1. Introduction
Over the past decade, the age-adjusted incidence rates of breast and cervical cancer have decreased from 134.7 to 123.1 and 9.7 to 7.9 per 100,000 women, respectively (U.S. Department of Health and Human Services, 2012). Data suggest that screening offers an opportunity for early detection and treatment of breast and cervical cancer thus decreasing health disparities (Howlader N, et al., 2012; Kohler, et al., 2011). In fact, efforts such as the CDC-sponsored National Breast and Cervical Cancer Early Detection Program and a multitude of smaller-scale programs have been successful in increasing cancer screening (Tangka, et al., 2010) and eliminating screening disparities by race (Klabunde, et al., 2012; National Center for Health Statistics, 2011; Swan, et al., 2010). In 2010, the percentage of U.S. women 50 years and older who reported having a mammogram in the past two years was 72.4%, and the percentage of women 21 years and older who had a Papanicolaou (Pap test) in the past three years was 83.0% (Klabunde, et al., 2012).
Despite these gains, significant disparities in cancer screening by socioeconomic status (SES) persist. Women with lower incomes and fewer years of education are less likely to comply with recommended cancer screening guidelines (National Center for Health Statistics, 2011; Smith, Cokkinides, & Brawley, 2012). For example, in 2010 only 58.3% of women, age 40 and older, with less than a high school education reported having a mammogram in the past two years compared to 69.5% and 80.8% of those with high school and college degrees, respectively (Klabunde, et al., 2012). Population-based studies have also shown that women with lower household incomes and less than a college education were significantly less likely to have mammograms (Dailey, et al., 2011; Welch, Miller, & James, 2008). A similar picture emerges for cervical cancer screening: 69.4% of women, age 21 and older, with less than high school diploma reported having a Pap test in the past three years, compared to 77.7% and 89.0% of those with a high school diploma and with some college, respectively (Klabunde, et al., 2012). Further, the risk of dying from cancer has been found to be higher for breast cancer patients living in low SES communities (Yu, 2009). Likewise, women with invasive cervical cancer who live in poorer areas tend to have significantly shorter survival (36.9 months) than those living in more affluent areas (52.8 months) (Brookfield, Cheung, Lucci, Fleming, & Koniaris, 2009).
Interpersonal factors such as social relationships and social support also affect screening behaviors (Allen, Sorensen, Stoddard, Peterson, & Colditz, 1999; Allen, Stoddard, & Sorensen, 2008; Fite, Frank, & Curtin, 1996; Gamarra, Paz, & Griep, 2009; Kang, Bloom, & Romano, 1994; Silva, Griep, & Rotenberg, 2009; Suarez, et al., 2000). Social support has been shown to protect health (Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997; Cohen & Lemay, 2007) and promote healthy behaviors. Studies have found that some aspects of social support are directly related to cancer screening. Emotional/information support and positive social interaction have been shown to be associated with annual mammogram screening (Messina, et al., 2004), and both positive social interaction (Silva, et al., 2009) and emotional support (Gamarra, et al., 2009) have been associated with Pap test screening. Additionally, unspecified social support has been associated with mammogram screening (Fite, et al., 1996). Other studies found Pap test screening had no relationship with emotional/informational (Silva, et al., 2009), emotional (Kang, et al., 1994), or tangible (Kang, et al., 1994; Silva, et al., 2009) support. Finally, additional studies reported no relationship between mammogram screening and affectionate (Messina, et al., 2004), emotional (Kang, et al., 1994), tangible (Kang, et al., 1994; Messina, et al., 2004) or general social support (Allen, et al., 1999; Allen, et al., 2008). Because the evidence is somewhat mixed, there is a need for a better understanding of the associations between cancer screening and social support.
Social support may help to mitigate the effects of SES on health outcomes, what Vitaliano and colleagues (2001) call the “added value hypothesis.” This hypothesis states that the health promoting effects of social support may be modified by income or other resources. That is to say, those with the least resources may benefit most from social support. Applying this hypothesis to cancer screening, Messina and colleagues (2004) conducted a population-based, cross-sectional study of 55,278 postmenopausal women from 40 areas of the U.S. They found that there was a direct association between emotional-informational support and repeated participation in mammography screening. When testing for interactions between social support and SES, they found that, for women who were caregivers, emotional-informational support was significantly associated with repeated mammography among women with lower household incomes versus among women with higher household incomes. However, this study did not include data on Pap tests.
Clarifying the relationship between social support and screening is difficult because of the methodological heterogeneity across studies in measurement of social support, and the timeframe used to measure screening outcomes. Additionally, many studies have focused on mammograms (Allen, et al., 1999; Allen, et al., 2008; Fite, et al., 1996; Kang, et al., 1994; Katapodi, Facione, Miaskowski, Dodd, & Waters, 2002; Messina, et al., 2004), but few have examined the relationship between social support and Pap testing (Allen, et al., 1999; Gamarra, et al., 2009; Kang, et al., 1994; Silva, et al., 2009).
In sum, the role of social support in increasing breast and cervical cancer screening remains unclear. The objectives of this study were to a) determine if an association exists between social support and compliance with mammography and Pap test screening guidelines; and b) test whether social support moderates the effect of education on breast and cervical cancer screening. The hypotheses were: 1) that women who reported greater social support would be more likely to receive screening according to guidelines; and 2) that greater social support would be more strongly associated with screening among women with lower education.
2. Methods
Population
The data for this analysis were derived from the Allegheny County Health Survey (ACHS) (Documet, Bear, & Green, 2012), a county-wide, population-based telephone survey modeled after the CDC-sponsored Behavioral Risk Factor Surveillance System (BRFSS) (Holtzman, 2003). The ACHS dataset has a total of 5,442 interviews, conducted in 2009–2010, with a cooperation rate of 66.1%. The ACHS was approved by the University of Pittsburgh IRB and verbal informed consent was obtained from all participants.
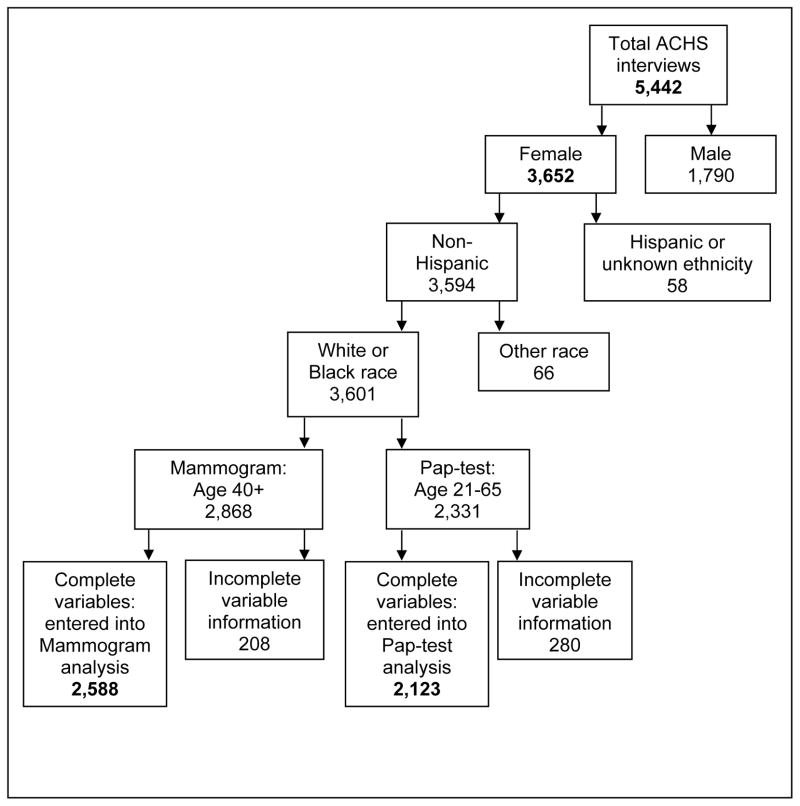
There were 3,652 women in the ACHS. The analysis included only non-Hispanic White or non-Hispanic Black women. There were 66 women who were “other race” and 58 who were either “Hispanic” or of an unknown ethnicity. They were excluded from the analysis because they do not constitute a homogeneous group. Furthermore, the small numbers (n=124) would have likely produced unreliable estimates. The population for this study was limited to women 40 and older for mammograms and to 21 to 65 years for analyses related to Pap test (Figure 1).
Figure 1.
Allegheny County Health Survey (ACHS) Participants and Number of Participants Entered in the Analysis
Variables
Compliance was determined based on screening guidelines in effect at the start of data collection (U. S. Preventive Services Task Force, 2010). Participants (women aged 40 and older) were classified as compliant if they reported having a mammogram in the past two years. This was assessed by asking participants “have you ever had a mammogram?” If they answered yes, they were asked “How long was it since your last mammogram?” Those who reported “Within the past year (anytime less than 12 months ago)” or “More than one year, but less than 2 years ago” were considered compliant. The questions about cervical cancer screening followed the same pattern. Women aged 21 to 29 were considered compliant with Pap test guidelines if they reported having had a Pap test in the past year or having a Pap test in the past three years if aged 30 to 65.
The independent variables were perceived social support and education. Social support was measured using a standardized scale with good internal consistency reliability (alpha=0.83) (Gjesfjeld, Greeno, & Kim, 2007). This 4-item scale was derived from a longer scale that has been widely used in other studies (Sherbourne & Stewart, 1991). This scale asks: “How often is each of the following kinds of support available to you when you need it?” The scale contains questions related to emotional-informational, tangible, and affectionate support as well as positive social interaction. The five-point, Likert scale response categories range from “none of the time” to “most of the time.” Reponses to the 4 items were summed to create a composite score with possible scores ranging from 0 to 16 and higher scores indicating higher social support. Social support scores in this sample were highly skewed to the left, thus social support was dichotomized into low and high social support. The bottom quartile defined low social support (score ≤11) and the upper three quartiles defined high social support (score >11).
SES was measured using education as a proxy, (LaVeist, 2005) categorized as: “less than or equal to a high school diploma,” “some college,” and “college graduate or more.” Education rather than household income was selected to avoid bias, as income was missing for a large percentage of participants eligible for the mammogram (18% missing income) and Pap test (11% missing income) analyses. Education was missing for less than 1% of respondents.
Variables were considered for inclusion in the model because they could be related both to social support and to screening and thus could potentially confound the relationship among them. Candidate variables included age (continuous variable) (Messina, et al., 2004; Welch, et al., 2008), race (white or black) (Messina, et al., 2004), health insurance (yes/no) (Messina, et al., 2004; Welch, et al., 2008), and having a regular health care provider (yes/no) (Messina, et al., 2004). Variables were included in multivariate analyses only if they were found to be associated in bivariate analysis with screening compliance and social support (p < .25) (Hosmer & Lemeshow, 2000). All potential confounders tested were included in the model because their association with the outcomes in bivariate analysis was not likely due to chance (p<0.25).
Statistical Analysis
Data analyses took place in 2012–2013. Cross-tabulations of the data served to identify potential cells with zero counts that would require data recoding (Hosmer & Lemeshow, 2000).
For the multivariate analyses, only records with complete data for all variables were included (Figure 1). For each screening outcome (mammogram and Pap test), a multiple regression was performed to test the association of social support and compliance with screening guidelines (Hypothesis 1), and models were adjusted for confounders identified in the bivariate analyses. To test the moderating effect of social support on the relationship between education and screening compliance (Hypothesis 2), an interaction term multiplying social support and education was added. In multivariate analysis, a p-value of <0.05 was considered significant. All analyses were conducted using the statistical package SAS 9.2; PROC SURVEY FREQ for cross-tabulations and PROC SURVEY LOGISTIC for logistic regressions to allow for proper consideration of required weights. The reference categories were “low social support” for hypothesis 1 and “an education less than or equal to high school diploma” for hypothesis 2. No adjustments were made for multiple comparisons due to the exploratory nature of this study.
3. Results
A total of 2,588 participants qualified for the mammogram analysis, and 2,123 for the Pap test analysis (Figure 1). Table 1 summarizes the characteristics of the final sample by compliance status.
Table 1.
Education, Social Support and Demographic Characteristics of the Sample by Screening Compliance (Percentage in Parentheses)
| Variable | Mammogram screening (n = 2,588)
|
Pap test screening (n = 2,123)
|
||
|---|---|---|---|---|
| Compliant | Not compliant | Compliant | Not compliant | |
| Education | ||||
| High school or less | 783 (72.20) | 292 (27.80) | 470 (75.41) | 145 (24.59) |
| Some college | 541 (74.52) | 174 (25.48) | 503 (80.60) | 119 (19.43) |
| College graduate | 643 (79.72) | 155 (30.28) | 780 (87.55) | 106 (12.45) |
| Social support | ||||
| Low | 565 (69.62) | 225 (30.38) | 460 (74.26) | 134 (25.34) |
| High | 1,402 (76.93) | 396 (23.07) | 1,293 (84.00) | 236 (16.00) |
| Age | ||||
| 21 – 29 | 132 (71.48) | 46 (28.54) | ||
| 30 – 39 | 334 (89.83) | 32 (10.17) | ||
| 40 – 49 | 347 (72.20) | 131 (27.80) | 413 (85.72) | 65 (14.28) |
| 50 – 59 | 549 (77.31) | 147 (22.69) | 551 (79.45) | 138 (20.55) |
| 60 – 69 | 489 (79.37) | 121 (20.63) | 313 (75.490 | 89 (23.51) |
| 70+ | 582 (70.81) | 222 (29.19) | ||
| Race | ||||
| White | 1,593 (74.46) | 518 (25.54) | 1,317 (81.04) | 262 (18.96) |
| Black | 374 (76.12) | 560 (23.88) | 436 (83.30) | 88 (16.70) |
| Health insurance | ||||
| No | 63 (50.24) | 61 (49.76) | 109 (60.00) | 66 (40.00) |
| Yes | 1,904 (76.12) | 560 (23.88) | 1,644 (83.56) | 304 (16.44) |
| Regular healthcare provider | ||||
| No | 58 (41.27) | 77 (58.73) | 118 (68.38) | 55 (31.62) |
| Yes | 1,909 (76.75) | 544 (23.25) | 1,635 (82.71) | 315 (17.29) |
Hypothesis 1: Association between social support and screening guidelines
Social support was significantly related to mammogram screening compliance in multivariate logistic regression after controlling for age, race, having a regular health care provider and insurance status (Adjusted OR 1.43; 95% CI=1.16, 1.77) (Table 2). Social support was also associated with Pap test compliance (Adjusted OR 1.71; 95% CI=1.27, 2.29). Education was also significant in the models, with college graduates being more likely to report compliance with mammogram and Pap test guidelines than those with less than or equal to a high school education.
Table 2.
Association of Social Support and Breast and Cervical Cancer Screening Compliance: Results of Multivariate Regression
| Variable | Breast Cancer Screening
|
Cervical Cancer Screening
|
||
|---|---|---|---|---|
| Adjusted OR [95% CI] | p | Adjusted OR [95% CI] | p | |
| Number of records in analysis | 2,588 | 2,123 | ||
| Social support a | 1.43 [1.16, 1.77] | <.001 | 1.74 [1.30, 2.34] | <.001 |
| Education b | ||||
| Some college | 1.07 [0.84, 1.36] | .58 | 1.26 [0.91, 1.74] | .17 |
| College graduate | 1.41 [1.09, 1.83] | .04 | 1.48 [1.06, 2.34] | <.001 |
Note. All analyses were adjusted for age, race, health insurance status and having a regular health care provider. OR = odds ratio; CI = confidence interval.
Reference group: low (<=11 on a 4-item social support scale).
Reference group: high school or less
Hypothesis 2: Social support as a moderator of the relationship between education and screening compliance
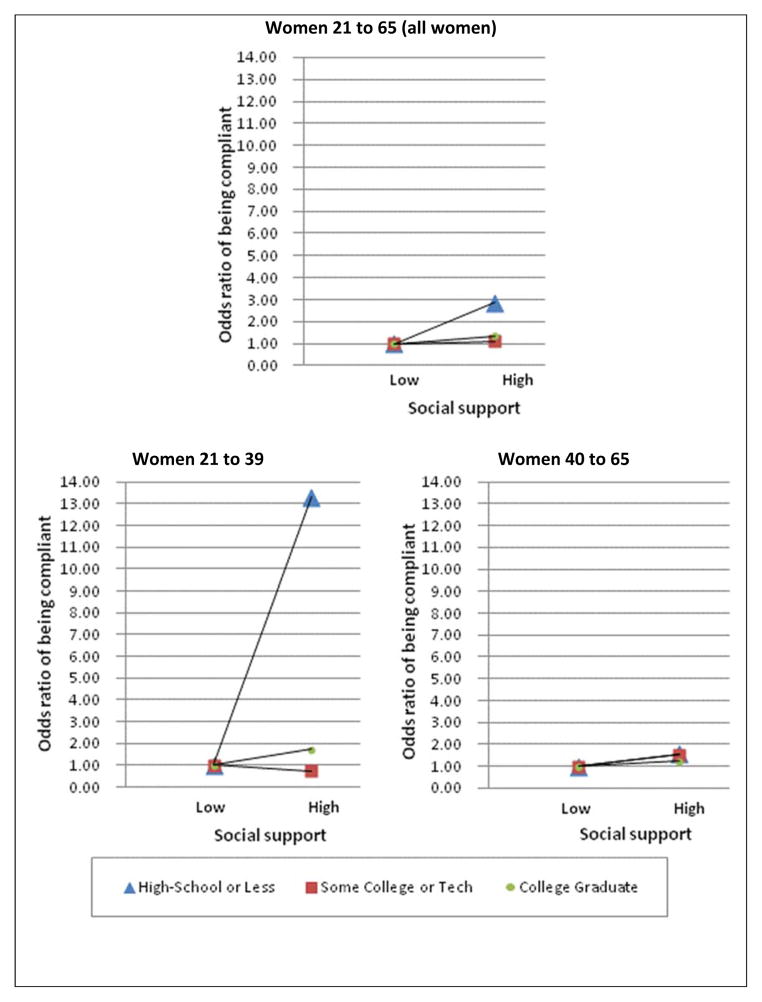
The interaction between social support and education had no significant effect on mammogram compliance. For Pap test compliance, there was a significant interaction between education and social support (p<0.05), with the effect of social support being more pronounced for women with less than or equal to a high school education than for more educated women. Among women with education less than or equal to high school diploma, those with high social support were 2.85 times (CI: 1.81, 4.49) more likely to be compliant with Pap test screening when compared to those with low social support. However, high social support did not make a difference for more educated women (Table 3).
Table 3.
Odds Ratios [and 95% Confidence Intervals] for Pap Test Compliance Split by Age Group for High Social Support Compared to Low Social Support by Level of Education
| Education | Adjusted OR [CI] | p |
|---|---|---|
| Age 21 to 65 a | ||
| High school or less | 2.85 [1.81,4.49] | <.001 |
| Some college | 1.12 [0.69,1.87] | .64 |
| College graduate | 1.36 [0.74,2.49] | .32 |
|
| ||
| Age 21 to 39 b | ||
| High school or less | 13.04 [4.21,42.04] | <.001 |
| Some college | 0.726 [0.23,2.24] | .58 |
| College graduate | 1.729 [0.51,5.82] | .38 |
| Age 40 to 65 b | ||
| High school or less | 1.55 [0.94,2.53] | .08 |
| Some college | 1.52 [0.89,2.58] | .12 |
| College graduate | 1.22 [0.71,2.10] | .47 |
Note. All analyses were adjusted for age, race, health insurance status and having a regular health care provider. Base was low social support. OR = odds ratios; CI = confidence intervals.
Original model.
Post-hoc analysis.
In view of the lack of significant effect on mammogram compliance, which is studied only with older women, it was considered that age might affect the way social support interacts with education. Therefore, post-hoc analyses were conducted for Pap test compliance: one model included only women 21 to 39 years of age and the other model included women 40 years and older to mirror the approximate age of the population in the mammogram analysis. Results showed that in the stratified analysis, the interaction between social support and education was significant only for the younger women (Table 3 and Figure 2).
Figure 2.
Adjusted odds ratios of Pap test compliance with interactions between education and social support, all sample and by age group.
4. Discussion
The results show that social support is associated with mammogram and Pap test compliance in multivariate analysis, confirming the first study hypothesis. This work builds on previous findings by Messina and colleagues (2004) regarding mammogram screening. To the authors’ knowledge, this is the first study showing that Pap tests are associated with social support in a population-based sample from the U.S. Two other studies have found similar results with social support and cervical cancer screening; however, their findings were based on limited sample sizes (Silva, et al., 2009) and from developing countries (Gamarra, et al., 2009; Silva, et al., 2009). Results regarding the second study hypothesis suggest that social support significantly moderates the relationship between education and Pap test compliance, but not for mammogram compliance. Furthermore, this moderation effect only holds for women younger than 40 years.
Different aspects of social support have been found to be associated with screening. In one U.S. study, an association was found between annual mammogram screening and emotional/informational support and positive social interaction (Messina, et al., 2004). In addition, having a lower level of positive social interaction was associated with lower odds of having a repeat mammogram for women who were caregivers. In a Brazilian study, Pap test screening was associated with reporting positive social interaction and affective support (Silva, et al., 2009). In both studies, no association was found between screening and tangible support.
Reasons for the potentially protective effects of social support on women’s cancer screening compliance are unclear. First, it has been suggested that social support could act as a buffer and may help to diminish the negative influence of stressful events (Cohen & Janicki-Deverts, 2009). Social support may help to relieve the stress associated with cancer screening as well as provide the necessary resources that encourage screening behaviors. Second, according to the “main effect” model, social support may affect health and health behaviors via a more direct pathway (Cohen & Janicki-Deverts, 2009). Social relationships may directly influence screening behavior via social norms or peer pressure. Aside from the main effect, others have theorized that social support could influence cancer screening by increasing knowledge, which, in turn may increase screening by providing women with a more accurate personal risk perception and by helping them to overcome barriers to screening (Katapodi, et al., 2002). Finally, it has been suggested that the health and health care habits of individuals with lower SES may benefit more from social support because it may be directly related to helping them overcome financial and educational barriers, as well as increasing access to social capital (Messina, et al., 2004; Suarez, et al., 2000; Vitaliano, et al., 2001).
There is a limited understanding of how social support interventions can be used to improve cancer screening compliance. Cohen and Janicki-DeVertis contend that natural social networks may hold the most promise for social support interventions (2009), yet they caution that public health professionals may find it challenging to activate such networks. Examples of interventions activating the social networks include two interventions (one individual and one group-based) aimed to increase mammogram screening by improving social support and knowledge (Paskett, et al., 1999; Slater, et al., 1998). One of the interventions utilized lay health advisors who reached out to African American, Native American and White women (Paskett, et al., 1999). The lay health advisors delivered social support and individual health education through three home visits and phone calls. Mammograms in the intervention group were 56% more frequent than in the control group. The other intervention aimed at increasing peer pressure and social support by organizing party-like events and small group discussions in public housing buildings (Slater, et al., 1998). Education about mammograms was also provided as were screening vouchers to uninsured women. Even though there was no significant increase in knowledge in either study, screening was significantly higher in the intervention groups. None of these studies formally measured social support, yet the results suggest that a potential reason for these findings may be due to the main effect of social support. Future research focusing on clarifying the effect of this mechanism is warranted.
The divergent results regarding Hypothesis 2 were puzzling. There was support for the added value hypothesis value hypothesis—i.e. that social support provides more benefits to those who are more disadvantaged only for Pap test yet not for mammogram. After separating the data by age, only younger women received an extra benefit from social support for Pap test screening. In a population-based study, Messina et al. (2004) found support for this hypothesis in a subgroup of postmenopausal women who were caregivers: emotional/informational support interacted with income to increase the likelihood of having an repeated mammogram. To our knowledge, this hypothesis has not been investigated in a population-based study with Pap tests as the outcome. One possibility is that the influence of social support varies over the lifespan, resulting in different effect from younger an older women (Wrzus, Hanel, Wagner, & Neyer, 2013). It is also possible that there are non-individual factors related to SES that were not addressed in the present study, such as residential area, that may influence breast and cervical cancer screening (Brookfield, et al., 2009; Dailey, et al., 2011; Pruitt, Shim, Mullen, Vernon, & Amick, 2009). This study only addresses the effects of individual-level factors. The finding that Pap test screening is associated with social support among younger women adds to the relatively small body of literature that indicates that social relationships could be important for cervical cancer screening compliance. Further study of the “added value hypothesis” in the context of Pap test screening is warranted.
This study has several strengths and limitations. Among the strengths is the inclusion of a large, population-based sample, with 18 to 24% of African Americans for each analysis. Other studies have used non-representative samples (i.e., mostly white, relatively affluent women) (Allen, et al., 1999; Allen, et al., 2008), only African Americans (Kang, et al., 1994), or non-population-based samples (Allen, et al., 1999; Allen, et al., 2008; Fite, et al., 1996; Katapodi, et al., 2002; Silva, et al., 2009). Further, to counter the bias introduced by using only landline telephone lines, the data were weighted for probability of selection, non-response and non-coverage.
Another strength is the use of a standardized scale to measure social support. In the past, while some studies used standardized scales (Gamarra, et al., 2009; Messina, et al., 2004; Silva, et al., 2009), others relied on non-standardized scales (Katapodi, et al., 2002), or subsets of scales (Allen, et al., 1999; Allen, et al., 2008). Furthermore, other studies measured only one dimension of support (Gamarra, et al., 2009) or the measure was not specified (Kang, et al., 1994). Measurement issues have been further complicated by how social support was defined--sometimes in terms of a supportive close relationship (Fite, et al., 1996), a supportive provider (Fite, et al., 1996), or family members and friends (Allen, et al., 1999; Kang, et al., 1994).
Finally, this study uses clear guidelines for measuring screening compliance, and the guidelines selected were based on those in effect at the time of data collection. In other studies, various timeframes for screening have been used as outcomes, often timeframes not directly related to screening guidelines, and have been not stated. Timeframes for mammograms included having one every two years (Allen, et al., 1999; Allen, et al., 2008) or annually in the past five years (Messina, et al., 2004), or it was measured on a scale as having a mammogram: “never” “once or twice” and “every one or two years” (Katapodi, et al., 2002). For Pap tests, studies have measured whether adult women, regardless of age, had a Pap test in the past three (Gamarra, et al., 2009), or past two years (Silva, et al., 2009). In at least one article the timeframe was not stated (Kang, et al., 1994). This is important because screening recommendations tend to vary over time and according to the organization issuing the recommendation. The recent changes in the United States Prevention Services Taskforce (USPSTF) recommendations (Moyer, 2012; U. S. Preventive Services Task Force, 2010) have also made it difficult to make comparisons across studies.
The main design limitation is that cross-sectional data does not permit analyses to determine causal inferences. Additionally, the brevity of the social support scale did not allow for exploration of the effect of different aspects of social support on screening. Finally, the use of education, which is only one aspect of SES, was a limitation. Even though education is a widely used proxy for SES (LaVeist, 2005; Oakes & Rossi, 2003), it is an incomplete measure of SES. In fact, authors debate what is the best measure of SES (Oakes & Rossi, 2003). It is possible that results would have been somewhat different if a composite of income, education and occupation had been available from the data.
Implications for practice
The evidence base for the use of social support to increase cancer screening is still emerging. Nevertheless, health educators and practitioners should consider individual or group-based interventions that activate natural social support networks as they may help to increase both breast and cervical cancer screening compliance. Benefits of social support interventions aimed at women with low education may be particularly effective in increasing Pap test screening. One promising way to increase social support includes the use of community health workers (CHW). CHW include lay individuals as well as semi-professionals (Love, Gardner, & Legion, 1997) and their use is currently encouraged by the Affordable Care Act (Plescia & White, 2013; Plescia, Wong, Pieters, & Joseph, 2014). CHW can better connect women to cancer screening services. If CHW are lay individuals who belong to the community they serve, they can be in a preferential position to harness the power of the natural social networks (Fisher, et al., 2014). Faith-based or community-based organizations, especially those based in low-income neighborhoods, may be able to use their existing rapport with community members to encourage screening through education and peer support (Elliott, Belinson, Ottolenghi, Smyth, & Belinson, 2013; Shirazi, Shirazi, & Bloom, 2013). Additionally, interventions in non-traditional health promotion venues such as beauty salons, where there is an established clientele, may prove to be successful. For example, interventions in these settings have addressed nutrition, cancer screening, and cardiovascular disease through education and social support (Kleindorfer, et al., 2008; Linnan & Ferguson, 2007; Wilson, et al., 2008). Some of these interventions have increased knowledge and intention (Kleindorfer, et al., 2008; Wilson, et al., 2008) Other options for innovative interventions that take advantage of natural social networks would include working with groups that provide non-professional social support, such as walking groups or disease-specific online support groups (e.g., for diabetics). Select members of these groups could be tapped as health champions to promote breast and cervical cancer screening among their peers. Finally, all of the potential interventions mentioned above may be strengthened by the utilization of a community-based participatory approach (Plescia & White, 2013; Shirazi, et al., 2013).
References
- Allen JD, Sorensen G, Stoddard AM, Peterson KE, Colditz G. The relationship between social network characteristics and breast cancer screening practices among employed women. Ann Behav Med. 1999;21(3):193–200. doi: 10.1007/BF02884833. [DOI] [PubMed] [Google Scholar]
- Allen JD, Stoddard AM, Sorensen G. Do social network characteristics predict mammography screening practices? Health Educ Behav. 2008;35(6):763–776. doi: 10.1177/1090198107303251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield KF, Cheung MC, Lucci J, Fleming LE, Koniaris LG. Disparities in survival among women with invasive cervical cancer: a problem of access to care. Cancer. 2009;115(1):166–178. doi: 10.1002/cncr.24007. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. Jama. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Can We Improve Our Physical Health by Altering Our Social Networks? Perspect Psychol Sci. 2009;4(4):375–378. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Lemay EP. Why would social networks be linked to affect and health practices? Health Psychol. 2007;26(4):410–417. doi: 10.1037/0278-6133.26.4.410. [DOI] [PubMed] [Google Scholar]
- Dailey AB, Brumback BA, Livingston MD, Jones BA, Curbow BA, Xu X. Area-level socioeconomic position and repeat mammography screening use: results from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2331–2344. doi: 10.1158/1055-9965.EPI-11-0528. [DOI] [PubMed] [Google Scholar]
- Documet PI, Bear TM, Green HH. Results from the 2009–2010 Allegheny County Behavioral Risk Factor Surveillance Survey (AC-BRFSS): Measuring the Health of Adult Residents. Pittsburgh, PA: Allegheny County Health Department and The Evaluation Institute, University of Pittsburgh; 2012. [Google Scholar]
- Elliott PF, Belinson SE, Ottolenghi E, Smyth K, Belinson JL. Community health workers, social support and cervical cancer screening among high-risk groups in rural Mexico. J Health Care Poor Underserved. 2013;24(4):1448–1459. doi: 10.1353/hpu.2013.0180. [DOI] [PubMed] [Google Scholar]
- Fisher EB, Coufal MM, Parada H, Robinette JB, Tang PY, Urlaub DM, et al. Peer support in health care and prevention: cultural, organizational, and dissemination issues. Annu Rev Public Health. 2014;35:363–383. doi: 10.1146/annurev-publhealth-032013-182450. [DOI] [PubMed] [Google Scholar]
- Fite S, Frank DI, Curtin J. The relationship of social support to women’s obtaining mammography screening. J Am Acad Nurse Pract. 1996;8(12):565–569. doi: 10.1111/j.1745-7599.1996.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Gamarra CJ, Paz EP, Griep RH. Social support and cervical and breast cancer screening in Argentinean women from a rural population. Public Health Nurs. 2009;26(3):269–276. doi: 10.1111/j.1525-1446.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- Gjesfjeld CD, Greeno CG, Kim KH. Research on Social Work Practice. 2007. A confirmatory factor analysis of an abbreviated social support instrument: The MOS-SSS. [Google Scholar]
- Holtzman DM. Analysis and Interpretation of Data from the U.S. Behavioral Risk Factor Surveillance System (BRFSS) Global Behavioral Risk Factor Surveillance. 2003:35–46. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- Kang SH, Bloom JR, Romano PS. Cancer screening among African-American women: their use of tests and social support. Am J Public Health. 1994;84(1):101–103. doi: 10.2105/ajph.84.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol Nurs Forum. 2002;29(5):845–852. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Brown M, Ballard-Barbash R, White MC, Thompson T, Plescia M, et al. Cancer screening - United States, 2010. Morb Mortal Wkly Rep Morbidity and Mortality Weekly Report. 2012;61(3):41–45. [PubMed] [Google Scholar]
- Kleindorfer D, Miller R, Sailor-Smith S, Moomaw CJ, Khoury J, Frankel M. The challenges of community-based research: the beauty shop stroke education project. Stroke. 2008;39(8):2331–2335. doi: 10.1161/STROKEAHA.107.508812. [DOI] [PubMed] [Google Scholar]
- Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA. Minority populations and health: an introduction to health disparities in the United States. San Francisco: Jossey-Bass; 2005. [Google Scholar]
- Linnan LA, Ferguson YO. Beauty salons: a promising health promotion setting for reaching and promoting health among African American women. Health Educ Behav. 2007;34(3):517–530. doi: 10.1177/1090198106295531. [DOI] [PubMed] [Google Scholar]
- Love MB, Gardner K, Legion V. Community health workers: who they are and what they do. Health Educ Behav. 1997;24(4):510–522. doi: 10.1177/109019819702400409. [DOI] [PubMed] [Google Scholar]
- Messina CR, Lane DS, Glanz K, West DS, Taylor V, Frishman W, et al. Relationship of social support and social burden to repeated breast cancer screening in the women’s health initiative. Health Psychol. 2004;23(6):582–594. doi: 10.1037/0278-6133.23.6.582. [DOI] [PubMed] [Google Scholar]
- Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–891. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. doi: 10.1016/s0277-9536(02)00073-4. S0277953602000734 [pii] [DOI] [PubMed] [Google Scholar]
- Paskett ED, Tatum CM, D’Agostino R, Jr, Rushing J, Velez R, Michielutte R, et al. Community-based interventions to improve breast and cervical cancer screening: results of the Forsyth County Cancer Screening (FoCaS) Project. Cancer Epidemiol Biomarkers Prev. 1999;8(5):453–459. [PubMed] [Google Scholar]
- Plescia M, White MC. The National Prevention Strategy and breast cancer screening: scientific evidence for public health action. Am J Public Health. 2013;103(9):1545–1548. doi: 10.2105/AJPH.2013.301305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plescia M, Wong F, Pieters J, Joseph D. The National Breast and Cervical Cancer Early Detection Program in the era of health reform: A vision forward. Cancer. 2014;120(Suppl 16):2620–2624. doi: 10.1002/cncr.28826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt SL, Shim MJ, Mullen PD, Vernon SW, Amick BC., 3rd Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2579–2599. doi: 10.1158/1055-9965.EPI-09-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Shirazi M, Shirazi A, Bloom J. Developing a Culturally Competent Faith-Based Framework to Promote Breast Cancer Screening Among Afghan Immigrant Women. J Relig Health. 2013 doi: 10.1007/s10943-013-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva IT, Griep RH, Rotenberg L. Social support and cervical and breast cancer screening practices among nurses. Rev Lat Am Enfermagem. 2009;17(4):514–521. doi: 10.1590/s0104-11692009000400013. [DOI] [PubMed] [Google Scholar]
- Slater JS, Ha CN, Malone ME, McGovern P, Madigan SD, Finnegan JR, et al. A randomized community trial to increase mammography utilization among low-income women living in public housing. Prev Med. 1998;27(6):862–870. doi: 10.1006/pmed.1998.0370. [DOI] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2012: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2012 doi: 10.3322/caac.20143. [DOI] [PubMed] [Google Scholar]
- Suarez L, Ramirez AG, Villarreal R, Marti J, McAlister A, Talavera GA, et al. Social networks and cancer screening in four U.S. Hispanic groups. Am J Prev Med. 2000;19(1):47–52. doi: 10.1016/s0749-3797(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Swan J, Breen N, Graubard BI, McNeel TS, Blackman D, Tangka FK, et al. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer. 2010;116(20):4872–4881. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka FK, O’Hara B, Gardner JG, Turner J, Royalty J, Shaw K, et al. Meeting the cervical cancer screening needs of underserved women: the National Breast and Cervical Cancer Early Detection Program, 2004–2006. Cancer Causes Control. 2010;21(7):1081–1090. doi: 10.1007/s10552-010-9536-3. [DOI] [PubMed] [Google Scholar]
- U. S. Preventive Services Task Force. Guide to clinical preventive services, 2010–2011 recommendations of the US Preventive Services Task Force. 2010 from http://www.ahrq.gov/clinic/pocketgd1011/
- U.S. Department of Health and Human Services, C. f. D. C. a. P., and National Cancer Institute. United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. 2012 from www.cdc.gov/uscs.
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Brummett B, Barefoot J, et al. Are the salutogenic effects of social supports modified by income? A test of an “added value hypothesis”. Health Psychol. 2001;20(3):155–165. [PubMed] [Google Scholar]
- Welch C, Miller CW, James NT. Sociodemographic and health-related determinants of breast and cervical cancer screening behavior, 2005. J Obstet Gynecol Neonatal Nurs. 2008;37(1):51–57. doi: 10.1111/j.1552-6909.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Fraser-White M, Feldman J, Homel P, Wright S, King G, et al. Hair salon stylists as breast cancer prevention lay health advisors for African American and Afro-Caribbean women. J Health Care Poor Underserved. 2008;19(1):216–226. doi: 10.1353/hpu.2008.0017. [DOI] [PubMed] [Google Scholar]
- Wrzus C, Hanel M, Wagner J, Neyer FJ. Social network changes and life events across the life span: a meta-analysis. Psychol Bull. 2013;139(1):53–80. doi: 10.1037/a0028601. [DOI] [PubMed] [Google Scholar]
- Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC Cancer. 2009;9:364. doi: 10.1186/1471-2407-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]