Abstract
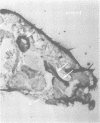
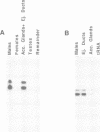
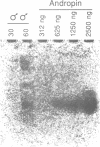
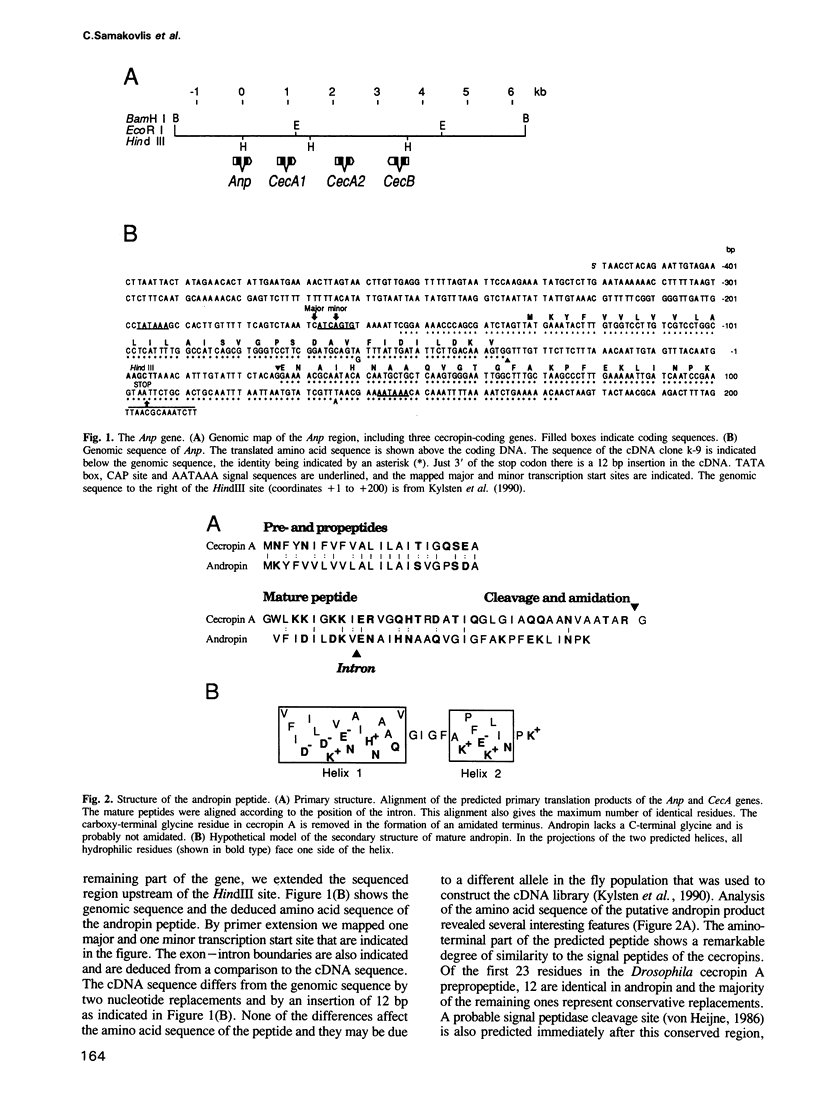
In our study of the cecropin locus in Drosophila we have found a gene for a new peptide, andropin, with antibacterial properties. Transcripts from this gene, Anp, could be detected in newly eclosed males and reached steady-state levels after 1 day. Transcription was strongly induced in response to mating and is strictly confined to the ejaculatory duct of adult males. The deduced peptide sequence reveals a hydrophobic amino terminus with striking similarity to the signal peptide of the cecropins. The sequence of the predicted mature andropin shows no direct homology with the cecropins, but the two peptides may have similar secondary structures. We have synthesized the predicted gene product and shown it to be antibacterial. Crude extracts from male genital tracts show a potent bactericidal activity, and electrophoretic separation revealed at least three antibacterial components, one with the same mobility as the synthetic peptide. It appears that insects have evolved a mechanism for the protection of the seminal fluid and the male reproductive tract against microbial infections.
Full text
PDF

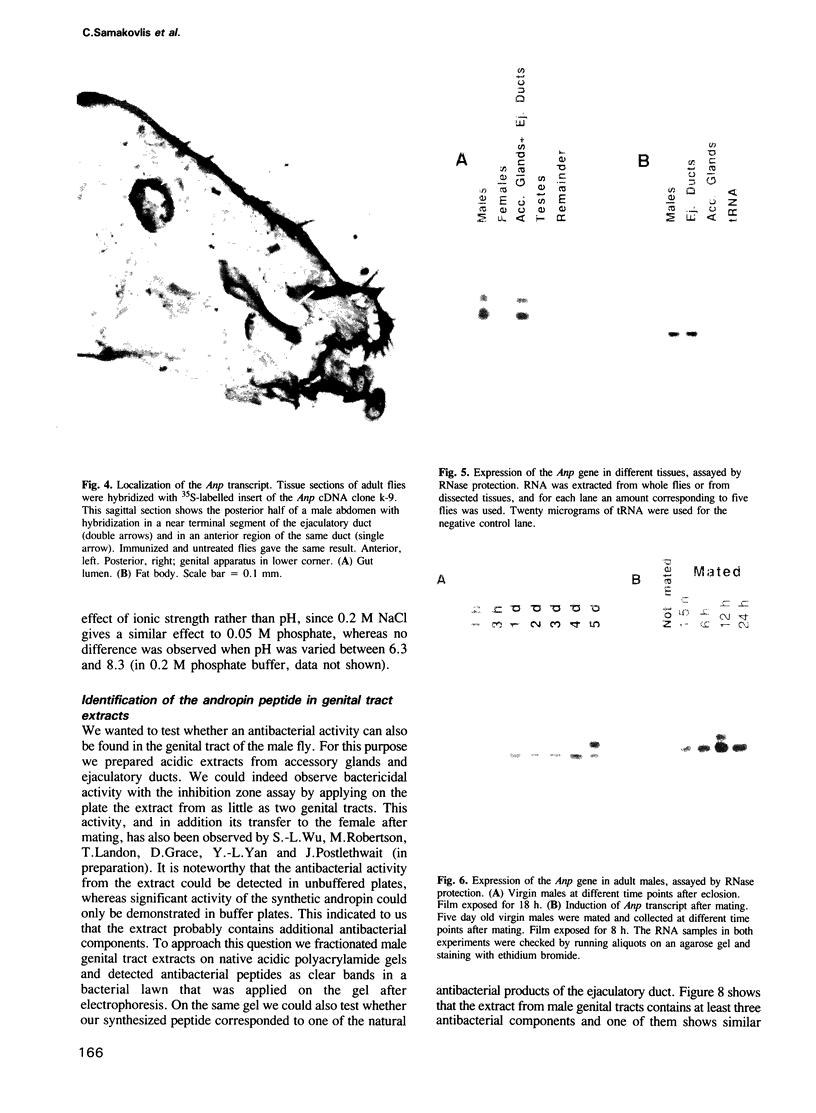
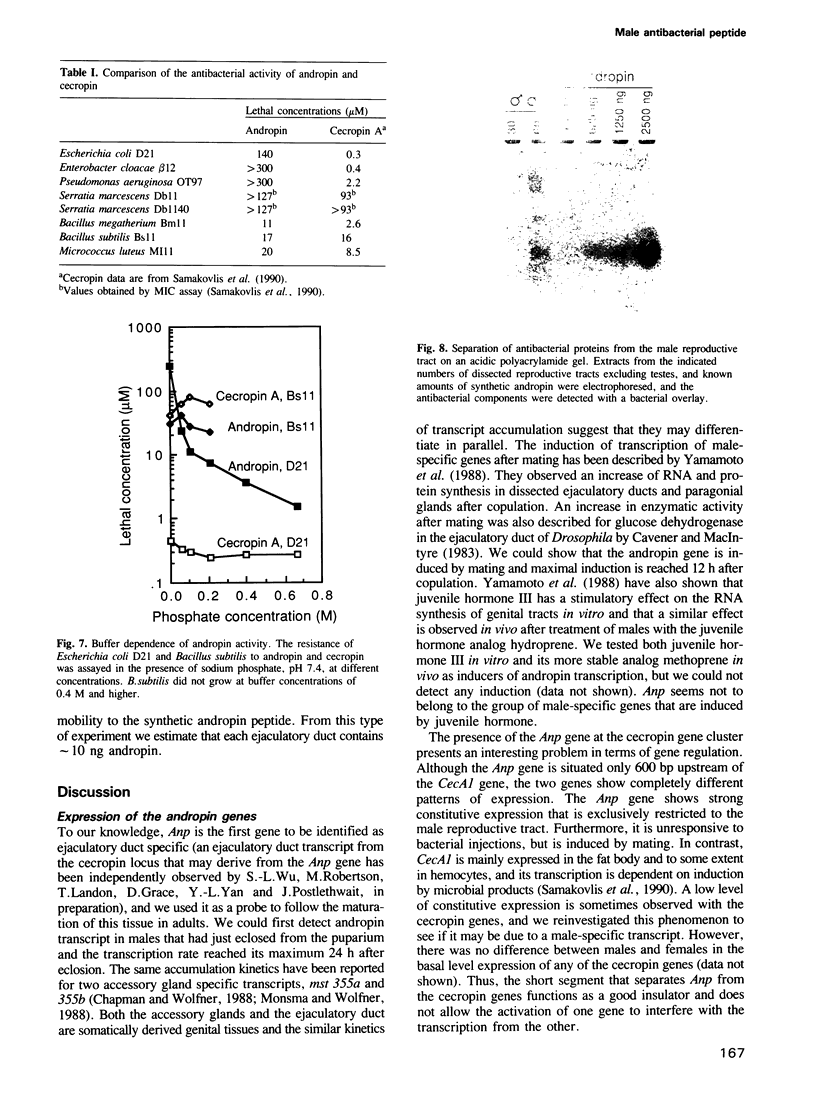


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. E., Martinez-Arias A. The distribution of Ultrabithorax transcripts in Drosophila embryos. EMBO J. 1985 Jul;4(7):1689–1700. doi: 10.1002/j.1460-2075.1985.tb03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. E. The location of Ultrabithorax transcripts in Drosophila tissue sections. EMBO J. 1983;2(11):2075–2084. doi: 10.1002/j.1460-2075.1983.tb01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Cavener D. R., MacIntyre R. J. Biphasic expression and function of glucose dehydrogenase in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6286–6288. doi: 10.1073/pnas.80.20.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K. B., Wolfner M. F. Determination of male-specific gene expression in Drosophila accessory glands. Dev Biol. 1988 Mar;126(1):195–202. doi: 10.1016/0012-1606(88)90253-9. [DOI] [PubMed] [Google Scholar]
- Chen P. S., Stumm-Zollinger E., Aigaki T., Balmer J., Bienz M., Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988 Jul 29;54(3):291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Ling N. C., Böhlen P. Microisolation of neuropeptides. Methods Enzymol. 1983;103:72–89. doi: 10.1016/s0076-6879(83)03007-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holak T. A., Engström A., Kraulis P. J., Lindeberg G., Bennich H., Jones T. A., Gronenborn A. M., Clore G. M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988 Oct 4;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem. 1982 Sep;127(1):207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Kreil G., Sures I. The genes for the frog skin peptides GLa, xenopsin, levitide and caerulein contain a homologous export exon encoding a signal sequence and part of an amphiphilic peptide. Eur J Biochem. 1989 Feb 1;179(2):281–285. doi: 10.1111/j.1432-1033.1989.tb14552.x. [DOI] [PubMed] [Google Scholar]
- Kylsten P., Samakovlis C., Hultmark D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990 Jan;9(1):217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma S. A., Wolfner M. F. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 1988 Sep;2(9):1063–1073. doi: 10.1101/gad.2.9.1063. [DOI] [PubMed] [Google Scholar]
- Richmond R. C., Gilbert D. G., Sheehan K. B., Gromko M. H., Butterworth F. M. Esterase 6 and reproduction in Drosophila melanogaster. Science. 1980 Mar 28;207(4438):1483–1485. doi: 10.1126/science.6767273. [DOI] [PubMed] [Google Scholar]
- Samakovlis C., Kimbrell D. A., Kylsten P., Engström A., Hultmark D. The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J. 1990 Sep;9(9):2969–2976. doi: 10.1002/j.1460-2075.1990.tb07489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Chadarevian A., Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988 Feb 19;239(4842):916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]