Abstract
Prokaryotes of the genus Mycoplasma are the smallest cellular organisms that persist as obligate extracellular parasites. Although mycoplasma infection is known to be associated with chromosomal instability and can promote malignant transformation, the mechanisms underlying these phenomena remain unknown. Since persistence of many cellular parasites requires suppression of apoptosis in host cells, we tested the effect of mycoplasma infection on the activity of the p53 and nuclear factor (NF)-κB pathways, major mechanisms controlling programmed cell death. To monitor the activity of p53 and NF-κB in mycoplasma-infected cells, we used a panel of reporter cell lines expressing the bacterial β-galactosidase gene under the control of p53-or NF-κB-responsive promoters. Cells incubated with media conditioned with different species of mycoplasma showed constitutive activation of NF-κB and reduced activation of p53, common characteristics of the majority of human tumor cells, with M. arginini having the strongest effect among the species tested. Moreover, mycoplasma infection reduced the expression level and inducibility of an endogenous p53-responsive gene, p21waf1, and inhibited apoptosis induced by genotoxic stress. Infection with M. arginini made rat and mouse embryo fibroblasts susceptible to transformation with oncogenic H-Ras, whereas mycoplasma-free cells underwent irreversible p53-dependent growth arrest. Mycoplasma infection was as effective as shRNA-mediated knockdown of p53 expression in making rodent fibroblasts permissive to Ras-induced transformation. These observations indicate that mycoplasma infection plays the role of a p53-suppressing oncogene that cooperates with Ras in cell transformation and suggest that the carcinogenic and mutagenic effects of mycoplasma might be due to inhibition of p53 tumor suppressor function by this common human parasite.
Keywords: oncogene, tumor suppressor, p21, senescence, transactivation
Introduction
Chronic inflammation associated with certain chronic infections has been defined as an important cancer-promoting condition (Karin et al., 2006; Correa and Houghton, 2007). Identification of additional chronic infections associated with cancer predisposition, as well as deciphering the mechanisms underlying their cancer-promoting activity, will be important for improving our understanding of environmental carcinogens and for developing new approaches to cancer prevention. In the current study, we addressed the role of mycoplasma infection in tumor suppressor function and cell transformation.
Mycoplasmas are prokaryotic obligate extracellular parasites of mammals. Although they have a significant impact on the cellular metabolism and physiology of their host (consumption of nutrients, production of reactive oxygen species (ROS) and so on) and are known to play causative roles in a number of diseases (for example, pneumonia, tracheitis, arthritis, uretritis and so on), mycoplasmas frequently persist as chronic, asymptomatic infections in humans and animals (Razin et al., 1998; Rottem, 2003). Similarly, mycoplasmas are common contaminants of laboratory cell cultures that usually do not affect cell growth and therefore remain undetectable in the absence of molecular or immunological assays. However, long-term mycoplasma infections in cell cultures are associated with increased frequency of chromosomal instability and malignant transformation (Tsai et al., 1995; Feng et al., 1999; Cimolai, 2001). Thus, long-term infection with Mycoplasma fermentans or M. penetrans was reported to induce spontaneous transformation of mouse embryo fibroblasts in concert with overexpression of the H-ras and c-myc protooncogenes (Zhang et al., 1997). Furthermore, R-Pam2 lipopeptides, essential components of the plasma membranes of mycoplasmas, act as agonists of the heterodimeric Toll-like receptors 2 and 6 (TLR2 and TLR6) (Takeda et al., 2002). Signaling through TLR2/6 results in activation of nuclear factor (NF)-κB, a major mediator of inflammatory responses (Karin, 2006) and antiapoptotic factor. These properties of mycoplasmas suggest that these agents might act as cancer-promoting factors and stimulated us to investigate the effects of mycoplasma infection on the major tumor suppressor p53 pathway.
Maintenance of genomic stability as well as suppression of aberrant oncogene activity is the function of the p53 pathway (Poyurovsky and Prives, 2006). p53 responds to different types of DNA damage by inducing temporary growth arrest, irreversible growth arrest (senescence) or apoptosis depending upon the cell type and the severity of damage. This is important for elimination of potentially genetically altered clones from proliferating cell populations (Efeyan and Serrano, 2007). In fibroblasts, DNA damage is usually translated by p53 into senescence. This process is mediated by DNA break-induced ATM-dependent phosphorylation of the p53 protein and its inhibitor, Mdm2. Phosphorylation leads to degradation of Mdm2 and accumulation and activation of p53. This results in transactivation of a number of p53-responsive genes that determine growth arrest at cell-cycle checkpoints.
p53 signaling is also activated with a similar outcome (senescence) in response to activation of dominant oncogenes, although through a different mechanism (Efeyan and Serrano, 2007). In this case, activation of Ras or Myc results in transcriptional activation of the gene encoding the p19Arf protein that competes with p53 for Mdm2 binding. Induction of p19Arf thus leads to stabilization and accumulation of p53. Suppression of p53 is a prerequisite for Ras- or Myc-mediated growth stimulation and is sufficient for full transformation of rodent cells by activated Ras (Ferbeyre et al., 2002). The cyclin-dependent kinase inhibitor p21waf1 is encoded by a p53-responsive gene and is important in both mechanisms of p53-mediated growth arrest (Gartel and Tyner, 1999); the scale of induction of p21 is an indicator of the strength of the p53 response.
The NF-κB pathway is a major regulator of immunity and holds primary responsibility for organismal reaction to extrinsic stresses (for example, presence of parasites) (Karin, 2006). Like the p53 pathway, the NF-κB pathway is also frequently deregulated in cancer. However, in contrast to inactivation of the tumor suppressor, proapoptotic activity of p53 in tumors, NF-κB is constitutively activated in the majority of tumors (Lu et al., 2004a, b; Lee et al., 2007) and generally functions as an oncogene with antiapoptotic effects. Moreover, constitutively active NF-κB is one of the mechanisms of functional inactivation of p53 in tumors (Gurova et al., 2005). Prolonged activation of NF-κB creates a condition of chronic inflammation and therefore plays a major role in cancer predisposition (Karin, 2006; Karin et al., 2006).
Abrogation of p53 function is an essential condition for successful replication of some viruses, such as human papilloma virus (HPV) and adenoviruses. HPV infection results in physical degradation of p53 through binding of p53 to the viral E6 protein (Mantovani and Banks, 2001). Adenoviral infection leads to p53 inactivation via the p53-binding viral protein, E1b (McCormick, 2000; Burgert et al., 2002). For both types of viruses, any deficiency in p53 inactivation strongly suppresses viral replication due to a rapid apoptotic response in the host cells. Viruses also frequently deregulate NF-κB in their host cells to take advantage of the antiapoptotic function of this pathway (Lisowska and Witkowski, 2003). Similarities in the general strategies of parasite–host interaction of latent viruses (for example, HPV) and mycoplasmas, as well as the chronic and asymptomatic nature of typical mycoplasma infections, suggest that mycoplasmas might employ similar mechanisms to suppress host responses that could be detrimental for the parasite and stimulated us to analyse the status of the p53 and NF-κB pathways in mycoplasma-infected cells.
To monitor the activity of p53 and NF-κB in mycoplasma-infected cells, we used a series of approaches, including detection of molecular indicators of p53 activation as well as functional assays for p53-dependent checkpoint control, apoptosis and cooperation with activated Ras in transformation. We found that various mycoplasma species cause different degrees of p53 suppression in infected cells. The most effective species, Mycoplasma arginini, was investigated further and found to induce constitutive activation of NF-κB and inhibit p53-mediated cell-cycle checkpoint control and apoptosis. Moreover, infection of rat fibroblasts with M. arginini was found to be sufficient for their Ras-induced oncogenic transformation, indicating that mycoplasma infection can act as the functional equivalent of a p53-suppressing oncogene.
Results
Infection with M. arninini suppresses the transcriptional activity of p53
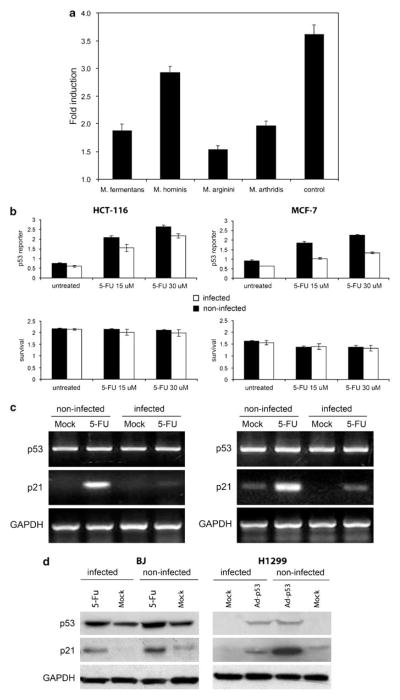
To detect whether mycoplasmas produce p53 inhibitory factors, we incubated mouse Balb 3T3 cells stably transfected with the p53-responsive β-galactosidase (β-gal) reporter gene (Komarova et al., 1997) with filtrates of cell-free broths conditioned with different in vitro grown mycoplasma species (M. fermentans, M. arginini, M. hominis, M. arthritidis). All filtrates were used in concentrations that do not display any growth-suppressive effects. p53 reporter activity in the filtrate-treated cells was measured in comparison with nontreated cells as the fold induction of β-gal activity following treatment with the DNA damage-inducing agent, doxorubicin. All four samples of broth conditioned with different mycoplasma species caused substantial inhibition of p53 reporter activity. Conditioned filtrate of broth from M. arginini had the strongest p53-suppressing effect (Figure 1a).
Figure 1.
Suppression of p53 transcriptional activity by mycoplasmas. (a) ConA-3T3 cells carrying a p53-dependent β-galactosidase (β-gal) reporter (ConALacZ) were treated overnight with 5% conditioned broths collected from cell-free cultures of the mycoplasma species indicated or with regular medium (‘control’). Doxorubicin was then added to a final concentration of 1 μg/ml and cells were cultured for an additional 24 h. The induction of β-gal activity in total cell lysates was determined by o-nitrophenyl β-D-galactopyranoside staining measured spectrophotometrically at 414 nm. The values plotted indicate the ratio of the β-gal activity in doxorubicin-treated cells infected with a given mycoplasma species to the β-gal activity in untreated cells infected with the same mycoplasma species. (b) p53-responsive reporter activity in control and M. arginini-infected HCT-116 and MCF-7 cells. HCT-116 and MCF-7 cells stably transfected with the ConALacZ reporter construct were plated into 96-well plates and incubated in medium containing the indicated concentration of 5-FU for 16 h. The cells were infected with M. arginini at least 2 weeks before the experiment. Presence of M. arginini in cell culture was confirmed by semiquantitative PCR vis-à-vis nuclear gene sequences. β-gal activity was measured by o-nitrophenyl β-D-galactopyranoside (ONPG) staining (upper panels) and cell survival was measured by methylene blue staining (bottom panels). Infected (M. arginini) cells (black bars), noninfected cells (white bars). (c) Reverse transcription (RT)–PCR analysis of p53 and p21 mRNA expression in control and M. arginini-infected HCT-116 and MCF-7 cells. Cells were left uninfected or were infected by growth in 5% M. arginini-conditioned medium overnight. To induce p53, cells were then treated with 5-FU (30 μM) for 24 h. Levels of p53 and p21 expression were measured by RT–PCR. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control to confirm the quality and amount of cDNA in each sample. HCT-116 cells (left panel), MCF-7 cells (right panel). (d) Effect of M. arginini infection on p53 and the p53-transcriptional target, p21. Lysates from control (noninfected) and M. arginini-infected BJ and H1299 cells were used for western blot analysis of p53 and p21 protein levels. GAPDH expression was used to control for protein concentration between lanes. To induce p53 activity, BJ cells were treated with 30 μM 5-FU for 16 h and H1299 cells were treated with 10 PFU (plaque-forming units) of Ad-p53 per cell.
To determine whether the activity of p53 is affected by mycoplasma infection in human cells, we infected human HCT-116/ConA and MCF-7/ConA cell lines carrying a p53-responsive β-gal reporter (Gurova et al., 2004) with M. arginini. p53 activity was measured in comparison with noninfected cells as the degree of reporter activity following treatment with the DNA damage-inducing agent, 5-FU. Reduced p53 reporter induction was evident in both infected cell lines (Figure 1b), yet there was no significant difference in cell viability between infected and noninfected cells. In addition to showing that p53-dependent reporter gene transactivation was suppressed by mycoplasma infection, we also demonstrated that transcription of an endogenous p53 target gene was similarly affected. The detection of the mRNA for the p53-regulated p21waf1 gene based on reverse transcription (RT)–PCR showed that its induction in response to DNA damage (5-FU) was reduced by infection with M. arginini in both HCT-116 and MCF-7 cells (Figure 1c).
To further characterize the p53-inhibitory properties of M. arginini infection, we analysed induction of the endogenous p53-responsive p21waf1 gene by endogenous p53 (in human diploid fibroblasts BJ treated with 5-FU) and ectopically expressed p53 (in p53-null human lung carcinoma cells H1299 infected with p53-expressing recombinant adenovirus 5). As shown in Figure 1d, mycoplasma infection resulted in a significant reduction in the degree of p21waf1 protein induction in both systems.
Constitutively active NF-κB in cells infected with M. arginini
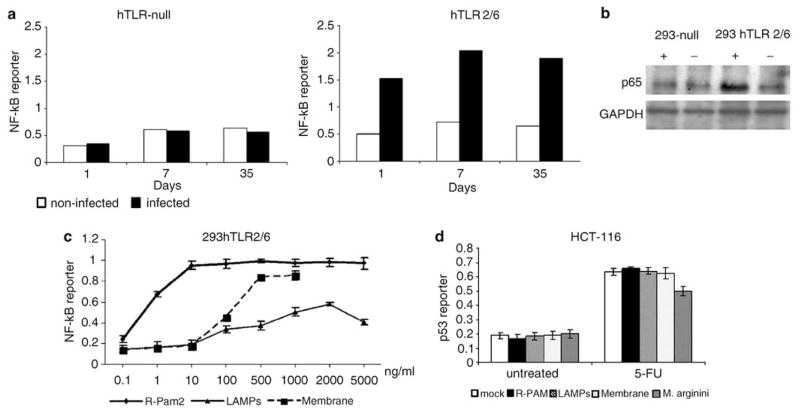
Constitutive activation of NF-κB signaling is a common feature of tumor cells that can contribute to their resistance to apoptosis and p53-mediated suppression (Lu et al., 2004a, b; Gurova et al., 2005; Lee et al., 2007). Since mycoplasma lipopeptides (R-Pam2) can induce NF-κB signaling via activation of TLR2/TLR6 (Takeda et al., 2002), we tested the status of NF-κB activity in a panel of cell lines that are derivatives of embryonic human kidney epithelial 293 cells differing in their content of expressed TLRs and stably transfected with an NF-κB-responsive reporter construct. Under normal growth conditions, NF-κB reporter activity was undetectable in 293-derived cells null for TLR expression as well as those expressing only TLR2/6. However, infection of TLR2/6-expressing cells, but not TLR-null cells, with M. arginini resulted in constitutive activation of the reporter for as long as the infection persisted (Figure 2a). Moreover, the amount of the p65 subunit of the NF-κB transcription complex was elevated in the nucleus of mycoplasma-infected cells only if they expressed TLR2/TLR6 (Figure 2b). The dependence of the observed NF-κB activation on TLR2/6 expression suggests that it is triggered by the lipopeptide component of the mycoplasma.
Figure 2.
Activation of nuclear factor (NF)-κB-dependent transcription in cells infected with M. arginini. (a) N F-κB-responsive reporter activity in control and M. arginini-infected 293hTLR-null cells (not expressing any Toll-like receptors (TLRs)) and 239hTLR2/TLR6 cells (expressing hTLR2 and TLR6 but not any other TLRs). 293hTLR-null and 239hTLR2/TLR6 cells bearing the NF-κB-LacZ reporter construct were infected with M. arginini. Next, cells were plated into 96-well plates and β-galactosidase (β-gal) activity was measured by o-nitrophenyl β-D-galactopyranoside (ONPG) staining 1, 7 and 35 days later. Infected cells (black bars), noninfected cells (white bars). (b) Influence of M. arginini infection on the nuclear localization of the p65 subunit of NF-κB. 293hTLR-null and 239hTLR2/TLR6 cells infected (+) or noninfected (−) with mycoplasma were collected 40 days post-infection and lysed for extraction of nuclear proteins. p65 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (control for equal protein loading) were detected by western blotting. (c) Effect of different mycoplasma components on NF-κB activity. N F-κB-responsive reporter activity in 293 cells expressing hTLR2/hTLR6 treated with different concentrations of mycoplasmal membranes, lipid-associated mycoplasmal proteins (LAMPs) and synthetic analogs of mycoplasmal MALP-2 lipopeptides (R-PAM2). (d) Induction of p53-responsive reporter activity in HCT-116 ConALacZ cells left untreated (‘mock’), treated with mycoplasmal membranes (‘membrane,’ 1μg/ml), lipid-associated membrane proteins (‘LAMPs,’ 2 μg/ml) or synthetic analogs of mycoplasmal MALP-2 lipopeptides (‘R-PAM,’ 0.1 μg/ml) or infected with M. arginini. p53 reporter activity was determined in cells grown in 15 μM 5-FU for 24 h and in cells grown without 5-FU (‘negative control’).
In order to check whether mycoplasma-induced NF-κB activation is responsible for the p53 inhibition observed in mycoplasma-infected cells, we compared p53 activity in mycoplasma-infected cells and in cells incubated with either whole mycoplasmal membranes, lipid-associated mycoplasmal proteins (LAMPs) or synthetic analogs of mycoplasmal R-Pam2 lipopeptides. Since all of these reagents are expected to induce NF-κB via TLR2 signaling (Chu et al., 2005), their functionality was confirmed by their ability to activate an NF-κB-dependent reporter in cells expressing TLR2/TLR6 (Figure 2c). Although all of the prepared mycoplasmal components activated NF-κB-dependent transcription, none of them, as opposed to mycoplasma infection, influenced p53 reporter activity (Figure 2d). These data suggest that mycoplasma-mediated inhibition of p53 does not depend upon activation of TLR2/6 and NF-κB signaling, but rather is triggered by an as yet unidentified component of the microbe.
Mycoplasma infection inhibits p53-mediated checkpoint control and apoptosis
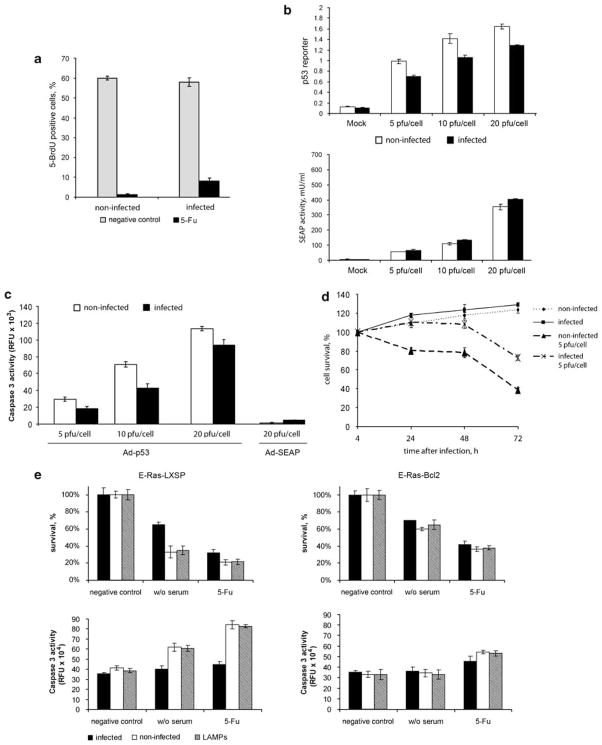
p53 is one of the determinants of cell transition through cell-cycle checkpoints. To test the effect of mycoplasmal infection on this p53 function, we analysed bromodeoxyuridine (BrdU) incorporation as a measure of DNA synthesis in p53 wild-type mycoplasma-infected and -noninfected REF52 cells. These immortalized nontransformed rat embryo fibroblasts can be arrested at the G1/S cell-cycle checkpoint in a p53-dependent manner (Sablina et al., 1999). Cultures of REF52 cells were synchronized by serum starvation (72 h) followed by cell-cycle release with simultaneous DNA-damaging treatment (5-FU). As shown in Figure 3a, only 2% of the uninfected cells overcame 5-FU-mediated growth arrest to enter S phase (1.2% of the 5-FU-treated cells were BrdU positive as compared to 60% of the 5-FU-untreated cells). In contrast, 13% of the cells in the mycoplasma-infected population entered S phase (8% of 5-FU-treated cells were BrdU positive as compared to 60% of the 5-FU-untreated cells). This result indicates that mycoplasma infection loosens the G1 block induced by 5-FU-treatment.
Figure 3.
M. arginini infection inhibits p53-mediated checkpoint control and apoptosis. (a) Effect of M. arginini infection on the ability of REF52 cells synchronized at G0/G1 by serum starvation (72 h) to enter S phase (incorporate 5-bromodeoxyuridine (BrdU) into DNA) following serum stimulation in the presence or absence of DNA-damaging treatment (30 μM 5-FU). After 7h of cell incubation with medium containing 10% fetal bovine serum (FBS) with 5-BrdU (‘negative control’, light gray bars) or 5-BrdU+5-FU (‘5-FU’, black bars), incorporation of 5-BrDU was detected by immunofluorescent staining with anti-BrdU antibodies. For each treatment, no fewer than 500 cells were analysed. (b) Infection of cells with M. arginini reduces the activity of ectopically expressed p53. Mycoplasma-infected (black bars) or -noninfected (white bars) p53-null H1299 cells containing the p53-responsive lacZ reporter construct were plated in 96-well plates and superinfected with Ad-p53 virus (top panel) or a control Ad-SEAP virus (bottom panel) at 5, 10 or 20 PFU per cell. p53 reporter transactivation and SEAP activities were measured 24 h after adenovirus infection. (c) Caspase 3 activity was measured in lysates of cells 24 h after the infection as described for (b) using a fluorogenic substrate. (d) M. arginini infection reduces the growth inhibitory effect of ectopically expressed p53 protein in H1299 cells. Mycoplasma-infected or -noninfected H1299 cells were plated in 96-well plates and superinfected with Ad-p53 virus at 5 PFU per cell. Cell survival was measured by methylene blue staining at the indicated times. (e) Effect of M. arginini infection on apoptosis in E-Ras cells triggered by serum deprivation or DNA damage (5-FU). E-Ras-LXSP and E-Ras-Bcl2 cells were plated in 96-well plates and incubated in serum-free medium for 16 h (w/o serum) or medium containing 5-FU (30 μM) for 24h. Cell survival was measured by methylene blue staining (upper panels). Activity of caspase-3 was measured using a specific fluorigenic substrate (bottom panels). M. arginini-infected cells (black bars), noninfected cells (white bars), lipid-associated mycoplasmal protein (LAMP)-treated (2 μg/ml of LAMP was added to cells 4 h before 5-FU for 28 h) cells (gray bars).
To assess the ability of mycoplasma infection to inhibit p53-dependent apoptosis, we used p53-negative H1299 (human lung adenocarcinoma) cells that are highly susceptible to apoptosis induced by ectopically expressed p53. M. arginini-infected and -noninfected H1299 cells were transduced with a recombinant adenoviral vector directing constitutive expression of the wild-type p53 gene (Ad-p53). To control for transduction efficiency, the cells were simultaneously infected with an adenoviral vector containing the SEAP (placental-secreted alkaline phosphatase) reporter gene. As shown in Figure 3b, SEAP activity was similar in noninfected and mycoplasma-infected cells, indicating that both adenovirus transduction efficiency and expression of a ‘housekeeping’ gene were not affected by concurrent mycoplasma infection. In contrast, transactivation of a p53-dependent reporter by the ectopically expressed p53 was reduced in mycoplasma-infected cells (Figure 3b, top panel). The effect of M. arginini infection on apoptosis induced by ectopic expression of p53 in this system was measured in terms of caspase 3 activation 24 h after adenovirus transduction. At several different multiplicities of infection of Ad-p53, caspase 3 activity was lower in mycoplasma-infected cells than in noninfected cells (Figure 3c). This indicates that the reduction in p53 transactivation observed in mycoplasma- infected cells translates into inhibition of a crucial p53 function, induction of apoptosis. Mycoplasma-mediated inhibition of p53-dependent apoptosis in this system was also demonstrated by methylene blue staining and quantitation of viable cells 24–72 h after adenovirus transduction (Figure 3d).
The effect of M. arginini infection on p53-dependent apoptosis was also checked in the E-Ras line of rat embryo fibroblasts that are transformed with a combination of E1a and Ras (Nelyudova et al., 2004). We used two variants of these cells: E-Ras-LXSP (transduced with an insert-free retroviral vector) and E-Ras-Bcl2 (transduced with a retroviral vector directing overexpression of the antiapoptotic Bcl-2 protein). E-Ras-LXSP cells are highly susceptible to a number of different proapoptotic stimuli, whereas E-Ras-Bcl2 cells are highly resistant to apoptosis due to inhibition of the mitochondrial apoptotic pathway by the over-expressed Bcl2 protein. Control and M. arginini-infected E-Ras-LXSP and E-Ras-Bcl2 cells were subjected to apoptosis-inducing stimuli (either serum deprivation or 5-FU treatment), and cell survival was quantitated 24 h later. As shown in the top panels of Figure 3e, mycoplasma infection resulted in increased survival of E-Ras-LXSP cells following induction of apoptosis by either serum deprivation or 5-FU treatment. The inhibitory effect of mycoplasma infection on apoptosis in this system was also illustrated by reduced induction of caspase 3 activity (as determined by cleavage of a caspase 3-specific fluorogenic substrate) in mycoplasma-infected cells as compared to noninfected cells (Figure 3e, bottom panels). E-Ras-LXSP cells infected with mycoplasma behaved similarly to E-Ras-Bcl2 cells that remained resistant to apoptotic stimuli regardless of mycoplasma infection. It should be noted that the reduction in cell numbers following serum deprivation or 5-FU treatment in E-Ras-Bcl2 cells is a result of growth arrest, not apoptosis (Nelyudova et al., 2004). In addition to infection of the cells with M. arginini, we also incubated the cells with LAMPs prior to treatment with the apoptotic stimuli. In both the cell viability and caspase 3 activity assays, LAMP-treated cells behaved the same as noninfected cells. This is consistent with our finding that LAMPs are not sufficient for p53 inhibition in HCT-116 cells (Figure 2d).
Taken together, the results of the functional assays presented demonstrate that M. arginini infection effectively suppresses major p53 functions, including control of cell-cycle checkpoints and regulation of apoptosis.
Mycoplasma infection is sufficient to allow for Ras-mediated transformation of rat embryo fibroblasts
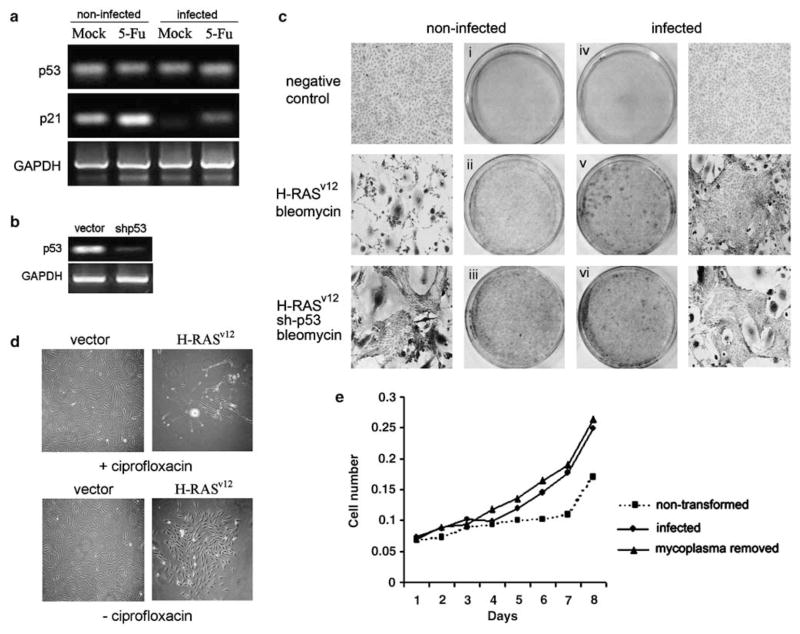
In rodent cells, p53 activity is the only mechanism that prevents full transformation of cells containing an activated Ras oncogene (Yaswen and Campisi, 2007). To determine whether the degree of p53 suppression by mycoplasma infection demonstrated above was sufficient to make cells permissive for Ras-mediated transformation, we used the nontransformed rat embryo fibroblast cell line REF52. These cells are known to be susceptible to senescence-like growth arrest in response to introduction of oncogenic H-Ras. Inactivation of p53 in the cells releases them from this growth arrest and leads to malignant transformation (Serrano et al., 1997). First, we confirmed that mycoplasma infection in fact resulted in decreased p53-dependent transcription of p21waf1 in REF52 cells as it did in the other cell lines tested above (Figure 4a). Next, we prepared a variant of REF52 cells (REF52/sh-p53) in which p53 expression is reduced due to lentiviral transduction of shRNA against p53 (Boiko et al., 2006). shRNA-mediated knockdown of p53 expression was confirmed by semiquantative RT–PCR analysis of p53 mRNA levels (Figure 4b). REF52 and REF52/sh-p53 cells were infected with M. arginini or kept uninfected. After 72 h, each population of cells was infected with lentivirus (LV-Ras-Bleo) bearing oncogenic Ras (H-Rasv12) in combination with the bleo resistance gene under the control of an internal H4 promoter. The transduced cells were subjected to selection in bleomycin to eliminate cells that were not transduced with oncogenic Ras and allowed to grow for 3 weeks to assess cell morphology and ability to form colonies. The results of a representative experiment are shown in Figure 4c. As expected, 21 days after selection for Ras transduction, parental REF52 cells (containing functional p53 and not infected with mycoplasma) remained growth arrested with cellular morphology consistent with their senescent state (Figure 4c, panel ii). In contrast, REF52/sh-p53 cells in which p53 expression was knocked down by shRNA expression formed a fully transformed culture indicative of Ras activity in the absence of p53 function (panel iii). Ras-transduced REF52/sh-p53 had a similar transformed phenotype regardless of whether they were mycoplasma-infected (panel vi) or -noninfected (panel iii). Strikingly, parental REF52 cells infected with M. arginini also formed a fully transformed culture, despite the fact that they express wild-type p53 (panel v). Thus, M. arginini infection mimicked shRNA-mediated p53 knockdown in this system to allow Ras transformation. Consistent with their lack of p53-inhibiting activity in previous assays, mycoplasma components (R-Pam2, membrane fraction and LAMPs) failed to cooperate with Ras in transformation of REF52 (data not shown). These data indicate that mycoplasma infection makes REF52 cells susceptible to transformation by Ras, presumably through functional inactivation of p53. Similar results were obtained in mouse embryo fibroblasts (data not shown).
Figure 4.
Decreased activity of p53 in mycoplasma-infected cells is accompanied by susceptibility to Ras-mediated transformation. (a) Reverse transcription (RT)–PCR analysis of p53 and p21 mRNA expression in REF52 cell-infected and -noninfected with M. arginini. For induction of p53, cells were treated with 5-FU (30 μM) for 16 h. Levels of p53 and p21 expression were measured by RT–PCR analysis. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control to confirm the quality and amount of cDNA in each sample. (b) Inhibition of p53 mRNA expression in REF52 cells transduced with a lentivirus expressing an inhibitory shRNA against p53. Levels of p53 were measured by RT–PCR analysis 72 h post-transduction. Expression of GAPDH was used as a positive control to confirm the quality and amount of cDNA in each sample. (c) Photomicrographs and photographs of methylene blue-stained REF52 cells. Noninfected (panels i, ii and iii) and M. arginini-infected (panels iv, v and vi) REF52 cells were transduced with an insert-free lentiviral vector (‘negative control,’ panels i and iv), the LV-Ras-Bleo lentivirus bearing the activated Ha-Rasv12oncogene (panels ii and v) or a combination of LV-Ras-Bleo and a lentivirus expressing an shRNA targeting p53 (‘shRNA-p53,’ panels iii and vi). Transduced cells (except for ‘negative control’ cells) were grown in the presence of bleomycin for 21 days to select Ras-expressing cells. Viable cells were stained with methylene blue and randomly selected areas on the plates were photographed. (d) Elimination of mycoplasma prevents Ras-mediated transformation of cells. Photomicrographs of monolayers of REF52 cells. Two sets of plates with REF52 cells chronically infected with M. arginini were transduced with either LV-Ras-Bleo expressing H-RASv12 or LV-Bleo (‘vector’). One set was treated with ciprofloxacin to remove mycoplasma (effect of antibiotic was confirmed by RT–PCR for mycoplasma DNA (data not shown)) and both sets were incubated 21 days with bleomycin-containing medium to selectively monitor lentivirus-transduced cells. On day 21, plates were fixed with methanol and stained with methylene blue. (e) Elimination of mycoplasma from REF52 cells that underwent Ras-mediated transformation by treatment with ciprofloxacin does not reverse their transformed phenotype. Nontransformed, transformed (by transduction of H-Rasv12 in the presence of M. arginini) or transformed cells treated with ciprofloxacin (transformed, mycoplasma removed) were plated in 96-well plates (5 × 102 per well). Cell numbers were determined by methylene blue assay at the indicated time points after plating.
To determine whether the susceptibility to Ras transformation conferred by mycoplasma infection is a reversible or irreversible trait, we used the antibiotic ciprofloxacin to eliminate mycoplasma from cell cultures. Ciprofloxacin was added to the culture medium shortly after transduction of REF52 cells with Ras-expressing lentivirus. The antibiotic effectively eliminated mycoplasma from the cultures as confirmed by PCR (data not shown). As shown in Figure 4d, ciprofloxacin treatment completely abolished Ras-mediated focus formation, indicating that persistent mycoplasma infection is needed for susceptibility of REF52 cells to Ras transformation. However, elimination of mycoplasma from cultures of cells that underwent Ras-mediated transformation did not result in their growth arrest or any other visible changes (Figure 4e). This suggests that some currently undefined, irreversible event(s) occur in the course of acquisition of the transformed phenotype. The nature of these events, as well as the extent of p53 functionality in mycoplasma+Ras- transformed mycoplasma-free cells will be addressed in future studies.
Discussion
In this study we have demonstrated that mycoplasmas, common contaminants of cell cultures that are frequently asymptomatically present in various mammals, including humans, can suppress p53 function and constitutively activate NF-κB in cultured cells. Importantly, deregulation of these major stress response pathways in this way—suppression of p53 and activation of N F-κB—mimics common features of tumor cells that contribute to their unconstrained growth, genomic instability and resistance to apoptotic stimuli (Karin, 2006; Efeyan and Serrano, 2007). Suppression of p53 is a prerequisite for mammalian cell transformation by dominant oncogenes; in rodents p53 activity is the only obstacle that must be overcome for activated Ras to induce a fully transformed tumorigenic phenotype in fibroblasts (Serrano et al., 1997). Consistent with this, we found that infection of rat and mouse fibroblasts by M. arginini was sufficient to allow their transformation by transduced H-Rasv12. In these experiments, M. arginini behaved as an oncogene, such as c-Myc, in its cooperation with oncogenic Ras in fibroblast transformation.
Suppression of the p53 pathway by mycoplasma infection provides a plausible explanation for earlier reports of chromosomal instability and frequent spontaneous transformation in cell cultures following mycoplasma infection (Tsai et al., 1995; Feng et al., 1999; Cimolai, 2001) and for a recent article describing mycoplasma-induced transformation of mouse lung epithelial cells (Jiang et al., 2007). In fact, p53 deficiency in mice is known to be associated with a high frequency of chromosomal instability in somatic cells (Baek et al., 2003) and spontaneous immortalization in culture (Donehower et al., 1996). It is noteworthy that, similar to p53−/− mouse embryo fibroblasts (MEFs), MEFs infected with M. arginini do not undergo massive growth arrest during their propagation in vitro (DY Logunov et al., unpublished data). This observation provides further evidence on p53 suppression in mycoplasma-infected cells.
Suppression of p53 and activation of NF-κB both result in cell resistance to apoptotic stimuli. Lack of p53 does not allow cells to activate transcription of p53-responsive proapoptotic members of the Bcl-2 family, including Bax, Puma and Noxa (Poyurovsky and Prives, 2006; Efeyan and Serrano, 2007). Active NF-κB induces transcription of genes encoding antiapoptotic proteins, such as Bcl-2 and IAPs (Takeda et al., 2002), and activates inhibitors of ROS, such as superoxide dismutase-2 and ferritin (Bubici et al., 2006). As expected based upon these known downstream effects of p53 and NF-κB deregulation, we found that apoptosis induced by genotoxic stress was suppressed in cells chronically infected with mycoplasma.
Overall, the association of mycoplasma infection with reduce control over genomic stability, loss of mechanisms arresting cells with increased activity of oncogenes in premature senescence, and suppression of apoptotic responses indicates that a radical loosening of major tumor suppressor mechanisms occurs in mycoplasma-infected cells. This raises the possibility of mycoplasma infection playing a role in cancer predisposition. Given the asymptomatic nature of mycoplasma infection and, therefore, its ability to persist in the organism for long periods of time, even partial deregulation of the above-described mechanisms might create conditions of high risk, similar to those associated, for example, with chronic infection with Helicobacter pylori, which is known to create conditions that promote gastric cancer development (Correa and Houghton, 2007).
Our study primarily focused on M. arginini as a model since this species showed the most prominent suppressive effects on p53 activation in vitro. Using this model, we were able to demonstrate in principle the effects of mycoplasma infection on the activity of two major stress response pathways, p53 and NF-κB. To what extent these effects are translated into mycoplasma pathogenicity remains to be determined.
It is important to note that the mechanism of mycoplasma-mediated p53-inhibition remains to be defined. Differential effect of mycoplasma infection on p21 and surrogate reporter (Figures 1b–d) suggests that mycoplasma infection may modulate post-translational modifications of p53 resulting in its distinct effect on different promoters.
M. arginini may not be the best pathogen to study in terms of mycoplasma effects on human health. M. arginini is a frequent pathogen of cows, goats and pigs, in which it persists either asymptomatically or associated with pneumonia. In contrast, M. arginini is only rarely found in humans (predominantly in animal care personnel) with no associated pathologies reported (Prozorovskiy et al., 1995). Revealing a possible cancer predisposition role for mycoplasma infection would require long-term observations, which are typically not feasible in the natural hosts of M. arginini.
Together with our detailed study of M. arginini, the fact that other tested mycoplasma species all demonstrated p53 inhibitory activity in cultured cells provides strong rationale for future studies aimed at investigating possible association of different mycoplasma infections with chronic human diseases, especially those known to be cancer-promoting conditions. Although in some of these cases (that is, viral hepatitis B and C for hepatocellular carcinomas or H. pylori-induced ulcer for gastric cancer) the carcinogenic role of the chronic infection has been well established, there are other instances in which the involvement of a latent infectious agent has been postulated (Cimolai, 2001) but never proven. In this regard, prostate cancer is an especially interesting disease since carcinogenesis in the prostate is frequently preceded by chronic inflammation of unknown nature (De Marzo et al., 2007). Frequent isolation of mycoplasmas from human prostates (for example, M. hominis and U. urealyticum (Oriel, 1983)) further argues for the importance of a large-scale correlative study to analyse possible correlations between mycoplasma infection and cancer predisposition.
Although the NF-κB-inducing activity of mycoplasmas can be attributed to stimulation of TLR2/TLR6 by theR-Pam2 lipopeptide components of their membranes (Takeda et al., 2002), the nature of the factor(s) responsible for their p53-suppressive function remains unknown. This is likely to be factor(s) originating from mycoplasma rather than from host cells since broths conditioned with different mycoplasma species grown in mammalian cell-free conditions possessed definite p53 inhibitory properties (Figure 1). Identification of such factor(s) and deciphering the mechanisms underlying their p53-suppressive function is another exciting direction of this study which is currently underway.
Materials and methods
Cells
H1299, MCF-7, HCT-116, BJ, REF52, 293 cells were obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, 1mM sodium pyruvate, 10mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer, 55 nM β-mercaptoethanol and antibiotics. Reporter cell lines with p53-responsive β-gal were described in Gurova et al. (2005). Reporter cell lines with NF-κB-dependent β-gal, with and without TLR2/TLR6, were obtained from Invivogen (San Diego, CA, USA) (www.invivogen.com).
Lentiviral transduction
293T cells were used for preparation of lentiviral stocks. Cells were transfected with vectors using Lipofectamin-Plus reagent (Invitrogen, Carlsbad, CA, USA). Two helper plasmids, pCMV_R8.2 and pVSV-G, were co-transfected along with the experimental vector. Supernatants containing infectious viral particles were harvested 24, 36 and 48 h post-transfection, pooled and filtered. Infection of exponentially growing cells was performed every 5 h for 2 days with various dilutions of virus-containing supernatant supplemented with 4 μg/ml polybrene (Sigma, St Louis, MO, USA).
Reporter assays
In total 2 × 104 cells with integrated reporter were plated per well in 96-well plates. At different time points, cell lysates were prepared using Reporter Lysis Buffer (Promega, Madison, WI, USA). β-gal activity was measured in aliquots of cell lysates using a standard kit (β-galactosidase Assay System; Promega). Colorimetric reactions were read on the Wallac 1420 plate reader (PerkinElmer, Waltham, MA, USA).
Cultivation of mycoplasmas
All mycoplasma species were cultivated at 37 °C in broth prepared from the tryptic digest of beef heart (PPLO broth; Diffco) with 10% fresh yeast extract, 20% normal horse serum, 500U/ml penicillin and 1% L-arginine, 1% glucose, pH—7.8. Specimens from tissue culture were inoculated directly into liquid medium, which was subsequently subcultured on agar (0.3 or 1.3%). Mycoplasma colonies are observed unstained using the low power of an ordinary light microscope. The titer of mycoplasma cells in broth was expressed as the number of colony-forming units in 1 ml of medium (CFU per ml).
Cell-survival assays
Cell survival was expressed as a percentage of intensity of methylene blue staining of treated cells as compared to untreated control cells. Methylene blue from stained colonies was extracted by 0.1% SDS and quantitated spectrophotometrically.
Western blot analysis
The following antibodies were used in a standard western blot protocol: anti-p53—mouse monoclonal DO1, anti-p21—mouse monoclonal F-5, anti-p65—rabbit polyclonal C20 and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All primary antibodies and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Analysis of 5-BrdU incorporation
After 7 h of incubation with 5-BrdU (10 μM; Serva, Heidelberg, Germany) cells were washed 2–3 times with phosphate-buffered saline at 35–37 °C, fixed with 4% formaldehyde for 15 min at room temperature, permeabilized with 1% Triton X-100 in buffer M (50mM imidazole, 50mM KCl, 0.5mM MCl2, 0.1mM EDTA, pH 6.8), containing 4% polyethylene glycol (Mr 40 000) for 3 min and hydrolysed with 4M HCl at room temperature for 10 min. 5-BrdU incorporation was visualized by indirect immunostaining with BU-33 mouse monoclonal antibody specific for 5-BrdU (Sigma) and tetramethyl Rhodamine isotiocyanate (TRITC)-conjugated anti-mouse immunoglobulin G (IgG; Sigma).
PCR detection of mycoplasmas
Total DNA from mycoplasma-infected cells was extracted with a DNA Purification kit (Promega) and used for PCR with the following primers for all types of mollicutes (16S RNA gene): forward primer GPO1 5′-act-c(/t)ct-acg-gg(/a) a-ggc-agc-agt-a, reverse primer MGSO 5′-tgc-acc-at(/c)c-tgt-ca (/t)c(/a/t)-t(/a/c)c(a/t)t(/g/c)-gt(/a)t(/a)-aac-ctc. PCR conditions were as follows: 5 min at 94 °C for 1 cycle; 1 min at 94 °C, 1 min at 63 °C, 1 min at 72 °C 1 min for 40 cycles.
RT–PCR analysis
Expression of the p53 and p21 genes in human/rat cell lines was analysed using RT–PCR. Total RNA was isolated using TRIZOL reagent (Invitrogen). Reverse transcription was performed using an RT System (Promega). p53 and p21 mRNA sequences were amplified by PCR with the following primers: human p53—forward 5′-CCC GAG TAT CTG GAA GAC AG-3′, reverse 5′-ATA GGT CGG CGG TTC AT-3′; human p21—forward 5′-ATG TCA GAA CCG GCT GGG GAT G-3′, reverse 5′-TTA GGG CTT CCT CTT GGA GAA G-3′; human GAPDH—forward 5′-TCT AGA CGG CAG GTC AGG TCC ACC-3′, reverse 5′-CCA CCC ATG GCA AAT TCC ATG GCA-3′; rat p53—forward 5′-TTC CTG CAG TCA GGG ACA G-3′, reverse 5′-ATA CGG ATT TCC TTC CAC CC-3′; rat p21—forward 5′-TGG CCT TGT CGC TGT CTT-3′, reverse 5′-CTA AGG CAG AAG ATG GGG AA-3′; rat GAPDH—forward 5′-CAT AGA CAA GAT GGT GAA GG-3′, reverse 5′-TCC ACA GTC TTC TGA GTG GC-3′. The number of PCR cycles for each gene was chosen to stay within a dynamic range of amplification.
Apoptosis assays
The caspase-3 activation assay was performed using the caspase-3-specific fluorigenic substrate Ac-DEVD-AMC, 30 μM, in lysing buffer pH 7.0 containing 10mM HEPES, 0.4mM EDTA, 0.1% [(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 2% glycerol, 2mM dithiothreitol. Fluorescence was measured at 0 and 6 h after adding the substrate using a Wallac 1420 plate reader (PerkinElmer). Background fluorescence determined at 0 h was subtracted from each value.
All described experiments were repeated at least two times with similar results. Collection of all data points was carried out in triplicate unless otherwise indicated.
Acknowledgments
We are grateful to Patti Baker for help in paper preparation and editing. We thank Tatiana Pospelova for the gift of E-Ras cells, Alex Shakhov for providing R-Pam2 and Galina Ilyinskaya for technical assistance. This work was supported by grants from Cleveland BioLabs Inc. and NIH (CA60730, CA75179 and CA098374) to AVG.
References
- Baek KH, Shin HJ, Yoo JK, Cho JH, Choi YH, Sung YC, et al. p53 deficiency and defective mitotic checkpoint in proliferating T lymphocytes increase chromosomal instability through aberrant exit from mitotic arrest. J Leukoc Biol. 2003;73:850–861. doi: 10.1189/jlb.1202607. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Porteous S, Razorenova OV, Krivokrysenko VI, Williams BR, Gudkov AV, et al. A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation. Genes Dev. 2006;20:236–252. doi: 10.1101/gad.1372606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M, Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, et al. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol. 2005;9:5713–5719. doi: 10.4049/jimmunol.174.9.5713. [DOI] [PubMed] [Google Scholar]
- Cimolai N. Do mycoplasmas cause human cancer? Can J Microbiol. 2001;47:691–697. [PubMed] [Google Scholar]
- Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:56–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D, et al. The role of p53 loss in genomic instability and tumor progression in a murine mammary cancer model. Prog Clin Biol Res. 1996;395:1–11. [PubMed] [Google Scholar]
- Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- Feng SH, Tsai S, Rodriguez J, Lo SC. Mycoplasmal infections prevent apoptosis and induce malignant transformation of interleukin-3-dependent 32D hematopoietic cells. Mol Cell Biol. 1999;12:7995–8002. doi: 10.1128/mcb.19.12.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbeyre G, de Stanchina E, Lin AW, Querido E, McCurrach ME, Hannon GJ, et al. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol Cell Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;48:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res. 2004;64:1951–1958. doi: 10.1158/0008-5472.can-03-1541. [DOI] [PubMed] [Google Scholar]
- Jiang S, Zhang S, Langenfeld J, Lo SC, Rogers MB. Mycoplasma infection transforms normal lung cells and induces bone morphogenetic protein 2 expression by post-transcriptional mechanisms. J Cell Biochem. 2007 doi: 10.1002/jcb.21647. e-pub ahead of print 4 Dec 2007. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, et al. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 1997;16:1391–1400. doi: 10.1093/emboj/16.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- Lisowska K, Witkowski JM. Viral strategies in modulation of NF-kappaB activity. Arch Immunol Ther Exp (Warsz) 2003;51:367–375. [PubMed] [Google Scholar]
- Lu T, Burdelya LG, Swiatkowski SM, Boiko AD, Howe PH, Stark GR, et al. Secreted transforming growth factor beta2 activates NF-kappaB, blocks apoptosis, and is essential for the survival of some tumor cells. Proc Natl Acad Sci USA. 2004a;18:7112–7117. doi: 10.1073/pnas.0402048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Sathe SS, Swiatkowski SM, Hampole CV, Stark GR. Secretion of cytokines and growth factors as a general cause of constitutive NFkappaB activation in cancer. Oncogene. 2004b;12:2138–2145. doi: 10.1038/sj.onc.1207332. [DOI] [PubMed] [Google Scholar]
- Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin Cancer Biol. 2000;10:453–459. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- Nelyudova AM, Tararova ND, Aksenov ND, Pospelov VA, Pospelova TV. Restoration of G1/S arrest in E1A+c-Ha-ras-transformed cells by Bcl-2 overexpression. Cell Cycle. 2004;11:1427–1432. doi: 10.4161/cc.3.11.1204. [DOI] [PubMed] [Google Scholar]
- Oriel JD. Role of genital mycoplasmas in nongonococcal urethritis and prostatitis. Sex Transm Dis. 1983;10:263–270. [PubMed] [Google Scholar]
- Poyurovsky MV, Prives C. Unleashing the power of p53: lessons from mice and men. Genes Dev. 2006;20:125–131. doi: 10.1101/gad.1397506. [DOI] [PubMed] [Google Scholar]
- Prozorovskiy SV, Rakovskaya IV, Vulfovich YuV. Medical Mycoplasmology. Medicine Publishers; Moscow, Russia: 1995. pp. 187–188. (in Russian) [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Agapova LS, Chumakov PM, Kopnin BP. p53 does not control the spindle assembly cell cycle checkpoint but mediates G1 arrest in response to disruption of microtubule system. Cell Biol Int. 1999;23:323–334. doi: 10.1006/cbir.1999.0362. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. 2002;8:459–463. doi: 10.1179/096805102125001073. [DOI] [PubMed] [Google Scholar]
- Tsai S, Wear DJ, Shih JW, Lo SC. Mycoplasmas and oncogenesis: persistent infection and multistage malignant transformation. Proc Natl Acad Sci USA. 1995;22:10197–10201. doi: 10.1073/pnas.92.22.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P, Campisi J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell. 2007;128:233–234. doi: 10.1016/j.cell.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shih JW, Wear DJ, Tsai S, Lo SC. High-level expression of H-ras and c-myc oncogenes in mycoplasma-mediated malignant cell transformation. Proc Soc Exp Biol Med. 1997;214:359–366. doi: 10.3181/00379727-214-44104. [DOI] [PubMed] [Google Scholar]