exp Brain Res. 2003;150:385–94. doi: 10.1007/s00221-003-1468-7. [DOI] [PubMed] [Google Scholar]
- 45.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–90. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 46.Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall CB, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire [letter] Age Ageing. 2004;33:628–32. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- 47.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 48.Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learn Verbal Behav. 1973;12:543–50. [Google Scholar]
- 49.Wechsler D. Wechsler Adult Intelligence Scale—Revised (WAIS-R) New York: Psychological Corporation; 1981. [Google Scholar]
- 50.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–8. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 51.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 52.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–23. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 53.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- 54.Strozyk D, Dickson DW, Lipton RB, et al. Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging. 2010;31:1710–20. doi: 10.1016/j.neurobiolaging.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–35. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathuranath PS, George A, Ranjith N, et al. Incidence of Alzheimer’s disease in India: a 10 years follow-up study. Neurol India. 2012;60:625–30. doi: 10.4103/0028-3886.105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayeux R, Reitz C, Brickman AM, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment, part 1. Alzheimers Dement. 2011;7:15–34. doi: 10.1016/j.jalz.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seshadri S, Beiser A, Au R, et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment, part 2. Alzheimers Dement. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003;58:70–5. doi: 10.1093/gerona/58.1.m70. [DOI] [PubMed] [Google Scholar]
- 60.The prevention of falls in later life: a report of the Kellogg International Work Group on the Prevention of Falls by the Elderly. Dan Med Bull. 1987;34:1–24. [PubMed] [Google Scholar]
- 61.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 62.Locascio JJ, Growdon JH, Corkin S. Cognitive test performance in detecting, staging, and tracking Alzheimer’s disease. Arch Neurol. 1995;52:1087–99. doi: 10.1001/archneur.1995.00540350081020. [DOI] [PubMed] [Google Scholar]
- 63.Schauer M, Mauritz KH. Musical motor feedback (MMF) in walking hemiparetic stroke patients: randomized trials of gait improvement. Clin Rehabil. 2003;17:713–22. doi: 10.1191/0269215503cr668oa. [DOI] [PubMed] [Google Scholar]
- 64.Horvath J, Czigler I, Birkas E, Winkler I, Gervai J. Age-related differences in distraction and reorientation in an auditory task. Neurobiol Aging. 2009;30:1157–72. doi: 10.1016/j.neurobiolaging.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Dennis NA, Cabeza R. Neuroimaging of healthy cognitive imaging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York: Psychology Pr; 2008. pp. 1–54. [Google Scholar]
- 66.Getzmann S, Gajewski PD, Falkenstein M. Does age increase auditory distraction? Electrophysiological correlates of high and low performance in seniors. Neurobiol Aging. 2013;34:1952–62. doi: 10.1016/j.neurobiolaging.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Getzmann S, Falkenstein M. Understanding of spoken language under challenging listening conditions in younger and older listeners: a combined behavioral and electrophysiological study. Brain Res. 2011;1415:8–22. doi: 10.1016/j.brainres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 69.Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood—what it is and how it interacts with cognitive performance. Curr Dir Psychol Sci. 2005;14:144–8. [Google Scholar]
- 70.Zhang XQ, Min BQ, Ma YC, Yang PJ, Li DP. [Relationship between three neuropsychological tests and cerebral glucose metabolism in Alzheimer’s disease] [Chinese] Zhonghua Yi Xue Za Zhi. 2003;83:100–2. [PubMed] [Google Scholar]
- 71.Wittwer JE, Webster KE, Hill K. Effect of rhythmic auditory cueing on gait in people with Alzheimer disease. Arch Phys Med Rehabil. 2013;94:718–24. doi: 10.1016/j.apmr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35:715–28. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–39. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- 74.Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M. On the neural basis of focused and divided attention. Brain Res Cogn Brain Res. 2005;25:760–76. doi: 10.1016/j.cogbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herholz K. Cerebral glucose metabolism in preclinical and pro-dromal Alzheimer’s disease [review] Expert Rev Neurother. 2010;10:1667–73. doi: 10.1586/ern.10.136. [DOI] [PubMed] [Google Scholar]
- 77.Huijbers MJ, Bergmann HC, Olde Rikkert MGM, Kessels RP. Memory for emotional pictures in patients with Alzheimer’s dementia: comparing picture-location binding and subsequent recognition. J Aging Res. 2011;2011:409364. doi: 10.4061/2011/409364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klein-Koerkamp Y, Beaudoin M, Baciu M, Hot P. Emotional decoding abilities in Alzheimer’s disease: a meta-analysis. J Alzheimers Dis. 2012;32:109–25. doi: 10.3233/JAD-2012-120553. [DOI] [PubMed] [Google Scholar]
- 79.McCade D, Savage G, Naismith SL. Review of emotion recognition in mild cognitive impairment. Dement Geriatr Cogn Disord. 2011;32:257–66. doi: 10.1159/000335009. [DOI] [PubMed] [Google Scholar]