Abstract
α-2,9-Di-, tri-, tetra-, and pentasialic acids were prepared and conjugated with a carrier protein. The resultant glycoconjugates elicited robust T cell-mediated immunity in mice. α-2,9-Trisialic acid was identified as a promising antigen for developing glycoconjugate vaccines against group C Neisseria meningitidis.
Neisseria meningitidis is an important human pathogen and a major cause of bacterial meningitis and sepsis.1, 2 So far, 13 serogroups of N. meningitidis have been identified and are classified according to the structure of their cell surface capsular polysaccharides (CPSs).3, 4 Five of these strains, A, B, C, Y, and W135, are the most frequent causes of meningococcal diseases.5–7 In industrialized countries, groups B and C are mainly repressible for meningitis epidemics.8, 9 In developing countries in Asia and the “Africa meningitis belt”,10, 11 most infections are associated with group A, and the remaining cases in developing countries are caused by groups Y and W135.
For the control of endemic and epidemic meningitis, vaccination is considered an important and effective strategy.12 Regarding vaccine design, CPSs on the meningococcal cell surface are considered the ideal targets, as they are not only the major and the most exposed but also the most conserved components on bacterial cells owing to their important biological roles.13 The first CPS-based meningitis vaccine was developed by GSK, which was plain polysaccharide.14 However, polysaccharides typically induce only T cell-independent immunities with poor immunological memory, especially in infants and young children, and are thus not appropriate for sustained protection against infectious diseases.15 To address the issue, CPSs have been coupled with immunologically active carrier proteins, such as a diphtheria toxin mutant CRM197, to form conjugate vaccines that have exhibited improved efficiency and, more importantly, elicited T cell-dependent immunities. Glycoconjugate vaccines have been used for meningitis control.16 However, conjugate vaccines currently in clinical uses are composed of heterogeneous and easily contaminated natural CPSs that can barely meet modern quality and safety standards and demands.16 To overcome these limitations, conjugate vaccines made of synthetic carbohydrate antigens, which have defined structures, uncompromised purity and reproducibility, and free of bacterial contaminants, have received increasing attention.17–19 Vaccines composed of synthetic oligosaccharides also offer the opportunity to decipher their detailed structure-immunogenicity relationships to guide rational design and further optimization of antigenic epitopes for vaccine development.19 Consequently, we are interested in exploring anti-meningitis conjugate vaccines derived from synthetic oligosaccharide antigens.
The most characteristic CSP isolated from group C N. meningitidis is α-2,9-ploysialic acid with occasional and sporadic 8-O-acetylation (Figure 1). Reports have shown that while de-O-acetylation of this antigen could improve its immunogenicity, the provoked immune response could still recognize and kill the bacterium,20–22 thus current glycoconjugate vaccines against group C meningitis are composed of α-2,9-ploysialic acid free of O-acetylation. Accordingly, we designed and prepared a series of α-2,9-oligosialic acids without 8-O-acetylation and coupled them with a carrier protein to formulate glycoconjugate vaccines (Figure 1), which were evaluated in mice to analyze their structure-activity relationships. In this study, the carrier protein used was keyhole limpet hemocyanin (KLH), as it is inexpensive and easily accessible. Although KLH is not necessarily the ideal carrier protein for antibacterial vaccines, it is perfectly suitable for structure-activity relationship studies, as demonstrated in cancer vaccine research.23 In addition, the human serum albumin (HSA) conjugates of these α-2,9-oligosialic acids were also prepared and used as capture reagents for enzyme-linked immunosorbent assays (ELISA) of α-2,9-oligosialic acid-specific antibodies.
Figure 1.
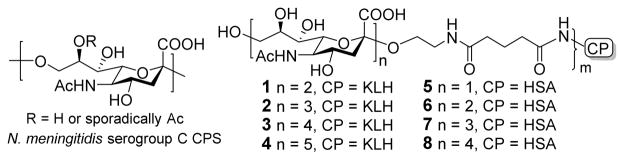
Structures of group C N. meningitidis CPS and the designed oligosaccharide antigens and their protein conjugates
Our first challenge in this study was to synthesize α-2,9-oligosialic acids having a reactive 2-aminoethyl group as an appendage at the reducing end to facilitate their coupling with carrier proteins. The synthesis of oligosialic acids is challenging because of the unique structure of sialic acid, e.g., the presence of an electron-withdrawing carboxyl group at and the quaternary property of its anomeric carbon and the absence of any participating neighbouring group at its C-3 position. These have impeded the reactivity of sialyl donors, affected the stereochemistry of glycosylation reactions, and allowed for side reactions. To address this issue, several creative synthetic strategies have been developed in recent years.24 Some of these strategies have been successfully used in oligo-sialic acid synthesis.25–30 Notably, an α-2,9-dodecasialic acid was effectively prepared with N5,O4-carbonyl-protected α-sialyl phosphate as a sialyl donor.31 However, most of the reported α-2,9-oligosialic acids were not completely deprotected and coupled with carrier proteins to form conjugate vaccines.
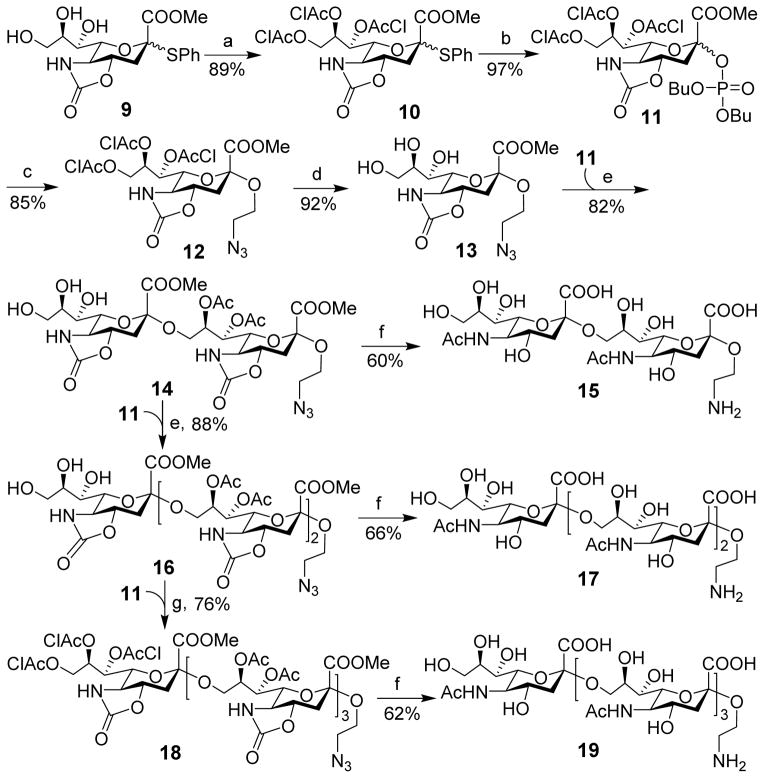
We planned to use in our synthesis the strategy with N5,O4-carbonyl-protected sialyl phosphates as donors due to its great success in the synthesis of oligosialic acids.31 Our synthesis of disialic acid 15, as shown in Scheme 1, was commenced with the preparation of 9 from sialic acid according to a reported procedure.29 It was then converted into the key building block, sialyl phosphate 11,31 as an α,β-mixture in two steps and an 86% yield. Rather than spending much effort on separating the two anomers, we probed the direct use of this mixture for sialylation. Delightfully, after exploring a series of conditions, we found that the reaction between 2-azidoethanol and 11 in a mixture of CH2Cl2 and CH3CN (2/1)32 at −78 °C to −40 °C with trimethylsilyl trifluoromethanesulfonate (TMSOTf) as the promoter was α-specific to give the desired anomer 12 exclusively in an excellent 85% yield. The anomeric configuration of 12 was proved as its 1H and 13C NMR data agreed well with that of reported similar structures.29, 32–34 This result suggested that both isomers of 11 could be activated and react with the glycosyl acceptor to give α-product. Next, the chloroacetyl (ClAc) groups in 12 were selectively removed with triethylamine (Et3N) in MeOH to produce triol 13. Taking advantage of the higher reactivity of the primary hydroxyl group than secondary hydroxyl groups in 13, it was directly used for sialylation with 11 under the above condition to furnish regioselective glycosylation. The product was acetylated and then de-O-chloroacetylated as described above to produce partially protected disialic acid 14 in an 82% yield in three steps. The newly formed α-sialyl bond in 14 was confirmed by comparing its NMR spectra with that of the reported.31 Moreover, the chemical shifts of its H-3eq signals (δ: 2.94 and 2.89 ppm) were consistent with the empirical rules about the anomeric configurations of N5,O4-carbonyl oligosialic acids described in the literature.29, 31, 35 The α,β-mixture of 11 as a sialyl donor was again very efficient and gave exclusively α-sialylation. In previous studies, usually pure α-sialyl phosphates were used as sialyl donors,31 as β-sialyl phosphates were shown to give low reactivity and α-selectivity,36, 37 especially in oligosialic acid synthesis.30, 31 We believed that the solvent used for the reaction might have a significant impact, as the reaction of 11 and 2-azidoethanol performed in pure dichloromethane gave a mixture (α/β 10:1). The partially protected disialic acid 14 was finally subjected to a series of reactions including deacylation with LiOH in MeOH/H2O, peracetylation with Ac2O, selective de-O-acetylation with NaOMe in MeOH, and then reduction of the azide group to obtain free disialic acid 15 in a 60% overall yield, which was purified by size exclusion column chromatography. The final product, as well as all synthetic intermediates, was fully characterized with 1D, 2D NMR and HR MS, which further confirmed the α-sialyl linkages in 15.
Scheme 1.
Synthesis of di-, tri, and tetrasialic acids 15, 17, and 19
Reagents and conditions: (a) ClCH2COCl, pyridine, CH2Cl2, 0 °C, 3 h; (b) dibutyl phosphate, NIS, TfOH, CH2Cl2, 0 °C, 12 h; (c) 2-azidoethanol, TMSOTf, CH2Cl2/CH3CN (2:1), −78 °C, 1 h; (d) Et3N, MeOH, rt, 10 min; (e) TMSOTf, CH2Cl2/CH3CN (2:1), −78 °C, 1 h; then AC2O, TfOH, CH2Cl2, 20 min; finally Et3N, MeOH, rt, 10 min; (f) LiOH, MeOH/H2O, reflux, 24 h; then NaHCO3, AC2O, H2O, rt, 3 h; then NaOMe, MeOH, rt, 24 h; finally Pd/C, H2, H2O, rt, 12 h. (g) TMSOTf, CH2Cl2/CH3CN (2:1), −78 °C, 1 h; then AC2O, TfOH, CH2Cl2, 20 min.
Trisialic and tetrasialic acids were prepared from disialic acid 14 by the same strategy (Scheme 1). Glycosylation of 14 with 11 followed by protecting group manipulation gave 16 in an excellent overall yield (88%). Compared to the reaction of 13, the longer sugar chain in 14 did not affect the efficiency of glycosylation. Thereafter, a part of 16 was deprotected to obtain free trisialic acid 17, and the remaining 16 was sialylated with 11 and acetylated to provide 18 in a 76% overall yield. Finally, 18 was deprotected by the above protocol to furnish free tetrasialic acid 19. Compounds 17 and 19 were characterized, and both sialylation reactions were α-selective.
For pentasialic acid synthesis (Scheme 2), we adopted a convergent [2+3] glycosylation strategy, rather than direct linear elongation of the sugar chain of 18. First, 9 was sialylated with 11 under the conditions established above to obtain disialic acid 20 that was then converted into sialyl phosphate 21 as a glycosyl donor. The coupling reaction between disialic acid donor 21 and trisialic acid acceptor 16 in the presence of TMSOTf was smooth, followed by O-acetylation to give 22 in a good yield (70%). Evidently, the size of sialyl donor did not significantly affect the glycosylation efficiency either. These results indicated that more complex oligosialic acids may be prepared via a convergent [n+n] or [n+m] strategy. Finally, 22 was deprotected as described above to furnish free pentasialic acid 23, which was fully characterized with 1D, 2D 1H and 13C NMR and HR MS.
Scheme 2.
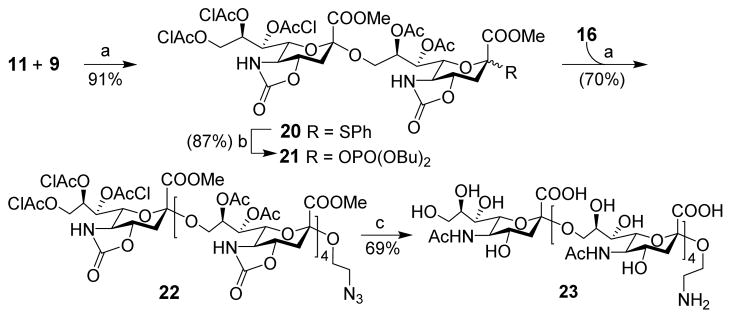
Synthesis of pentasialic acid 23
Reagents and conditions: (a) TMSOTf, CH2Cl2/CH3CN (2:1), −78 °C, 1 h, then AC2O, TfOH, CH2Cl2, 20 min; (b) dibutyl phosphate, NIS, TfOH, CH2Cl2, 0 °C, 12 h; (c) LiOH, MeOH/H2O, reflux, 24 h, and NaHCO3, AC2O, H2O, rt, 3 h, then NaOMe, MeOH, rt, 24 h, finally Pd/c, H2, H2O, rt, 12 h.
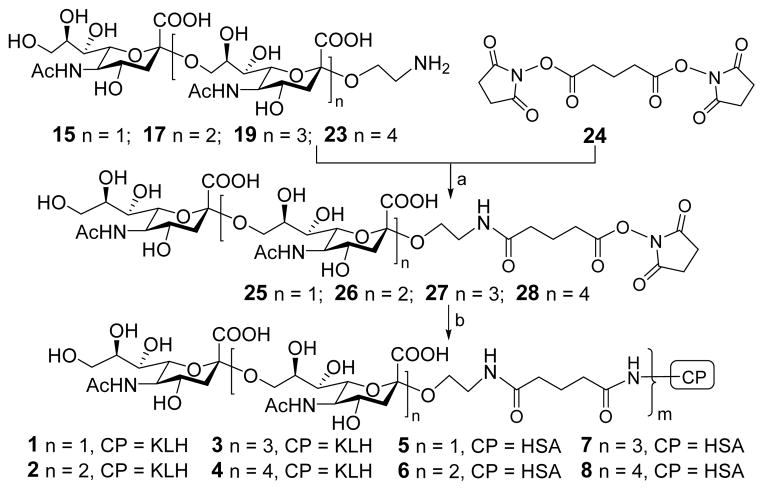
Once the oligosialic acids were available, they were conjugated with KLH and HSA via the bifunctional glutaryl linker (Scheme 3). This simple linker was selected because its conjugation reactions are easy and effective by means of activated glutaryl esters and it is not likely to affect the immunological property of resulting conjugates.38, 39 First, 15, 17, 19 and 23 were treated, respectively, with a large excess (15 equiv.) of disuccinimidal glutarate (DSG, 24) in a mixture of DMF and PBS buffer (4:1) to generate corresponding activated monoesters 25–28. Then, 25–28 reacted with KLH or HSA in 0.1 M PBS buffer to afford conjugates 1–8 that were purified by size exclusion column chromatography and then dialysis. According to our experience, the column chromatography is more effective than dialysis to remove the unreacted oligosaccharides. The sialic acid contents of the resultant glycoconjugates were determined by the Svennerholm method,40 and the results of HSA conjugates 5–8 were also validated with MS. The sialic acid loadings of 1–8 were 7.5–11.5% (Supporting Information), indicating that the conjugation reactions were efficient and that the antigen loading levels were in the desired range for glycoconjugate vaccines or for capture reagents used in ELISA.41
Scheme 3.
Conjugation of oligosialic acids with carrier proteins
Reagents and conditions: (a) DMF, PBS buffer (4:1), rt, 4 h; (b) KLH or HSA, PBS buffer, rt, 2.5 days.
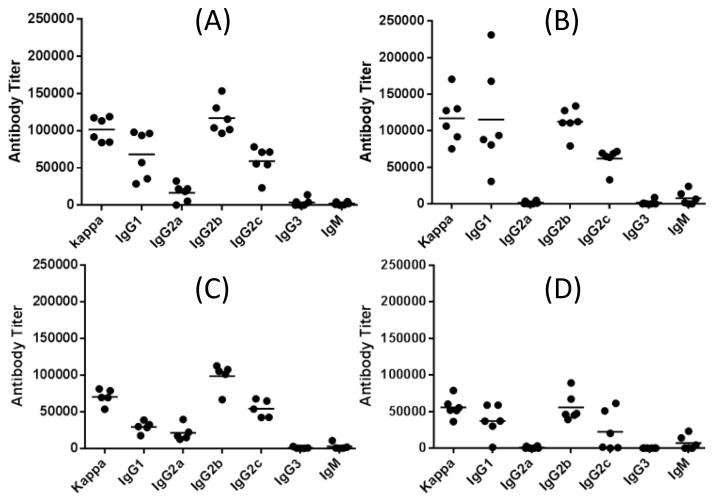
Immunological evaluations of glycoconjugates 1–4 were carried out with 5/6-week-old female C57BL/6J mouse. Each group of 5–6 mice was initially immunized through intramuscular (i.m.) injection of an emulsion (0.1 mL) of a conjugate (3 μg of sialic acid per injection) and the Titermax Gold adjuvant. Later, the mice were boosted three times by subcutaneous (s.c.) injection of the same conjugate on days 14, 21 and 28. Blood samples were collected from each mouse on day 0 before initial inoculation and on days 27 and 38 after boosting immunizations and were treated according to the standard protocols to prepare antisera that were studied by ELISA to determine antigen-specific antibodies with corresponding HSA conjugates 5–8 as capture reagents. Titers of total antibodies and individual antibody isotypes including IgG1, IgG2a, IgG2b, IgG2b, IgG3, and IgM were assessed. Antibody titer was calculated based on linear regression analysis of the curve obtained by drawing the ELISA optical density (OD) value against serum dilution number in logarithmic scale, and defined as the dilution number yielding an OD value of 0.2. Figure 2 gave the ELISA results of day 38 antisera obtained from mice inoculated with 1–4. All of the conjugates elicited high titers of antigen-specific total antibodies, indicating that they induced strong immune responses.
Figure 2.
ELISA results of various isotypes of antigen-specific antibodies in day 38 antisera of mice immunized with 1 (A), 2 (B), 3 (C) and 4 (D). Each black dot represents the antibody titer of an individual mouse, and the black bar shows the average antibody titer of a group of five or six mice.
The assessment of individual antibody isotypes revealed that all of the conjugates elicited mainly IgG1, IgG2b, and IgG2c antibodies (Figure 2) and only low levels of IgM antibodies were observed. In consistent with literature report that C57BL/6 mouse does not have the IgG2a gene but expresses the IgG2c isotype instead,42, 43 no significant level of IgG2a antibody was observed with the antisera. The production of IgG antibodies indicated the induction of T cell-mediated immunities and the switching of carbohydrate antigens from traditionally T cell-independent to T cell-dependent antigens through conjugation with a carrier protein.44 It was also reported that IgG antibody responses were associated with cellular immunity, long-term immunological memory, maturation of antibody affinity, and improved antibody-mediated cell or complement-dependent cytotoxicity,45, 46 which are important and desirable for prophylactic vaccines. The subclasses of IgG antibodies are defined according to their different Fc regions and differ in their ability to activate the immune system. It was reported that the activity hierarchy for IgG antibodies was: IgG2a ~ IgG2b > IgG1 ≫ IgG3.47 The incitement of high titers of IgG1, IgG2b, and IgG2c antibodies, the latter of which is allelic to IgG2a,48 by 1–4 suggested their likely protective activity against N. meningitidis. Moreover, among various subclasses of IgG antibodies, IgG2b and IgG2a are believed to be the most potent ones for the activation of effector response and antiviral immunity,45, 46 which further supports the protective activity of these conjugates as antibacterial vaccines.
Figure 2 also disclosed that 2 elicited a higher level of IgG1 antibody than 1, but their IgG2b and IgG2c antibody levels were similar. Both elicited significantly higher IgG1, IgG2b, and IgG2c antibody titers than 3 and 4. It was further revealed that the total IgG antibody titer for 2 was slightly higher than that for 1 and significantly higher than that for 3 and 4 (Figure 3). These results clearly suggested that the immunogenicity of the tested oligosialic acids followed the order of tri- > di- > tetra- > penta. Consequently, trisialic acid was identified as the most promising oligosialic acid antigen for the development of group C meningitis vaccines.
Figure 3.
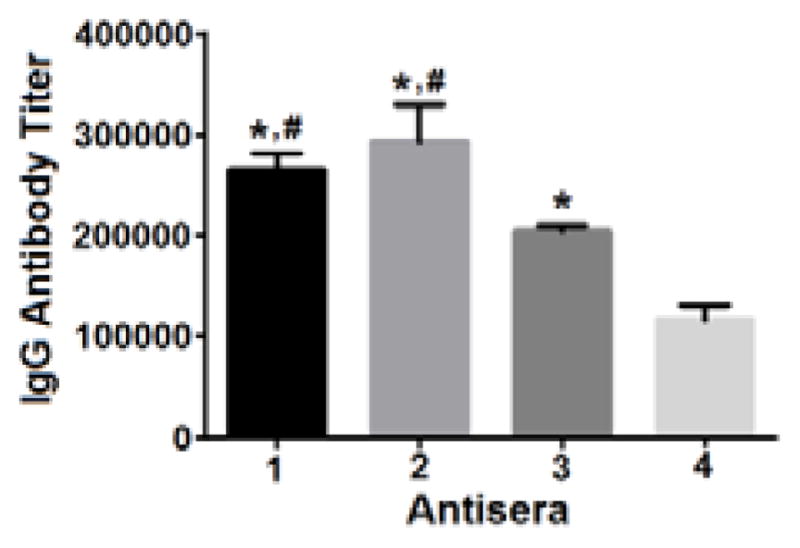
The average titers of antigen-specific total IgG antibodies in the day 38 antisera of individual mice inoculated with 1, 2, 3, and 4. Error bar shows the standard error of mean for each group of mice. The difference is statistically significant (P < 0.05) as compared to 4 (*) or 3 (#).
The next important question was whether the elicited antibodies or immunities could recognize and target group C N. meningitidis. This is directly related to the efficacy of the glycoconjugates as vaccines. To answer this question, we studied the binding between the antisera and group C N. meningitidis cell using normal mouse serum as the negative control. As shown in Figure 3, all of the antisera obtained from mice inoculated with 1, 2, 3 and 4 had very strong binding to N. meningitidis cell, but no significant binding to cells not expressing α-2,9-poly/oligosialic acids, although these cells carry sialoglycans. Moreover, the antisera did not bind to silaoglycans sTn, GM3, GM2, and α-2,8-linked polysialic acid either. These results indicated that the antibodies induced by 1, 2, 3 and 4 could specifically recognize and target α-2,9-linked polysialic acid and group C N. meningitidis.
Figure 3.
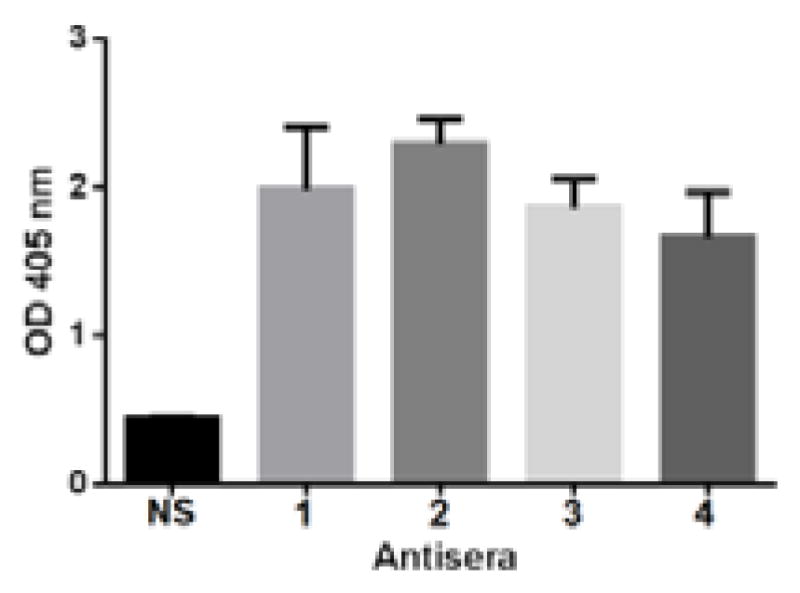
The results of binding assays of group C N. meningitidis cell with 1:100 diluted normal serum (NS) or 1:100 pooled antisera derived from mice immunized with 1, 2, 3 and 4. The error bar shows the standard deviation of three parallel experiments. The difference between NS and all of the antisera against 1–4 was statistically significant (P < 0.05).
In summary, α-2,9-di-, tri-, tetra- and pentasialic acid derivatives were efficiently synthesized and coupled with KLH. The immunological properties of the resulting glycoconjugates 1, 2, 3 and 4 were studied in mice. It was discovered that all of the conjugates elicited robust T cell-mediated immunities desirable for prophylactic vaccines. It was also found that the order of immunogenicity of the oligosialic acids was tri- > di- > tetra- > penta, suggesting that larger glycans are not necessarily better immunogens. To the best of our knowledge, this is the first systematic immunologic study of oligosialic acids, although several oligosialic acids were synthesized previously. It was further demonstrated that the elicited antibodies or immunities were specific to α-2,9-polysialic acid-expressing group C N. meningitidis cell. The binding of antibody to bacterial cell was very strong even with 1:100 and more diluted antisera, while usually original antisera were used for similar study in the literature.49 It was concluded that α-2,9-trisialic acid is a promising antigen for the development of functional vaccines for group C meningitis, and we are currently optimizing the carrier molecule for α-2,9-trisialic acid-based vaccines.
Supplementary Material
Acknowledgments
This work was supported by an NIH/NCI grant (R01 CA95142). We thank Dr. B. Ksebati (WSU) for some 2D NMR measurements. The animal protocol for this research was approved by WSU IACUC.
Footnotes
Electronic Supplementary Information (ESI) available: Synthetic procedures for oligosialic acids 15, 17, 19, and 23 and glycoconjugates 1–8; protocols for immunological and binding studies of 1–4 and for antigen loading analysis of 1–8 and related results; NMR spectra of 15, 17, 19, and 23 and intermediates; MS spectra of 5–8. See DOI: 10.1039/c000000x/
References
- 1.Stephens DS. FEMS Microbiol Rev. 2007;31:3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Khatami A, Pollard AJ. Expert Rev Vaccines. 2010;9:285–298. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 3.Girard MP, Preziosi MP, Aguado MT, Kieny MP. Vaccine. 2006;24:4692–4700. doi: 10.1016/j.vaccine.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Yogev R, Tan T. Hum Vaccin. 2011;7:828–837. doi: 10.4161/hv.7.8.16270. [DOI] [PubMed] [Google Scholar]
- 5.Morley SL, Pollard AJ. Vaccine. 2001;20:666–687. doi: 10.1016/s0264-410x(01)00410-8. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas P, Norheim G, Garnotel E, Djibo S, Caugant DA. J Clin Microbiol. 2005;43:5129–5135. doi: 10.1128/JCM.43.10.5129-5135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison LH, Mohan N, Kirkpatrick P. Nat Rev Drug Discov. 2010;9:429–430. doi: 10.1038/nrd3194. [DOI] [PubMed] [Google Scholar]
- 8.Harrison LH. Clin Infect Dis. 2010;50:S37–S44. doi: 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens DS, Greenwood B, Brandtzaeg P. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 10.Achtman M. Trends Microbiol. 1995;3:186–192. doi: 10.1016/s0966-842x(00)88918-0. [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, Clark TA, Memish ZA. J Travel Med. 2010;17:3–8. doi: 10.1111/j.1708-8305.2010.00448.x. [DOI] [PubMed] [Google Scholar]
- 12.Berti F, Adamo R. ACS Chem Biol. 2013;8:1653–1663. doi: 10.1021/cb400423g. [DOI] [PubMed] [Google Scholar]
- 13.Spinosa MR, Progida C, Tala A, Cogli L, Alifano P, Bucci C. Infect Immun. 2007;75:3594–3603. doi: 10.1128/IAI.01945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao PL, Chang LY, Hsieh SM, Chang SC, Pan SC, Lu CY, Hsieh YC, Lee CY, Dobbelaere K, Boutriau D, Tang HW, Bock HL, Huang LM. J Formos Med Assoc. 2009;108:539–547. doi: 10.1016/S0929-6646(09)60371-5. [DOI] [PubMed] [Google Scholar]
- 15.Broker M, Veitch K. Travel Med Infect Dis. 2010;8:47–50. doi: 10.1016/j.tmaid.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Astronomo RD, Burton DR. Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boltje TJ, Buskas T, Boons GJ. Nat Chem. 2009;1:611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peri F. Chem Soc Rev. 2013;42:4543–4556. doi: 10.1039/c2cs35422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anish C, Schumann B, Pereira CL, Seeberger PH. Chem Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, Cartwright K, Miller E. J Infect Dis. 2001;183:160–163. doi: 10.1086/317646. [DOI] [PubMed] [Google Scholar]
- 21.Borrow R, Longworth E, Gray SJ, Kaczmarski EB. Fems Immunol Med Mic. 2000;28:189–191. doi: 10.1111/j.1574-695X.2000.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 22.Richmond P, Borrow R, Findlow J, Martin S, Thornton C, Cartwright K, Miller E. Infect Immun. 2001;69:2378–2382. doi: 10.1128/IAI.69.4.2378-2382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Z, Huang X. J Carbohydr Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Meo C, Priyadarshani U. Carbohyd Res. 2008;343:1540–1552. doi: 10.1016/j.carres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Yu CS, Niikura K, Lin CC, Wong CH. Angew Chem Int Ed. 2001;40:2900–2903. doi: 10.1002/1521-3773(20010803)40:15<2900::AID-ANIE2900>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Tateno Y, Nishiura Y, Takahashi T. Org Lett. 2008;10:5597–5600. doi: 10.1021/ol802207e. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Nishiura Y, Takahashi T. J Org Chem. 2009;74:4383–4386. doi: 10.1021/jo900176e. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Nishiura Y, Takahashi T. J Am Chem Soc. 2006;128:7124–7125. doi: 10.1021/ja0613613. [DOI] [PubMed] [Google Scholar]
- 29.Lin CC, Lin NP, Sahabuddin LS, Reddy VR, Huang LD, Hwang KC, Lin CC. J Org Chem. 2010;75:4921–4928. doi: 10.1021/jo100824s. [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Huang KT, Lin CC. Org Lett. 2005;7:4169–4172. doi: 10.1021/ol0515210. [DOI] [PubMed] [Google Scholar]
- 31.Chu KC, Ren CT, Lu CP, Hsu CH, Sun TH, Han JL, Pal B, Chao TA, Lin YF, Wu SH, Wong CH, Wu CY. Angew Chem Int Edit. 2011;50:9391–9395. doi: 10.1002/anie.201101794. [DOI] [PubMed] [Google Scholar]
- 32.Crich D, Li WJ. J Org Chem. 2007;72:7794–7797. doi: 10.1021/jo7012912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crich D, Li WJ. J Org Chem. 2007;72:2387–2391. doi: 10.1021/jo062431r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Meo C, Farris M, Ginder N, Gulley B, Priyadarshani U, Woods M. Eur J Org Chem. 2008:3673–3677. [Google Scholar]
- 35.Hori H, Nakajima T, Nishida Y, Ohrui H, Meguro H. Tetrahedron Lett. 1988;29:6317–6320. [Google Scholar]
- 36.Plante OJ, Andrade RB, Seeberger PH. Org Lett. 1999;1:211–214. doi: 10.1021/ol9905452. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CH, Chu KC, Lin YS, Han JL, Peng YS, Ren CT, Wu CY, Wong CH. Chem Eur J. 2010;16:1754–1760. doi: 10.1002/chem.200903035. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Ekanayaka SA, Wu J, Zhang J, Guo Z. Bioconjugate Chem. 2008;19:2060–2068. doi: 10.1021/bc800243f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buskas T, Li Y, Boons GJ. Chem Eur J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 40.Svennerholm L. Biochim Biophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 41.Jennings HJ, Sood RK. Neoglycoconjugates, Preparation and Applications. Academic Press; San Diego, San Diego: 1994. [Google Scholar]
- 42.Martin RM, Silva A, Lew AM. Immunogenetics. 1997;46:167–168. doi: 10.1007/s002510050258. [DOI] [PubMed] [Google Scholar]
- 43.Martin RM, Brady JL, Lew AM. J Immunol Meth. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Fernandez A, Faro J, Fernandez C. Vaccine. 2008;26:292–300. doi: 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 45.Fossati-Jimack L, Ioan-Facsinay A, Reininger L, Chicheportiche Y, Watanabe N, Saito T, Hofhuis FM, Gessner JE, Schiller C, Schmidt RE, Honjo T, Verbeek JS, Izui S. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mond JJ, Lees A, Snapper CM. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Ravetch JV. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Goldschmidtb T, Salter H. Mol Immunol. 2012;50:169–171. doi: 10.1016/j.molimm.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Wang CH, Li ST, Lin TL, Cheng YY, Sun TH, Wang JT, Cheng TJ, Mong KK, Wong CH, Wu CY. Angew Chem Int Ed. 2013;52:9157–9161. doi: 10.1002/anie.201302540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.