Abstract
The growing diversity of heritable skin diseases, a practical challenge to clinicians and dermatonosologists alike, has nonetheless served as a rich source of insight into skin biology and disease mechanisms. I summarize below some key insights from the recent gene-driven phase of research on Werner syndrome, a heritable adult progeroid syndrome with prominent dermatologic features, constitutional genomic instability and an elevated risk of cancer. I also indicate how new insights into skin biology, disease and aging may come from unexpected sources.
INTRODUCTION
Werner syndrome (WS) is an autosomal recessive disease that first captured wide attention due to its prominent premature aging (or progeroid) features. WS is also of considerable biomedical science interest in light of the pairing of these progeroid features with constitutional genomic instability and an elevated risk of many clinically important, age-dependent human diseases.
The progeroid features of WS were first well-described by Otto Werner (Werner, 1985), who described a North German family of four siblings, ages 31 – 40, with short stature, prematurely gray hair, bilateral cataracts, atrophy of the extremities, hyperkeratosis and scleroderma-like changes together with foot and ankle skin ulceration. He noted one of the siblings, a 36 yr old male, gave “…the impression of extreme senility.” These observations were published as part of Werner’s doctoral thesis prior to his embarking on a career in a small North Sea village. Werner never again returned to study his syndrome (Pehmoeller, 2001).
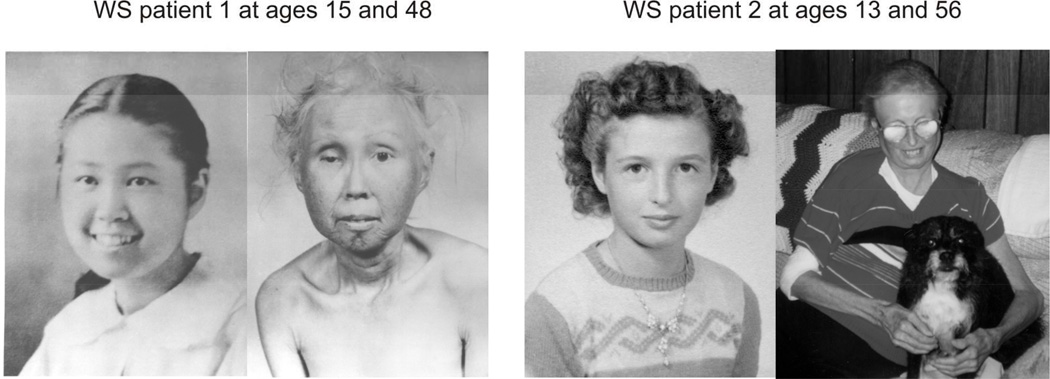
The term ‘Werner’s syndrome’ was first used in a subsequent report of an additional patient who resembled the family members seen by Werner (Oppenheimer and Kugel, 1934). This case report together with a more comprehensive study by Thannhauser of five additional patients (Thannhauser, 1945) provided a detailed description of WS. Werner syndrome was next ‘rediscovered’ by colleagues at the University of Washington who described three Japanese-American sisters with WS (one of whom is shown in Figure 1). Their analysis established the autosomal recessive inheritance of WS and delineated key differences between WS and normal aging (Epstein et al., 1966).
Figure 1.
Clinical features and progression of Werner syndrome. Left photo panels are of Case 1, a Japanese-American WS patient reported by Epstein et al., 1966, at ages 15 (left) and 48 (right). Right photo panels are of a second Caucasian WS patient at ages ~13 (left) and 56 (right). Key clinical features of WS are present in both sets of photos, including the rounded face; sharp facial features; graying, thinning and loss of scalp and eyebrow hair; and in Patient 2 right panel thin, atrophic forearms and right elbow ulceration. Patient 1 archival photos, kindly provided by Drs. George Martin and Nancy Hanson of the International Registry of Werner Syndrome, were digitized and restored by Alden Hackmann. They are used courtesy of Lippincott Williams & Wilkins. Patient 2 photos were provided by Dr. George Martin, and are used here courtesy of the patient’s spouse with informed consent of the patient, and of Elsevier Press where they were originally published in different form (Martin, GM (2005) Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120:523–532).
Werner syndrome as a clinical entity
The most consistent and earliest noted findings are premature graying and loss of hair together with bilateral cataracts, short stature and progressive, scleroderma-like skin changes (Table 1)(Epstein et al., 1966; Goto, 1997; Tollefsbol and Cohen, 1984). Hair graying and loss begin in the second decade with the scalp and eyebrows, as do bilateral ocular cataracts. The short stature of WS patients reflects the absence of a pubertal growth spurt. Short stature together with progressive limb thinning, atrophy and a stocky trunk give many patients a ‘cushingoid’ appearance (see Figure 2 of (Goto, 2001)).
Table 1.
Werner syndrome diagnostic criteria
| Category | WS signs and symptoms* |
|---|---|
| Cardinal** | 1. cataracts (bilateral) |
| 2. sclerodermalike skin changes | |
| 3. short stature | |
| 4. parental consanguinity | |
| 5. premature greying and/or thinning of scalp hair | |
| Additional | 1. diabetes mellitus |
| 2. hypogonadism | |
| 3. osteoporosis | |
| 4. osteosclerosis (distal phalanges/fingers and/or toes) | |
| 5. soft tissue calcification | |
| 6. premature atherosclerosis | |
| 7. neoplasia | |
| 8. thin, highpitched voice |
| Category | Diagnostic confidence | Diagnostic criteria |
|---|---|---|
| Definite | High confidence | all cardinal signs + two additional signs OR confirmed pathogenic WRN mutations in both alleles |
| Probable | High confidence | first 3 cardinal signs + any 2 others |
| Possible | Low confidence | either cataracts or dermatological changes + any 4 additional signs |
| Exclusion** | Exclude | signs or symptoms before adolescence (except stature) |
notes:
WS signs and symptoms are from the diagnostic criteria established by the International Registry of Werner Syndrome: www.wernersyndrome.org/registry/diagnostic.html, with additional discussion and application provided in Lauper et al. (2013).
The scleroderma-like skin changes of WS (Thannhauser, 1945) consist of a mix of atrophic and proliferative changes: epidermal atrophy that includes skin appendages in conjunction with focal hyperkeratosis and basal hypermelanosis. Dermal subcutaneous atrophy is often found with dermal fibrosis underlying atrophic skin (Epstein et al., 1966; Goto, 1997; Hatamochi, 2001; Thannhauser, 1945). These changes give skin a ‘tight, white and shiny’ appearance, with a progressive sharpening of facial features to give a ‘pinched’, ‘beaked’ or ‘bird-like’ appearance (see Figure 3 in (Goto, 2001)). The lower extremities, especially the feet, may be markedly deformed with ulceration and calcification of soft tissue and tendons (Hatamochi, 2001).
Many of these changes are readily apparent in patient photos taken in early adulthood and later in life (Figure 1). The progressive development of phenotype makes the diagnosis of WS challenging, especially in young adults. However cardinal features are often present early, and can be used together with molecular confirmation to confirm or exclude a diagnosis (Table 1)(Hisama et al., 2014).
Related RECQ helicase deficiency syndromes
The positional cloning of the WRN locus (Yu et al., 1996) and other members of the RECQ helicase gene family led to recognition of deeper links between WS and two additional genodermatoses: Bloom syndrome (BS)(Ellis et al., 1995) and Rothmund-Thomson syndrome (RTS)(Kitao et al., 1999). Bloom syndrome patients display congenital short stature and a characteristic sun-sensitive ‘butterfly’ rash across the bridge of the nose and cheeks that may involve hands and forearms (Bloom, 1954). Many patients display cellular and humoral immune deficits; an elevated risk of otitis media, pneumonia and diabetes mellitus with reduced fertility. Cancer is the leading cause of premature death (Bloom, 1954; German, 1979, 1993, 1997).
Rothmund-Thomson syndrome (RTS) was first described as a familial occurrence of skin changes with bilateral juvenile cataracts (Rothmund, 1868; Taylor, 1957; Thomson, 1936). A characteristic sun-sensitive rash with redness, swelling and blistering appears in the first year of life, and may involve the buttocks and extremities while sparing the chest, back and abdomen. The rash may further develop variable pigmentation, telangiectasia and focal atrophy. Hair, eyelashes and eyebrows are often sparse or absent. Congenital short stature is common, though less severe than in BS. Developmental abnormalities include dysplastic, malformed or absent bones, often in the hand or thumbs; delayed bone formation or bone density loss; and malformed, missing or extra teeth. Cataracts have been documented in only a minority of contemporary RTS patients (Larizza et al., 2010; Wang et al., 2001). Cancer risk is largely limited to osteosarcoma (Siitonen et al., 2009; Wang et al., 2003). Immunologic function appears intact, but fertility may be reduced. Additional diseases associated with RECQL4 mutations are RAPADILINO and Baller-Gerold (BGS) syndromes. RAPADILINO syndrome patients have joint dislocations and patellar hypoplasia or aplasia, but lack skin changes. BGS patients have craniosynostosis with radial aplasia, and RTS-like skin changes (Siitonen et al., 2003; Siitonen et al., 2009; Van Maldergem et al., 2006). Life expectancy appears normal in the absence of cancer (Larizza et al., 2010; Wang et al., 2001).
Elevated acquired disease risk in Werner syndrome
Many WS patients prematurely develop age-dependent diseases such as myocardial infarction and stroke; cancer; osteoporosis; diabetes mellitus; and hypogonadism. Cardio-vascular disease and cancer are leading causes of premature death (Goto, 1997; Goto et al., 2013). The elevated risk of neoplasia is quite selective: two-thirds of neoplasms in WS patients were of 6, not obviously related, tumor types: thyroid epithelial carcinomas, melanomas, meningiomas, soft tissue sarcomas, hematologic neoplasia, chiefly leukemias, and osteosarcoma (Lauper et al., 2013; Lauper and Monnat Jr, 2013; Monnat Jr, 2013). Multiple neoplasms were common: 22% of 189 patients in our series had 1 to 4 concurrent or sequential neoplasms, often of unusual types or at unusual sites. For example, melanomas were almost exclusively less common variants: acral lentiginous melanomas arising on the palms, soles or in nail beds; and mucosal melanomas arising in the nasal cavity or esophagus. Thyroid neoplasms, in similar fashion, were disproportionately less common follicular carcinomas (Lauper et al., 2013). The excess risk of these specific neoplasms, estimated using a combination of standardized incidence ratio (SIR), proportional incidence ratio (SPIR) analyses, ranged from nearly 60-fold for melanoma to 1.5-fold for leukemia and pre-leukemic disorders (Lauper et al., 2013). This spectrum of neoplasia overlaps with, but is distinct from, the neoplasms observed in BS and RTS (German, 1997; Monnat, 2001; Siitonen et al., 2009). BS is unusual among heritable cancer predispositions as many different tumor types are involved (German, 1997; Monnat, 2001). The cancer risk in the RTS-associated RECQL4 syndromes is, conversely, restricted largely to osteosarcoma and lymphoma (Siitonen et al., 2009; Wang et al., 2003; Wang et al., 2001).
Physiologic roles of RECQ helicases
All of the human RECQ helicases hydrolyze ATP, unwind double-stranded DNA and possess good DNA strand annealing activity. WRN alone possesses an additional, 3' to 5' exonuclease activity. Despite their common biochemical activities, the three disease-associated RECQ helicases have differing substrate preferences and different sets of protein partners (reviewed in (Bachrati and Hickson, 2003; Brosh Jr, 2013; Croteau et al., 2014; Sidorova and Monnat Jr, 2015)). These biochemical data together with functional and cellular data begin to indicate how these seemingly similar proteins fill different physiologic ‘niches’ in human cells and, by extension, how the loss of function of one RECQ protein may lead to distinct cellular and organismal phenotypes.
Functional characterizations have identified distinct roles in specific RECQ helicase proteins in DNA replication. For example, RECQL4 and to a lesser extent RECQL bind replication origins and contribute to DNA replication initiation (Thangavel et al., 2009; Xu et al., 2009). Single molecule DNA replication track analyses we and others have performed revealed roles for BLM, WRN and RECQL in replication fork rate maintenance and fork restart (Berti et al., 2013; Sidorova et al., 2013; Sidorova et al., 2008). RECQL4 has an interesting additional role in mtDNA maintenance: it is co-imported with TP53, and appears to limit mtDNA damage in a replication-dependent manner (Croteau et al., 2012; De et al., 2012; Gupta et al., 2014).
Several RECQ helicases also help maintain telomeres, though again display apparent functional specialization. Telomeric DNA poses a dual challenge to the DNA replication machinery as it is composed of repeated (the human telomeric repeat sequence is TTAGGG), GC-rich DNA organized into a unique chromatin ‘cap’ at the ends of chromosomes (Sfeir et al., 2009). G-rich lagging strands may form G4 DNA quadruplex structures (Maizels and Gray, 2013) which are a good biochemical substrates for WRN and BLM. Only WRN helicase activity is required for complete replication of telomeric G-rich lagging strands, whereas cells lacking RECQL, RECQL4 or BLM also show telomere breakage and loss. It is unclear whether these effects are via a common mechanism (Barefield and Karlseder, 2012; Crabbe et al., 2007; Crabbe et al., 2004; Ghosh et al., 2012; Popuri et al., 2014). In contrast, WRN and BLM both participate in the recombination-mediated ‘alternative lengthening of telomeres’ (ALT) pathway used by many tumor cells to gain replicative immortality (Mendez-Bermudez et al., 2012). One phenomenon that is not yet well-understood is the sensitivity of WRN+ cells to telomere-homologous DNA oligonucleotides (‘T-oligos’). The ability to respond to T-oligos may depend upon WRN exonuclease activity, and may have therapeutic potential in light of protective or deleterious responses in different cell types (Eller et al., 2006; Gilchrest and Eller, 2009).
All five of the human RECQ helicases also participate in DNA double strand break repair by non-homologous DNA end joining (NHEJ) or homology-dependent recombination (HDR or HR). WRN and RECQL4 participate in base excision repair, and RECQL5 may play an additional role in ssDNA break repair (reviewed in (Brosh Jr, 2013; Croteau et al., 2014; Sidorova and Monnat Jr, 2015).
‘Rewritten in the skin’ – RECQ helicases in transcription
Previous analyses had suggested a role for the WRN and BLM RECQ helicases in transcription (Johnson et al., 2010; Kyng et al., 2003). In order to better understand this role, we analyzed gene and miRNA expression in mutation-typed WS and BS primary fibroblasts and in isogenic control primary fibroblasts depleted of the WRN or BLM protein. These analyses identified −3,000 genes and dozens of miRNAs whose expression was significantly altered by the loss of WRN and BLM function. Among the subset (−25%) of genes altered in both WS and BS cells, a surprisingly high fraction (>90%) had expression altered in the same direction (Nguyen et al., 2014)(Tang, Robles et. al., in preparation). Many WRN- and BLM-responsive downregulated genes contained G quadruplex (G4) DNA motifs in their 5' ends, providing strong evidence that G4 DNA structures are physiologic as well as biochemical substrates for WRN and BLM.
The genes and miRNAs altered in WS and/or BS cells play important roles in pathways that drive cell growth, proliferation, death and survival. BS patient cells had gene expression patterns predicted to alter DNA replication recombination and repair, as well as immune function and tumorigenic/DNA damage signaling. These make good sense in light of our understanding of the biochemical, cellular and organismal phenotype of BS (Nguyen et al., 2014)(Tang, Robles et. al., in preparation). WS appears more complex, and thus intriguing. One remarkable—and as yet not fully understood—finding in WS cells was coordinate upregulation of nearly all of the cytoplasmic tRNA synthestase (ARS) and synthetase-associated interacting protein (AIMP) genes (Kim et al., 2011; Park et al., 2010; Wallen and Antonellis, 2013; Yao and Fox, 2013).
The mechanism of ARS/AIMP upregulation is not yet understood but may include MYC, which can alter and in turn be modulated by ARSs (Shi et al., 2014) while driving expression of WRN and telomerase (Grandori et al., 2003). ARS and AIMP overexpression in WS could perturb protein homeostasis by altering global protein turnover and/or translational fidelity (Lee et al., 2014; Wolff et al., 2014). Altered tRNA charging could affect the balance between mitochondrial and nuclear protein synthesis to promote mitochondrial dysfunction and oxidative stress (Jovaisaite and Auwerx, 2015). It could also drive disease pathogenesis via the growing list of ARS/AIMP ‘non-canonical’ functions that modulate disease-related metabolic, developmental, angiogenic, tumorigenic, immune and inflammatory pathways (Paul and Schimmel, 2013; Son et al., 2014). All of these areas are ripe for further exploration using a combination of new genomic and proteomic approaches.
The origins of phenotype
The biochemical and cellular specializations of the individual RECQ helicases outlined above begin to indicate how the loss of function of a single RECQ protein may lead to specific RECQ deficiency syndromes and their associated disease risks. As noted above, RECQ-deficient cells display cell proliferation defects in conjunction with genomic instability (Dhillon et al., 2007; Mao et al., 2010; Martin et al., 1970; Sharma et al., 2007; Sidorova et al., 2013; Thangavel et al., 2009; Warren et al., 1981). These cellular defects in turn are likely part of the explanation for why BS and RTS patients are often small though proportionately developed. BLM or RECQL4 loss can both interfere with DNA replication and impair cell production throughout development. Despite this, development appears largely normal in both syndromes and responds by proportionately scaling output (the fetus) to reflect inadequate substrate (cells). This proportional dwarfing is particularly striking in BS, where patients are born and often remain at or below the 5th percentile for height and weight (Keller et al., 1999).
The progressive development of progeroid features in WS only after development is largely complete may reflect the starkly different outcome of replication arrest, which leads to high levels of cell death in BS though not in WS cells (Mao et al., 2010; Sidorova et al., 2013). WRN loss, in contrast, has a more profound effect on transcription than does BLM loss, and thus may have a correspondingly more prominent role in transcription and tissue maintenance (see above). Disrupted DNA metabolism in WS patient cells could drive the progressive accumulation of mutant and senescent cells in many tissues, with acquisition of a senescence-associated secretory phenotype that could in turn promote the elevated risk of many clinically important age-associated diseases (Campisi, 2013). Cellular senescence in the RECQ helicase syndromes may have one modest silver lining: it is an effective, albeit non-specific, tumor-suppressive mechanism (Adda di Fagagna, 2008; Collado and Serrano, 2010). Altered RECQ expression, as opposed to mutation, may be frequent in many tumor types (Lao et al., 2013). However the previous suggestion that these changes may be largely methylation-driven (Agrelo et al., 2006) has not been consistent enough in our hands to serve as a reliable marker for altered WRN expression in tumors (Lao et al., 2013)(Bosch, Luo et al, in preparation).
More systematic collection of patient data, longitudinal study of patients, and the collection and distribution of well-characterized clinical samples should all aid our understanding of the RECQ deficiency syndromes. We also have a growing range of options to capture and analyze patient-derived cells and cell lines, including improved short term primary culture, organoid culture and the generation of cell lines and iPS cells (Cheung et al., 2014; Martin et al., 1970; Shimamoto et al., 2014; Wyllie et al., 2000). These analyses and materials have the potential to identify genetic and environmental modifiers of disease progression and acquired disease risk.
New clues to skin biology, disease and therapy
The above analyses emphasize the complexity of disease pathogenesis in even ‘simple’ monogenic genetic diseases such as Werner syndrome. They also emphasize how new insights into disease pathogenesis from rare heritable diseases may improve our understanding of skin biology while identifying potential new therapies. One example comes from another skin disease, recessive dystrophic epidemolysis bullosa (RDEB). RDEB results from COL7A1 mutations leading to loss of Type VII collagen, a marked reduction in anchoring fibrils and extreme skin fragility with loss and scaring (Tolar and Wagner).
The potential for genetic therapies of EB and a handful of other heritable diseases was emphasized over two decades ago by the identification of patients who had undergone spontaneous reversion of causative mutations with partial or full correction of disease-specific defects in skin, blood, lymphoid or liver (Hirschhorn 2003). A deeper understanding of the role of Type VII collagen in skin (Tolar and Wagner) had led to a diversity of therapeutic approaches: complementation (GENEGRAFT 2014) or targeted correction of causative COL7A1 mutations in epidermal cells (Sebastiano et al., 2014); use of patient-derived, mutation-reverted keratinocytes (Tolar et al., 2014); and the repair in trans of anchoring fibrils using allogeneic fibroblasts (Venugopal et al., 2013), mutation-corrected, iPS-derived fibroblasts (Wenzel et al., 2014) or bone marrow transplantation (Tolar and Wagner; Wagner et al., 2010). Repair in trans may be a viable option for dealing with the scleroderma-like skin changes seen in WS, as might aminoglycoside suppression of WRN missense mutations, a strategy that has been used in RDEB (Cogan et al., 2014).
Another unusual example of where we may find new clues to treating heritable or acquired skin disease as well as age-associated changes comes from comparative genetics, more specifically the African spiny mice Acomys kempi and Acomys percivali. Acomys mice have the remarkable ability to shed–and then regenerate without scaring–large segments of skin, and may have evolved this ability to escape predators (Seifert et al., 2012). While scarless wound healing also occurs in humans, it is largely restricted to the fetus (Yates et al., 2012). Acomys mice, in contrast, are able to continuously regenerate skin without scaring in the face of injury, inflammation and infection. Understanding the mechanistic basis for this remarkable example of epimorphic regeneration may identify new ways to maintain or rejuvenate skin, and to help individuals with injuries that lead to disfiguring scaring. Nature undoubtedly holds more examples of remarkable cutaneous biology. Finding these and turning them to good use will require imagination, together with a willingness to look—and think—a bit beyond our usual comfort zone.
Acknowledgments
Work in the author’s lab has been supported by the US National Institutes on Aging, Cancer and Environmental Health Sciences and by the Nippon Boehringer Ingelheim Virtual Research Institute of Aging. The author thanks the many individuals who have contributed to the basic science of RECQ helicases, and patients, families and clinicians for their friendship, generosity and support. The preparation of this manuscript was supported in part by US NIH NCI Award CA77852 to the author, and Montagna Symposium on the Biology of Skin NIAMS/NIA award R13-AR009431-48 to M. Kulesz-Martin.
Footnotes
Conflicts of Interest
The author has no relevant conflicts of interest to declare.
References
- Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- Agrelo R, Cheng WH, Setien F, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proceedings of the National Academy of Sciences. 2006;103:8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Hickson ID. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochemical Journal. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefield C, Karlseder J. The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures. Nucleic Acids Res. 2012;40:7358–7367. doi: 10.1093/nar/gks407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti M, Chaudhuri AR, Thangavel S, et al. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013;20:347–354. doi: 10.1038/nsmb.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs. American Journal of Diseases of Children. 1954;88:754–758. [PubMed] [Google Scholar]
- Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–558. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annual Review of Physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H-H, Liu X, Canterel-Thouennon L, et al. Telomerase protects Werner syndrome lineage-specific stem cells from premature aging. Stem Cell Reports. 2014;2:534–546. doi: 10.1016/j.stemcr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan J, Weinstein J, Wang X, et al. Aminoglycosides restore full-length Type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther. 2014;22:1741–1752. doi: 10.1038/mt.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe L, Jauch A, Naeger CM, et al. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proceedings of the National Academy of Sciences. 2007;104:2205–2210. doi: 10.1073/pnas.0609410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe L, Verdun RE, Haggblom CI, et al. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- Croteau DL, Popuri V, Opresko PL, et al. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 2014;83:519–552. doi: 10.1146/annurev-biochem-060713-035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Rossi ML, Canugovi C, et al. RECQL4 localizes to mitochondria and preserves mitochondrial DNA integrity. Aging Cell. 2012;11:456–466. doi: 10.1111/j.1474-9726.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Kumari J, Mudgal R, et al. RECQL4 is essential for the transport of p53 to mitochondria in normal human cells in the absence of exogenous stress. Journal of Cell Science. 2012;125:2509–2522. doi: 10.1242/jcs.101501. [DOI] [PubMed] [Google Scholar]
- Dhillon KK, Sidorova J, Saintigny Y, et al. Functional role of the Werner syndrome RecQ helicase in human fibroblasts. Aging Cell. 2007;6:53–61. doi: 10.1111/j.1474-9726.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Eller MS, Liao X, Liu S, et al. A role for WRN in telomere-based DNA damage responses. Proceedings of the National Academy of Sciences. 2006;103:15073–15078. doi: 10.1073/pnas.0607332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis NA, Groden J, Ye TZ, et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Martin GM, Schultz AL, et al. Werner's syndrome: A review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine. 1966;45:177–221. doi: 10.1097/00005792-196605000-00001. [DOI] [PubMed] [Google Scholar]
- GENEGRAFT. Phase I/II ex vivo gene therapy clinical trial for recessive dystrophic epidermolysis bullosa using skin equivalent grafts genetically corrected with a COL7A1-encoding SIN retroviral vector (GENEGRAFT) Human Gene Therapy Clinical Development. 2014;25:65–66. doi: 10.1089/humc.2014.2508. [DOI] [PubMed] [Google Scholar]
- German J. Bloom's syndrome. VIII. Review of clinical and genetic aspects. In: Goodman RM, Motulsky AG, editors. Genetic Diseases Among Askenazi Jews. Vol. 1. New York: Raven Press; 1979. pp. 121–139. [Google Scholar]
- German J. Bloom syndrome: a Mendelian prototype of somatic mutational disease. Medicine. 1993;72:393–406. [PubMed] [Google Scholar]
- German J. Bloom's syndrome: XX. The first 100 cancers. Cytogenetics and Cell Genetics. 1997;93:100–106. doi: 10.1016/s0165-4608(96)00336-6. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Rossi ML, Singh DK, et al. RECQL4, the protein mutated in Rothmund-Thomson syndrome, functions in telomere maintenance. J Biol Chem. 2012;287:196–209. doi: 10.1074/jbc.M111.295063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS. Cancer therapeutics: smart and smarter. Drugs of the Future. 2009;34:205–216. [Google Scholar]
- Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mechanisms of Ageing and Development. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Goto M. Clinical characteristics of Werner syndrome and other premature aging syndromes: pattern of aging in progeroid syndromes. Gann Monograph Cancer Res. 2001;49:27–39. [Google Scholar]
- Goto M, Ishikawa Y, Sugimoto M, et al. Werner syndrome: A changing pattern of clinical manifestations in Japan (1917−2008) BioScience Trends. 2013;7:13–22. [PubMed] [Google Scholar]
- Grandori C, Wu KJ, Fernandez P, et al. Werner syndrome protein limits MYC-induced cellular senescence. Genes and Development. 2003;17:1569–1574. doi: 10.1101/gad.1100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, De S, Srivastava V, et al. RECQL4 and p53 potentiate the activity of polymerase γ and maintain the integrity of the human mitochondrial genome. Carcinogenesis. 2014;35:34–45. doi: 10.1093/carcin/bgt315. [DOI] [PubMed] [Google Scholar]
- Hatamochi A. Dermatological features and collagen metabolism in Werner syndrome. In: Goto M, Miller RW, editors. From Premature Gray Hair to Helicase-Werner Syndrome: Implications for Aging and Cancer. Tokyo: Japan Scientific Societies Press; 2001. pp. 51–59. [Google Scholar]
- Hirschhorn R. In vivo reversion to normal of inherited mutations in humans. Journal of Medical Genetics. 2003;40:721–728. doi: 10.1136/jmg.40.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisama FM, Kubisch C, Martin GM, et al. Clinical utility gene card for: Werner Syndrome. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Cao K, Ryvkin P, et al. Altered gene expression in the Werner and Bloom syndromes is associated with sequences having G-quadruplex forming potential. Nucleic Acids Research. 2010;38:1114–1122. doi: 10.1093/nar/gkp1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovaisaite V, Auwerx J. The mitochondrial unfolded protein response-synchronizing genomes. Current Opinion in Cell Biology. 2015;33:74–81. doi: 10.1016/j.ceb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Keller KR, Shew SB, et al. Growth deficiency and malnutrition in Bloom syndrome. The Journal of Pediatrics. 1999;134:472–479. doi: 10.1016/s0022-3476(99)70206-4. [DOI] [PubMed] [Google Scholar]
- Kim S, You S, Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: more than housekeeping. Nat Rev Cancer. 2011;11:708–718. doi: 10.1038/nrc3124. [DOI] [PubMed] [Google Scholar]
- Kitao S, Shimamoto A, Goto M, et al. Mutations in RECQ4L cause a subset of cases of Rothmund-Thomson syndrome. Nature Genetics. 1999;22:82–84. doi: 10.1038/8788. [DOI] [PubMed] [Google Scholar]
- Kyng KJ, May A, Kolvraa S, et al. Gene expression profiling in Werner syndrome closely resembles that of normal aging. Proceedings of the National Academy of Sciences. 2003;100:12259–12264. doi: 10.1073/pnas.2130723100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao VV, Welcsh P, Luo Y, et al. Altered RECQ helicase expression in sporadic primary colorectal cancers. Translational Oncology. 2013;6:458–469. doi: 10.1593/tlo.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet Journal of Rare Diseases. 2010;5:2. doi: 10.1186/1750-1172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauper JM, Krause A, Vaughan TL, et al. Spectrum and risk of neoplasia in Werner syndrome: A systematic review. PLoS ONE. 2013;8:e59709. doi: 10.1371/journal.pone.0059709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauper JM, Monnat RJ., Jr Diabetes mellitus and cancer in Werner syndrome. Acta Diabetologica. 2013;51:159–161. doi: 10.1007/s00592-013-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Kim DG, Kim B-G, et al. Promiscuous methionyl-tRNA synthetase mediates adaptive mistranslation to protect cells against oxidative stress. Journal of Cell Science. 2014;127:4234–4245. doi: 10.1242/jcs.152470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9:e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao FJ, Sidorova JM, Lauper JM, et al. The human WRN and BLM RecQ helicases differentially regulate cell proliferation and survival after chemotherapeutic DNA damage. Cancer Research. 2010;70:6548–6555. doi: 10.1158/0008-5472.CAN-10-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Sprague CA, Epstein CJ. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Laboratory Investigation. 1970;23:86–92. [PubMed] [Google Scholar]
- Mendez-Bermudez A, Hidalgo-Bravo A, Cotton VE, et al. The roles of WRN and BLM RecQ helicases in the alternative lengthening of telomeres. Nucleic Acids Res. 2012;40:10809–10820. doi: 10.1093/nar/gks862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat RJ., Jr . Werner syndrome. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO/IARC Monograph on Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2013. pp. 393–394. [Google Scholar]
- Monnat RJ., Jr Cancer pathogenesis in the human RecQ helicase deficiency syndromes. Gann Monograph Cancer Res. 2001;49:83–94. [Google Scholar]
- Nguyen GH, Tang W, Robles AI, et al. Regulation of gene expression by the BLM helicase correlates with the presence of G-quadruplex DNA motifs. Proceedings of the National Academy of Sciences. 2014;111:9905–9910. doi: 10.1073/pnas.1404807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer BS, Kugel VH. Werner's syndrome - a heredo-familial disorder with scleroderma, bilateral juvenile cataract, precocious graying of hair and endocrine stigmatization. Trans Assoc Am Physician. 1934;49:358. [Google Scholar]
- Park SG, Choi E-C, Kim S. Aminoacyl-tRNA synthetase–interacting multifunctional proteins (AIMPs): A triad for cellular homeostasis. IUBMB Life. 2010;62:296–302. doi: 10.1002/iub.324. [DOI] [PubMed] [Google Scholar]
- Paul M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9:145–153. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehmoeller G. Memory of my father, Otto Werner. Gann Monograph Cancer Res. 2001;49:vii–viii. [Google Scholar]
- Popuri V, Hsu J, Khadka P, et al. Human RECQL1 participates in telomere maintenance. Nucleic Acids Res. 2014;42:5671–5688. doi: 10.1093/nar/gku200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmund A. Ueber cataracten in verbindung mit einer eigent mlichen hautdegeneration. Arch Klin Exp Ophtal. 1868;14:159–182. [Google Scholar]
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Science Translational Medicine. 2014;6:264ra163. doi: 10.1126/scitranslmed.3009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561–565. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Stumpo DJ, Balajee AS, et al. RECQL, a Member of the RecQ Family of DNA Helicases, Suppresses Chromosomal Instability. Molecular and Cellular Biology. 2007;27:1784–1794. doi: 10.1128/MCB.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Xu X, Zhang Q, et al. tRNA synthetase counteracts c-Myc to develop functional vasculature. eLife. 2014;3:e02349. doi: 10.7554/eLife.02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto A, Kagawa H, Zensho K, et al. Reprogramming suppresses premature senescence phenotypes of Werner syndrome cells and maintains chromosomal stability over long-term culture. PLoS ONE. 2014;9:e112900. doi: 10.1371/journal.pone.0112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Kehrli K, Mao F, et al. Distinct functions of human RECQ helicases WRN and BLM in replication fork recovery and progression after hydroxyurea-induced stalling. DNA Repair. 2013;12:128–139. doi: 10.1016/j.dnarep.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Li N, Folch A, et al. The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle. 2008;7:796–807. doi: 10.4161/cc.7.6.5566. PMID:18250621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Monnat RJ., Jr Human RECQ helicases: roles in cancer, aging, and inherited disease. Advances in Genomics and Genetics. 2015;5:19–33. [Google Scholar]
- Siitonen HA, Kopra O, Kaariainen H, et al. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Human Molecular Genetics. 2003;12:2837–2844. doi: 10.1093/hmg/ddg306. [DOI] [PubMed] [Google Scholar]
- Siitonen HA, Sotkasiira J, Biervliet M, et al. The mutation spectrum in RECQL4 diseases. Eur J Hum Genet. 2009;17:151–158. doi: 10.1038/ejhg.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S, Park M, Kim S. Extracellular activities of aminoacyl-tRNA synthetases: new mediators for cell–cell communication. In: Kim S, editor. Aminoacyl-tRNA Synthetases in Biology and Medicine. Vol. 344. Netherlands: Springer; 2014. pp. 145–166. [DOI] [PubMed] [Google Scholar]
- Taylor WB. Rothmund's syndrome–Thomson's syndrome: Congenital poikiloderma with and without juvenile cataracts. A review of the literature,report of a case, and discussion of the relationship of the two syndromes. AMA Archives of Dermatology. 1957;75:236–244. doi: 10.1001/archderm.1957.01550140080013. [DOI] [PubMed] [Google Scholar]
- Thangavel S, Mendoza-Maldonado R, Tissino E, et al. The human RECQ1 and RECQ4 helicases play distinct roles in DNA replication initiation. Molecular and Cellular Biology. 2009;30:1382–1396. doi: 10.1128/MCB.01290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannhauser SJ. Werner's syndrome (progeria of the adult) and Rothmund's syndrome: two types of closely related hederofamilial atrophic dermatoses with juvenile cataracts and endocrine features; a critical study of five new cases. Annals of Internal Medicine. 1945;23:559–626. [PubMed] [Google Scholar]
- Thomson MS. Poikiloderma congenitale. British Journal of Dermatology. 1936;48:221–234. [Google Scholar]
- Tolar J, McGrath JA, Xia L, et al. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2014;134:1246–1254. doi: 10.1038/jid.2013.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolar J, Wagner JE. Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: repair of the extracellular matrix. The Lancet. 382:1214–1223. doi: 10.1016/S0140-6736(13)61897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsbol TO, Cohen HJ. Werner's sydrome: an underdiagnosed disorder resembling premature aging. Age. 1984;7:75–88. [Google Scholar]
- Van Maldergem L, Siitonen HA, Jalkh N, et al. Revisiting the craniosynostosis-radial ray hypoplasia association: Baller-Gerold syndrome caused by mutations in the RECQL4 gene. Journal of Medical Genetics. 2006;43:148–152. doi: 10.1136/jmg.2005.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SS, Yan W, Frew JW, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. Journal of the American Academy of Dermatology. 2013;69:898–908.e7. doi: 10.1016/j.jaad.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. New England Journal of Medicine. 2010;363:629–639. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen RC, Antonellis A. To charge or not to charge: mechanistic insights into neuropathy-associated tRNA synthetase mutations. Current Opinion in Genetics & Development. 2013;23:302–309. doi: 10.1016/j.gde.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Gannavarapu A, Kozinetz CA, et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome. J Natl Cancer Inst. 2003;95:669–674. doi: 10.1093/jnci/95.9.669. [DOI] [PubMed] [Google Scholar]
- Wang LL, Levy ML, Lewis RA, et al. Clinical manifestations in a cohort of 41 Rothmund- Thomson syndrome patients. American Journal of Human Genetics. 2001;102:11–17. doi: 10.1002/1096-8628(20010722)102:1<11::aid-ajmg1413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Warren ST, Schultz RA, Chang Cc, et al. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Science Translational Medicine. 2014;6:264ra165. doi: 10.1126/scitranslmed.3010083. [DOI] [PubMed] [Google Scholar]
- Werner O. On cataract in conjunction with scleroderma (translated by H. Hoehn) In: Salk D, Fujiwara Y, Martin GM, editors. Werner's Syndrome and Human Aging. 1st. New York: Plenum Press; 1985. pp. 1–14. [Google Scholar]
- Wolff S, Weissman Jonathan S, Dillin A. Differential scales of protein quality control. Cell. 2014;157:52–64. doi: 10.1016/j.cell.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Wyllie FS, Jones CJ, Skinner JW, et al. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nature Genetics. 2000;24:16–17. doi: 10.1038/71630. [DOI] [PubMed] [Google Scholar]
- Xu X, Rochette PJ, Feyissa EA, et al. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J. 2009;28:3005–3014. doi: 10.1038/emboj.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P, Fox PL. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Molecular Medicine. 2013;5:332–343. doi: 10.1002/emmm.201100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Research Part C: Embryo Today: Reviews. 2012;96:325–333. doi: 10.1002/bdrc.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CE, Oshima J, Fu YH, et al. Positional cloning of the Werner's syndrome gene. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]