Abstract
Objective
Inflammation is associated with the formation of aortic aneurysm. This study investigates the role of Cys-X-Cys chemokine receptor (CXCR)3 and its ligands, in the pathogenesis of arterial aneurysms.
Methods
Plasma samples from patients with or without a diagnosis of thoracic aortic aneurysms were analyzed by ELISA for the Th1 cytokine interferon (IFN)-γ as well as the IFN-γ-inducible CXCR3 ligands, IP-10, I-TAC, and Mig. Patient charts were reviewed for demographics, initial aortic diameter, and growth rates. Aneurysm diameter and growth rates were correlated with plasma cytokine and chemokine levels using linear regression analysis. We used an animal model of aneurysm formation, where calcium chloride is applied topically to carotid arteries of wild type and CXCR3−/− mice. After 10 weeks, the arteries were harvested and analyzed by histology and immunohistochemistry.
Results
Patients with thoracic aortic aneurysms had significant elevations in circulating IFN-γ, IP-10, I-TAC, and Mig compared to referent patients (p<0.001). Cytokine and chemokine plasma levels did not correlate with aneurysm size or growth rates. CXCR3−/− mice were protected from aneurysm formation and showed decreased vascular infiltration by CD45+ leukocytes.
Conclusions
Elevated plasma levels of IFN-γ and CXCR3-binding chemokines are present in patients with thoracic aortic aneurysms. The CXCR3 receptor is necessary for vascular inflammation and the formation of arterial aneurysms in mice.
Keywords: aortic diseases, cytokines, chemokines, interferon-γ
Aortic aneurysms may cause severe complications or death as a consequence of progressive enlargement, dissection, and rupture of the vessel wall.1 Analyses of clinical specimens and experimental models have led to an increased understanding of the role of inflammation in the pathogenesis of aneurysmal disease where medial degeneration and vascular cell death in aneurysmal aortas is associated with leukocytic infiltrates.2–4 The recruitment of leukocytes into the aortic media is promoted by chemokines and elastin degradation peptides whereas activated macrophages and T cells within the aortic wall produce pro-inflammatory factors that contribute to aortic wall injury and expansion.5–8 Activated T cells differentiate from naïve precursors into different effector populations characterized by the production of particular cytokines, including that of Th1 cells which secrete their signature cytokine, interferon (IFN)-γ. Chemokines, such as IP-10, I-TAC and Mig, are induced by IFN-γ and bind to and activate the Cys-X-Cys chemokine receptor (CXCR3), preferentially expressed by Th1 cells (Figure 1). We have previously reported that Th1-type immune responses are associated with human thoracic aortic aneurysms; specifically, transmural aortic inflammation correlates with increased aneurysm diameter and is characterized by activated T cells, increased expression of IFN-γ, IP-10 and Mig, and the selective recruitment of CXCR3-expressing Th1 cells.9 The importance of Th1 immune responses in the development of abdominal aortic aneurysms has been demonstrated in IFN-γ−/− mice using a model of topical calcium chloride application and in patients with abdominal aortic aneurysm where serum levels of INF-γ are elevated.10,11 These clinical and experimental data indicate that Th1-type immune responses are important in the pathogenesis of aortic aneurysms and argue against the hypothesis that arterial enlargement result from Th2-dominant immune responses.12
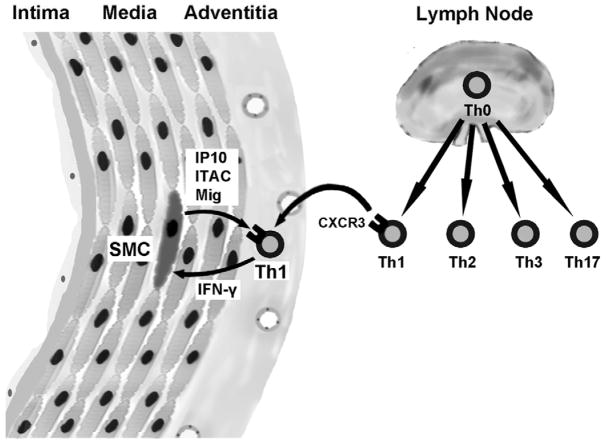
Figure 1.
Schema of Th1 immune responses and the IFN-γ cytokine axis in arterial immune responses. Activation of naïve T cell precursors, or Th0 cells, occurs in draining lymph nodes of the aorta and/or peri-adventitial tertiary lymphoid organs that organize around chronically inflamed arteries. The cytokine milieau at the time of cognate recognition of antigen skews the development of CD4+ effector T cells into different lineages characterized by the polarization of secreted cytokines, viz. IFN-γ-producing Th1 cells, IL-4-producing Th2 cells, IL-10-producing Th3 cells, and IL-17-producing Th17 cells. Recirculating T cells reach the vessel wall via the luminal endothelium or from microvessels of the adventitia and occasionally outer media of the thoracic aorta. Th1 cells preferentially express the chemokine receptor, CXCR3 and their recruitment, retention, and activation is augmented by the IFN-γ-inducible CXCR3 ligands, IP-10, Mig, and ITAC that are robustly produced by vascular cells, such as medial smooth muscle cells (SMC). The positive feedback loop of IFN-γ-inducing factors, IFN-γ, and IFN-γ-inducible chemokines is referred to as the IFN-γ cytokine axis.
An improved understanding of the role of cytokines and chemokines in aneurysm pathophysiology may lead to novel therapies. To determine the role of CXCR3 chemokines in aneurysmal diasease we measured their plasma levels in patients with thoracic aortic aneurysm and we examined the formation of arterial aneurysm in CXCR3−/− mice.
METHODS
Patients
Patients with thoracic aortic aneurysms and referent controls were enrolled at VA Connecticut Healthcare Systems, West Haven, CT, between January 2005 and April 2006. The sizes of the thoracic aortic aneurysms were measured by computed tomography. Histories were obtained from chart reviews and interviews performed by an investigator who was blinded to the results of plasma evaluations. Subjects with a history of chronic infection, who were immunocompromised, or taking oral corticosteroids were excluded from the study. Referent patients of similar age and gender to the experimental group, but without a known diagnosis of aortic aneurysms were randomly enrolled at the West Haven VA primary care clinic. The referent patients did not undergo imaging studies to definitively excluded aortic aneurysm. Peripheral blood samples were collected in EDTA tubes, the plasma was separated by centrifugation and the specimens were stored at −80 °C until analyzed. Informed consent was obtained on all study participants. The research was approved by the Institutional Review Boards.
ELISA
Measurements of IFN-γ, IP-10, I-TAC, and Mig were performed using ELISA kits according to the manufacturer’s directions (R&D Systems). The lower limit of detection (i.e., the lowest concentration of cytokine standard used to construct a reference line) was 15.6 pg/ml for IFN-γ and Mig, 7.8 pg/ml for IP-10 and 1.9 pg/ml for I-TAC.
Mice
CXCR3−/− mice were obtained from Paul Noble, M.D. (Yale University) and used at 8–12 weeks of age. These animals have been previously described in detail13 and were crossed onto a C57BL/6J background for more than five generations. Age and sex-matched wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animal experiments were approved by the Institutional Animal Care and Use Committee at Yale University.
Model of aneurysm formation
Aneurysms were induced in the carotid artery by topical application of calcium chloride, as previously described.14 Briefly, mice were anesthetized and the right carotid artery was accessed through a midline anterior neck incision. Calcium chloride (0.5 M) was applied to the entire length of the right carotid artery for 15 minutes and then the vessel was irrigated with 0.9% sterile saline. The left carotid was then exposed and 0.9% sterile saline was similarly applied. The incision was closed. At 10 weeks post-operational, the mice were sacrificed by cardiac perfusion with saline followed by 4% paraformaldehyde. The right and left carotid arteries were harvested en bloc, as a single specimen attached to the aorta, and then fixed in 10% formalin for 3 hours and then in 15% glucose overnight. The carotid arteries were then placed in parallel in Tissue-Tek O.C.T. Compound (Miles, Inc., Elkhard, Ind).
Morphometry
Transverse sections of the carotid arteries over 5 mm length were stained with hematoxylin and eosin. From these sections, the internal and external elastic lamina perimeters were measured using Image 1.62c software (Scion, Frederick, MD). Vessel area was calculated from perimeter measurements assuming that the vessels were circular in shape. The medial thickness was determined by measuring four separate areas of each vessel and averaging these measurements.
Immunhistochemistry
Formalin-fixed and O.C.T. embedded carotid artery specimens were sectioned at a thickness of 8 μm. The sections were incubated with monoclonal antibodies to mouse: CD45, F4/80, CD3 (Abcam, Cambridge, MA), IgG, and α-actin (Sigma). Binding of secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were detected with peroxidase/3-amino ethyl carbazole kits (Vector, Burlingame, CA).
Statistical analysis
Statistical analyses were performed using Prism version 4 (GraphPad Software, San Diego, CA) statistical software. Subject characteristics were compared using Chi-square or Fisher’s exact test for proportions. Mean comparisons between two groups and multiple groups were performed respectively with a Student’s t-test and one-way ANOVA. Correlation coefficients were calculated using Pearson correlation. P values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Twenty-six patients with thoracic aortic aneurysms and 54 reference patients were enrolled. The two groups had similar age, gender and race and had similar risk factors and diagnosis of atherosclerosis (Table 1).
Table 1.
Clinical characteristics of human subjects without and with thoracic aortic aneurysms.
| Controls (n=54) | Aneurysms (n=26) | P value | |
|---|---|---|---|
| Age (years) | 68.4±10.5 | 68.8±12.4 | 0.89 |
| Gender (male/female) | 50(93%)/4(7%) | 23(88%)/3(12%) | 0.67 |
| Race (caucasian/other) | 21(81%)/5(19%) | 49(91%)/5(9%) | 0.28 |
| Risk factors for atherosclerosis | |||
| Diabetes | 12(22%) | 5(19%) | 0.76 |
| Hyperlipidemia | 14(26%) | 4(15%) | 0.29 |
| Hypertension | 30(56%) | 17(65%) | 0.47 |
| Current smoker | 5(9%) | 4(15%) | 0.42 |
| Diagnosis of atherosclerosis* | 4(7%) | 4(15%) | 0.27 |
includes coronary artery disease, cerebrovascular disease, peripheral vascular disease Data are means±SD or N (% of total). P values for comparison between controls and aneurysm subjects calculated by Fisher exact test or Chi-square for proportions and non-paired t-test for means. P<0.05 considered significant.
The average aneurysm size was 5.03 cm with the majority of aneurysms located in the ascending thoracic aorta (81%). Open surgical replacement was performed in 38% of patients with aneurysm, while the remaining patients were either not surgical candidates due to aneurysm size or medical comorbidities, or refused surgical intervention. Patients who did not have operative repair were followed with serial radiologic imaging. Growth rates were calculated for patients with multiple imaging modalities over time. Expansion rates ranged from 0 to 5.2 cm/year with a mean of 0.69±1.4 cm/yr.
Elevated circulating IFN-γ and IFN-γ-inducible CXCR3 ligands in aneurysm patients
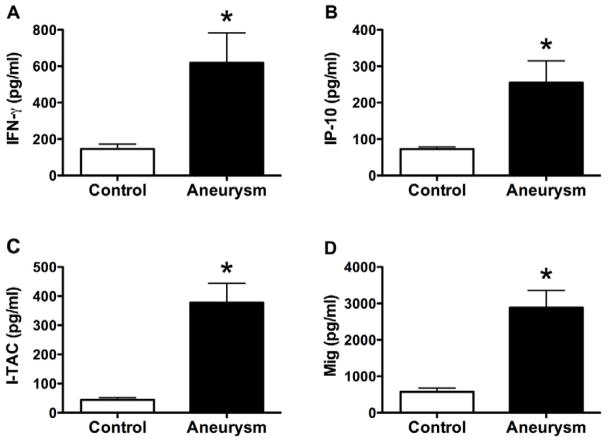
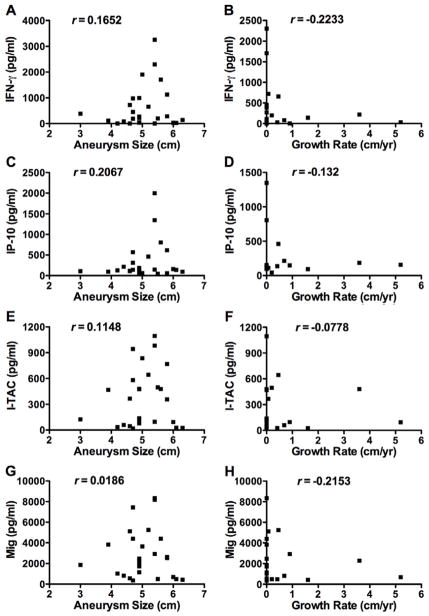
To determine whether circulating markers of adaptive immunity were present in patients with thoracic aortic aneurysms, we measured plasma levels of IFN-γ and the IFN-γ-inducible CXCR3 ligands, IP-10, I-TAC, and Mig. Patients with thoracic aortic aneurysms had elevated plasma levels of IFN-γ, IP-10, I-TAC, and Mig compared to reference patients (Figure 2). No patients with aneurysms had undetectable I-TAC plasma levels (<1.9 pg/ml), while 47% of control patients had undetectable I-TAC levels. All patients with aneurysms had elevated plasma levels of at least one of the four circulating pro-inflammatory factors and 96% of aneurysm patients had elevated plasma values of two of the four cytokine/chemokines. There was no significant correlation between plasma levels of IFN-γ or any of the CXCR3 ligands with aneurysm diameter or growth rate (Figure 3). These results demonstrate that circulating chemokines are increased in patients with ascending aortic aneurysm but the degree of elevation does not correlate with either aneurysm size or its rate of growth, perhaps the more appropriate function to assess for vascular responses to inflammatory stimuli.
Figure 2.
Circulating chemokines in serum of humans with and without aortic aneurysms. (A), IFN-γ; (B), IP-10; (C), I-TAC; and (D), Mig plasma levels in pg/ml as measured by ELISA. Values are mean±SEM, *P<0.001.
Figure 3.
Circulating chemokines in patients with aortic aneurysms do not correlate with aneurysm diameter or growth rate. Scatter plots of IFN-γ (A, B), IP-10 (B, C), I-TAC (D, E), and Mig (F, G) plasma levels with aneurysm diameter (left panels) or growth rates (right panels). Growth rates were determined for patients with serial images (73%, n=19). Cytokine and chemokine levels are expressed in pg/ml as measured by ELISA. r is the correlation coefficient calculated using Pearson correlation.
CXCR3 is required for arterial aneurysm formation in mice
We used a well described mouse model of aneurysm formation to determine whether CXCR3 ligands play a role in the early pathogenesis of aneurysmal disease14. Using topical application of calcium chloride, we evaluated aneurysm formation and growth in WT vs. CXCR3−/− mice over 10 week period (Figures 4A). WT mice showed significant aneurysm formation in treated common carotid arteries, and the vessel area increased by 47%. However, CXCR3−/− mice did not demonstrate any aneurysm formation in response to calcium chloride treatment (Figure 4B). CXCR3−/− mice had slightly larger carotid arteries at baseline compared to WT mice; however, this was not statistically significant. There was no difference in the thickness of the media in any of the groups.
Figure 4.
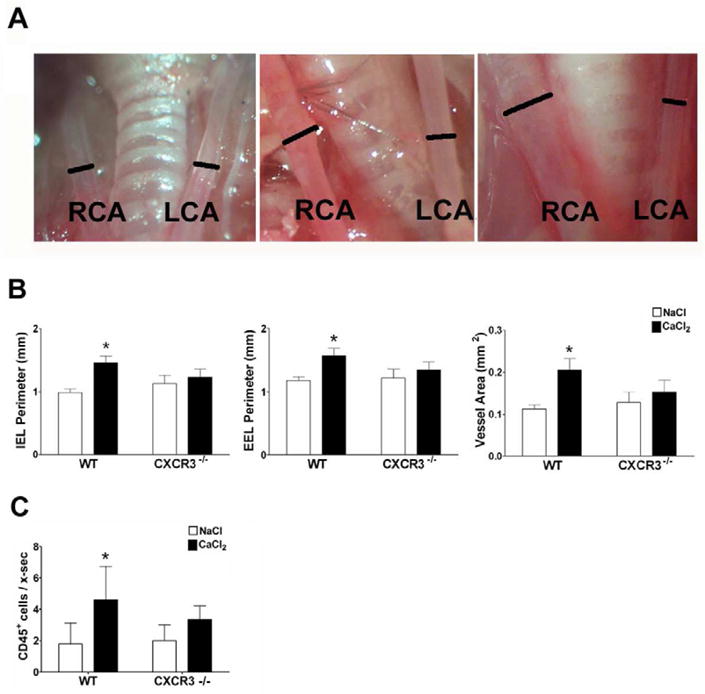
CXCR3−/− mice do not develop aneurysms. (A) Gross photographs of aneurysm development in calcium chloride-treated right common carotid arteries vs. saline-treated left common carotid arteries in WT mice at day 0, day 28, and day 70 (right to left panels), bars represent artery diameter. (B) Bar graphs showing the average IEL perimeters, EEL perimeters, and vessel area of the calcium chloride-treated (black bars) and saline-treated (white bars) carotid arteries. WT, n=8; CXCR3−/−, n=8. Values are mean±SEM. *P<0.05. (C) Infiltrating CD45+ leukocytes in calcium chloride-treated vs. saline-treated carotid arteries in WT and CXCR3−/− mice were detected by immunohistochemistry and bar graphs represents the mean number of CD45+ cells per artery cross-section. WT, n=6; CXCR3−/−, n=5. Values are mean±SEM. *P<0.05.
To determine whether inhibition of aneurysm formation in CXCR3−/− mice was associated with reduced inflammation, we examined the carotid arteries of WT and CXCR3−/− mice for the presence of inflammatory cells. There were increased number of infiltrating cells that expressed the pan-leukocyte marker CD45+ in the arteries of WT animals treated with calcium chloride vs. sodium chloride; however, there was no significant increase of CD45+ cells in treated arteries of CXCR3−/− mice (Figure 4C). The inflammatory cells were localized to the adventitia, without any infiltration of the media and consisted of both F4/80+ macrophages and CD3+ T cells (data not shown). This demonstrates that CXCR3 is required for recruitment and/or retention of CD45+ leukocytes, initiation of inflammatory response within the vessel wall and subsequent formation of arterial aneurysm.
DISCUSSION
We demonstrate that patients with thoracic aortic aneurysms have elevated plasma levels of circulating IFN-γ as well as the IFN-γ-inducible chemokines, IP-10, ITAC, and Mig compared to referent subjects. To extend these descriptive observations, we find that CXCR3, the cognate receptor for IP-10, ITAC, and Mig, is necessary for calcium chloride-induced aneurysm formation in mice. These results suggest that Th1-type immune responses and their effector chemokines are essential in the pathogenesis of the disease.
A role for inflammation in aneurysm progression and rupture has been established.15 In particular, the Th1 immune response has been identified by a number of groups as a critical determinant of pathogenesis of human aortic aneurysms.9,16 However, downstream effectors of the Th1 response that are involved in aneurysm pathogenesis have not been previously reported. We previously reported that IFN-γ and IFN-γ-inducible CXCR3 ligands co-localize in the walls of thoracic aortic aneurysms.9 Here we extend these observations and identify elevated plasma levels of circulating IFN-γ, IP-10, I-TAC, and Mig in patients with thoracic aortic aneurysms. It has been previously reported that cytokines such as IL-1β, IL-6, and TNF-α are elevated in the serum of patients with abdominal aortic aneurysms compared to patients with coronary artery disease and vascular disease-free controls.10,17 IFN-γ was also found to be elevated in the serum of women with abdominal aortic aneurysm.10 We were unable to detect a correlation between plasma levels of IFN-γ-inducible chemokines with either aneurysm diameter or growth rate, two potential surrogate measurements of disease severity. Lack of correlation could simply reflect variation in human subjects in a relatively small study population; in fact there is no documentation of cytokines whose levels correlate with aneurysm diameter or expansion rate.10 In addition, circulating levels may not reflect tissue levels or activity. On the other hand, the lack of correlation suggests an advanced disease state of human aneurysms once clinically diagnosed and these chemokines may be responsible for early initiation rather than progression of the disease. Arguably, we may have increased the power of our study by including patients with aneurysms at any arterial location. There are several similarities between thoracic and aortic aneurysms regarding leukocytic infiltrates of the vessel wall predicting similar vascular responses to inflammatory stimuli.2–6 However, distinct differences have been documented in their pathogenesis, e.g. Th1-dominant immune responses in thoracic aortic aneurysms vs. Th2-dominant immune responses in abdominal aortic aneurysms.9,12 We therefore focused our study on thoracic aortic aneurysms exclusively to avoid confounding factors and variability between disease processes. An additional limitation of this study pertains to the control group. The referent patients were not known to have aneurysmal disease, but this was not definitely excluded by imaging studies. However, in a population-based study the incidence of thoracic aortic aneurysm was 5.9 per 100,000 person-year and is therefore unlikely to be present in our randomly selected control group.18
Since elevated circulating CXCR3 ligands in patients with aneurysmal disease may reflect late stage disease or co-existent atherosclerotic disease, we also examined an animal model of aneurysm formation to test our hypothesis that CXCR3 ligands are essential in the initiating phases of aneurysm pathogenesis. Lack of aneurysm formation in CXCR3−/− mice support that hypothesis and is consistent with previous reports that the Th1-type responses play a role in aneurysm formation.9,11 In addition, our finding of inflammatory and chemokine involvement in aneurysm formation using the calcium chloride model of aneurysm formation agrees with findings of the elastase model.19 These results suggest the importance of inflammation and Th1-type immune responses in the pathogenesis of aneurysmal disease and support a role of CXCR3 in aneurysmal diseases. Previous reports using similar animal model, revealed no attenuation of abdominal aortic aneurysm formation in CXCR3−/− mice.20 Pathogenesis of thoracic and abdominal aortic might be different or site-specific, since ascending thoracic aneurysms are associated with preservation, not loss, of medial smooth muscle cells.21 Different segments of the aorta have different embryological origin where ascending aorta and arch are of neural crest origin but more distal part correspond to the embryological dorsal aorta. Our animal model of aneurysm formation used the carotid arteries, which may have different results than in the aorta. However, similar results have been reported in the aorta and carotid arteries and carotid arteries are of embryologically similar derivation as thoracic aorta but not abdominal aorta.22–24 Other studies have documented a role for IFN-γ in calcium chloride-induced aneurysms of the abdominal aorta.11 Additionally, deletion of certain chemokine receptors, such as CCR2 prevented aortic aneurysm formation in the same murine model.20 One problem with extrapolating results from knockout murine models to clinical disease is that genetic deletion strategies affect the development of the immune system, whereas pathological conditions are generally acquired during adult life.
We conclude that Th1-type immune responses are essential in the pathogenesis of aortic aneurysms and that modulation of these immune responses may attenuate aneurysm progression and complications. Detection of circulating chemokines may represent a sensitive serologic assay of underlying local arterial inflammation through amplification of T cell-derived factors on vascular cell responses.
Acknowledgments
Funding source: NIH (PO1 HL70295).
Footnotes
Disclosures: No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–91. doi: 10.1016/S0022-5223(97)70360-X. [DOI] [PubMed] [Google Scholar]
- 2.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–8. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.He R, Guo DC, Sun W, Papke CL, Duraisamy S, Estrera AL, et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg. 2008;136:922–9. doi: 10.1016/j.jtcvs.2007.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C. Characterization of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 5.Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–61. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- 6.Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–6. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 7.Busuttil RW, Abou-Zamzam AM, Machleder HI. Collagenase activity of the human aorta. A comparison of patients with and without abdominal aortic aneurysms. Arch Surg. 1980;115:1373–8. doi: 10.1001/archsurg.1980.01380110105016. [DOI] [PubMed] [Google Scholar]
- 8.Newman KM, Ogata Y, Malon AM, Irizarry E, Gandhi RH, Nagase H, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14:1315–20. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 9.Tang PC, Yakimov AO, Teesdale MA, Coady MA, Dardik A, Elefteriades JA, et al. Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J. 2005;19:1528–30. doi: 10.1096/fj.05-3671fje. [DOI] [PubMed] [Google Scholar]
- 10.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–7. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 11.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–12. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–8. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pimiento JM, Maloney SP, Tang PC, Muto A, Westvik TS, Fitzgerald TN, et al. Endothelial nitric oxide synthase stimulates aneurysm growth in aged mice. J Vasc Res. 2008;45:251–8. doi: 10.1159/000112940. [DOI] [PubMed] [Google Scholar]
- 15.Treska V, Kocova J, Boudova L, Neprasova P, Topolcan O, Pecen L, et al. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell Mol Ther. 2002;7:91–7. doi: 10.1080/13684730310001652. [DOI] [PubMed] [Google Scholar]
- 16.Galle C, Schandene L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, et al. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol. 2005;142:519–27. doi: 10.1111/j.1365-2249.2005.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson J, Cockerill G, Choke E, Loftus I, Thompson MM. Circulating cytokines in patients with abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:324–6. doi: 10.1196/annals.1383.010. [DOI] [PubMed] [Google Scholar]
- 18.Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, et al. Thoracic aortic aneurysm: a population-based study. Surgery. 1982;92:1103–8. [PubMed] [Google Scholar]
- 19.Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, et al. Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg. 2006;43:1010–20. doi: 10.1016/j.jvs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.MacTaggart JN, Xiong W, Knispel R, Baxter BT. Deletion of CCR2 but not CCR5 or CXCR3 inhibits aortic aneurysm. Surgery. 2007;142:284–8. doi: 10.1016/j.surg.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Tang PC, Coady MA, Lovoulos C, Dardik A, Aslan M, Elefteriades JA, et al. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112:1098–105. doi: 10.1161/CIRCULATIONAHA.104.511717. [DOI] [PubMed] [Google Scholar]
- 22.Chiou AC, Chiu B, Pearce WH. Murine aortic aneurysm produced by periarterial application of calcium chloride. J Surg Res. 2001;99:371–6. doi: 10.1006/jsre.2001.6207. [DOI] [PubMed] [Google Scholar]
- 23.Gertz SD, Kurgan A, Eisenberg D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J Clin Invest. 1988;81:649–56. doi: 10.1172/JCI113368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majesky MW, Dong XR, Topouzis S. Smooth muscle cell diversity and the extracellular matrix in a rat model of restenosis. P R Health Sci J. 1996;15:187–91. [PubMed] [Google Scholar]