Abstract
Background
Epidemiologic research has yielded inconsistent evidence on whether use of hormonal contraception (HC) increases women’s risk of HIV acquisition. A robust meta-analysis of existing data can yield a valid summary estimate to inform guidelines, models and future studies.
Methods
We updated a recent systematic review to identify studies examining the relationship between various HC methods and women’s risk of HIV. We assessed statistical heterogeneity, and, when appropriate, combined point estimates using random effects models. We explored heterogeneity through subgroup and stratified analyses according to study populations and design features.
Findings
We identified 26 studies, 12 of which met inclusion criteria. There was evidence of a modest increase in HIV risk in the ten studies examining depot-medroxyprogesterone acetate (DMPA) [pooled relative risk (RR) =1.40, 95% CI: 1.16, 1.69]. This risk was lower in the eight studies conducted with women in the general population [pooled RR=1.31, 95% CI: 1.10, 1.57]. There was substantial between study heterogeneity in secondary analyses of trials (n=7, I2=51.1%). Although individual study estimates suggested an elevated risk, substantial heterogeneity between the two studies conducted with high risk women (I2=54%) precluded pooling estimates. There was no evidence of an elevated HIV risk in the ten studies examining oral contraceptive pills (OCPs) [pooled RR = 1.00, 95% CI: 0.86, 1.16] or the five studies examining norethisterone enanthate (Net-En) ([pooled RR=1.10; 95% CI: 0.88, 1.37].
Interpretation
The risks of HIV found here would not merit complete withdrawal of DMPA, OCPs, or Net-En from the contraceptive method mix in most settings for women in the general population.
INTRODUCTION
Despite over two decades of scientific inquiry, uncertainty remains regarding whether use of hormonal contraception (HC) increases women’s risk of HIV acquisition(1). The potential implications of an elevated risk are significant. Globally, 140 million women use HC, including 41 million injectable users and 100 million oral contraceptive pill (OCP) users(2). Use of these methods prevents unintended pregnancies, reduces maternal and infant morbidity and mortality, and enables women to achieve other life goals(3). Given high fertility levels and rates of maternal mortality, particularly in settings of high HIV prevalence, women must be able to avoid pregnancy without increasing their risk of HIV.
After reviewing available epidemiologic evidence, an expert panel convened by the World Health Organization (WHO) in 2012 recommended leaving HC a “Category 1” method with no restrictions for use. However, the panel also recommended that women using progestin-only injectables like DMPA be “strongly advised to also always use condoms”(4). Despite this guidance, some countries in sub-Saharan Africa (SSA) are considering withdrawing DMPA from their family planning programs, while modeling studies suggest that the effects of such a decision on unintended births and maternal and infant morbidity and mortality would be substantial in most settings (5–7). Thus, the decision to remove HC will depend not only on whether there is an actual association, but importantly its magnitude to determine whether the increased HIV risk outweighs the tremendous benefits of highly effective contraception.
Given the public health urgency of this question, it is critical to maximally leverage existing observational evidence. Several recent systematic reviews concluded that existing evidence suggests an increased risk of HIV associated with use of progestin-only injectables, potentially isolated to high risk women, but stopped short of quantitatively summarizing results due to perceived heterogeneity in study designs and populations (8–10). However, up to now, heterogeneity has never been quantitatively assessed, and even a moderate amount should not preclude moving forward with meta-analyses of observational data, especially when randomized control trial data are not available to address an urgent public health issue requiring policy decisions(11, 12). Furthermore, as research on this topic has intensified in recent years, the methodological approaches to answering this question have increased in rigor and similarity, making it an opportune time for meta-analysis.
Here, we build on one recent review(8) to quantitatively summarize observational evidence, offering a series of pooled estimates of the effect of HC use on HIV risk by method type. We focus our analyses on studies of sufficient quality and comparability, and explore heterogeneity through a series of a priori secondary analyses.
METHODS
This meta-analysis was conducted in accordance with the PRISMA guidance(13). All statistical analyses were guided by Egger, Davey-Smith, and Altman(14).
Study identification and selection
We used the WHO technical review (4) to identify studies.* We searched PubMed using the terms “hormonal contraception”, “HIV/acquisition”, “injectables” “progestin”, and “oral contraceptive pills”. In addition, we identified relevant abstracts presented at the 2011 through 2014 International AIDS Society and Conference on Retroviruses and Opportunistic Infections meetings and followed up with authors to determine if their analyses had been published. Finally, we reviewed lists of studies with experts in the field.
Two investigators (LR, KS) reviewed the full text of articles identified to determine if they met the following inclusion criteria: Assessed hormonal contraceptive use as an exposure, including at least one of the following categories: depot-medroxyprogesterone acetate (DMPA), norethisterone enanthate (Net-En), combined oral contraceptives (COCs), or progestin only pills (POPs); Employed a prospective design and excluded HIV positive women at baseline, ensuring exposure assessment preceded detection of an incident HIV infection; Analytic approach minimized confounding and selection bias by: Adjusting for confounders in multivariate models, including at a minimum age and condom use; Having minimal loss to follow up (defined as ≤ 30%); Published in a peer-reviewed journal by May 2014; Data collection took place in a low or middle income country as defined by the World Bank.
Data extraction and coding
Two reviewers independently extracted data using a custom, piloted spreadsheet. One investigator compared extractions to ensure inter-coder reliability; when discrepancies arose, a third investigator was brought in to arbitrate.
Given the array of hormonal contraceptive methods available, studies often differed in their classification of contraceptive types and many presented multiple effect estimates. We focused extraction on estimates disaggregated by hormone formulation (e.g, DMPA, Net-En, COCs, or POPs). When only method type (e.g., “injectable” or “pill”) was specified, we reviewed the article to identify whether a specific formulation (e.g., DMPA vs. NetEn) predominated. We coded how comparison groups were constructed, noting whether women using condoms (either alone or in addition to HC), other types of HC, or no contraception were included.
We extracted effect estimates and 95% confidence intervals (CIs) for each model. We made note of the confounders adjusted for in multivariate models and the analytic strategy used [e.g., Cox, inverse probability of treatment weighted marginal structural model (IPTW-MSM)]. In one instance, we also extracted a DMPA specific estimate and its 95% CI from a letter (15) submitted in response to an original manuscript (16).
We extracted information on features that might influence internal or external validity (and overall study quality) or explain heterogeneity, including: study retention rates, inter-survey intervals, the risk profile of study participants, and the study design underlying the estimate. For the risk profile of participants, we distinguished high-risk women or key populations (e.g., commercial sex workers, injection drug users, or women in serodiscordant [SD] partnerships) from women in the general population. Finally, we extracted details on the demographic characteristics of participants, recruitment sites, study durations, and exclusion criteria.
Statistical analysis
Effect estimates and their 95% CIs were log transformed and the standard error of each estimate was calculated. Funnel plots were generated to assess publication bias.
We selected one effect estimate per HC formulation per study† to include in primary pooled analyses.‡ When multiple effect estimates were available, we selected the estimate from the most fully adjusted multivariate model. Although four studies(16–19) presented estimates derived using IPTW-MSMs, we did not include these estimates in our primary pooled analyses as they estimate different parameters than traditional regression approaches and the two should not be compared or combined. Specifically, traditional Cox models estimate the average effect of treatment on an individual, whereas MSMs provide the average effect of treatment on the population(20). However, we performed separate analyses that combined only those estimates generated using IPTW-MSMs.
Evidence for statistical heterogeneity between studies was assessed for each HC formulation (DMPA, OCPs/COCs, NetEn) using the I2 statistic and its 95% CI; an I2 ≥ 50% was considered evidence of sufficient heterogeneity to contraindicate a pooled estimate. (21). When the I2 was less than 50%, pooled effect estimates were calculated using DerSimonian and Laird random effects models(22).
We assessed the robustness of findings and explored heterogeneity through a series of a priori secondary analyses. First, we conducted an influence analysis to identify whether any one study disproportionately affected the results. Second, we stratified meta-analyses according to: 1) the risk profile of the study population (high risk vs. general population), and 2) the original study design (prospective cohort vs. randomized trial). Third, given concerns that having a reference group that is composed largely of condom users may artificially inflate the risk of HIV acquisition for HC users(23), we explored whether our results were sensitive to the exclusion of condom users from the comparison group. Finally, we explored whether results were qualitatively different when studies with inter-survey intervals longer than the duration of the contraceptive methods under study (1 to 3 months) were excluded. All analyses were conducted in Stata 12.0.
We refer to effect estimates as hazard ratios (HRs) since all of the studies in our pooled analyses used this measure, with one exception (24). That study estimated an incidence rate ratio (IRR), which is comparable in practical interpretation to the HR (25, 26).
RESULTS
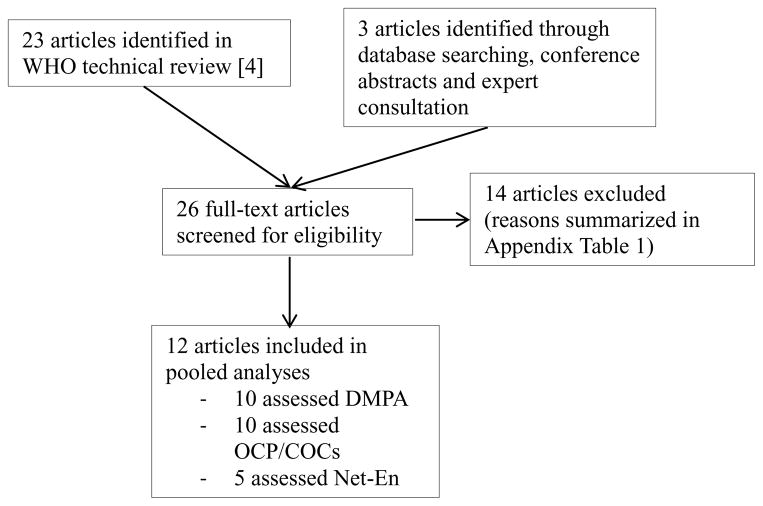
We identified 26 articles (16–19, 24, 27–47), 12 of which met our inclusion criteria (16–19, 24, 37–40, 44, 45, 47) [Figure 1 and Appendix Table 1]. Two represented analyses on the same population; however, since they employed different analytic approaches (Cox regression (40) vs. IPTW-MSM (17)), both were included but in separate pooled analyses to prevent double counting.
FIGURE 1.
Flowchart of study selection
All studies in the final sample were conducted in SSA. Three, all prospective cohort studies, were designed specifically to assess the HC-HIV relationship (37, 39, 40). The rest were secondary analyses on cohorts enrolled in randomized trials of various HIV (16, 18, 19, 24, 44, 45, 47) and one cervical cancer (38) prevention interventions. Two study populations consisted of high risk women, either CSWs (37) or women in SD partnerships (16). The remainder were composed of women in the general population, typically recruited at family planning or other health centers. The median age of participants ranged from 25 to 40. With the exception of two studies that surveyed women every six (38) or ten (24) months, the remainder surveyed women at least every three months. With the exception of one study which followed a subset of women for six months (38), all studies planned to follow women for at least one year. The median follow up ranged from 12 to 31.2 months. Given heterogeneity in how study authors presented estimates of loss to follow up, we did not quantitatively summarize this metric. However, in general, study retention was high, with a minimum of six of 12 studies having retention rates over 85% (Tables 1 and 2).
TABLE 1.
Descriptive characteristics of studies included in primary DMPA-HIV, COC-HIV, and NetEn-HIV pooled analyses
| First autho and citation |
Year | Study location(s) |
Study Participants (SDP=serodiscordant partnerships; CSW=commercial sex workers) |
Mean age of study popln. |
Exposures assessed* |
# of HIV sero- conversions |
Effect Estimate (adjusted hazard ratio, unless otherwise noted) |
Reference group |
Inter- survey interval (months) |
Duration of follow up (months) |
Present IPTW- MSM^ estimates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crook [47] | 2013 | S. Africa, Uganda, Tanzania, Zambia | N=8663 women in microbicide trial; 786 in SDPs | 27 | DMPA | 146 | 1.45 (1.09, 1.93) | Non-hormonal or no method | 1 | 12 (planned) | X |
| Net-En | 69 | 1.20 (0.84, 1.69) | |||||||||
| OCPs | 50 | 0.90 (0.63, 1.26) | |||||||||
| Ref | 117 | -- | |||||||||
| McCoy [19] | 2012 | Zimbabwe, S. Africa | N=4913 women in MIRA trial | 27.5 | DMPA | 63 | 1.22 (0.84, 1.74) | Non-hormonal or no method | 3 | 17.9 (median) | X |
| Net-En | 17 | 1.15 (0.58, 1.95) | |||||||||
| OCPs | 61 | 0.84 (0.57, 1.22) | |||||||||
| Ref | 108 | -- | |||||||||
| Morrison [18] | 2012 | S. Africa | N=5567 women in Carraguard trial | 28 | DMPA | 270 (total) | 1.27 (0.93, 1.73) | Non-hormonal or no method | 3 | 24 (planned) | X |
| Net-En | 0.87 (0.60, 1.25) | ||||||||||
| COCs | 0.88 (0.49, 1.30) | ||||||||||
| Ref | -- | ||||||||||
| Heffron [16] | 2012 | Bostwana, Kenya, Rwanda, S. Africa, Tanzania, Uganda, Zambia | N=1314 women in SDP in Partners in Prevention Trial | 30.2 | Inj | 10 | 1.80 (0.92, 3.52) | Non-hormonal or no method | 3 | 18 (median) | X |
| DMPA# | Not reported | 3.93 (1.38,11.22) | |||||||||
| OCPs | 3 | 1.80 (0.55, 5.82) | |||||||||
| Wand [45] | 2012 | S. Africa | N=2236 women in microbicide trial | 27 | Inj-DMPA& | Not reported | 2.02 (1.37, 3.00) | Non-hormonal method^^ | 3 | Not reported | |
| OCPs | Not reported | 0.95 (0.62, 1.46) | |||||||||
| Morrison [40][18] | 2007 2010 |
Uganda, Zimbabwe | N=4435 women recruited at health clinics | 25 | DMPA | 213 (total) | 1.25 (0.89, 1.78) | Non-hormonal method^^ | 3 | 21.5 (mean) | X |
| COCs | 0.99 (0.69, 1.42) | ||||||||||
| Reid [44] | 2010 | S. Africa, Zambia, Zimbabwe | N=1358 women in acyclovir trial (HPTN 039) | 31 | Inj | Not reported | 0.94 (0.46, 1.92) | No method&& | 3 | 18 (planned) | |
| OCs | Not reported | 0.91 (0.45, 1.83) | |||||||||
| Baeten [37] | 2007 | Kenya | N=1206 CSWs recruited at communicable disease clinics | 26 | DMPA | 79 | 1.73 (1.28, 2.34) | No method or tubal ligation@@ | 1 | 14.9 (median) | |
| OCPs | 38 | 1.46 (1.00, 2.13) | |||||||||
| Ref | 118 | -- | |||||||||
| Kleinschmidt [39] | 2007 | S. Africa | N=551 women recruited at family planning clinics | 27.7 | DMPA | 1 | 0.46 (0.06, 3.79) | Non-hormonal or no method | 3 | 12 (planned) | |
| Net-En | 10 | 1.76 (0.64, 4.84) | |||||||||
| Ref | 12 | -- | |||||||||
| Myer** [38] | 2007 | S. Africa | N=4200 women in cervical cancer prevention trial | 40 | DMPA | Not reported | 0.75 (0.33, 1.68) | Non-hormonal or no method | 6–12** | 14.3** (median) | |
| Net-En | Not reported | 1.60 (0.63, 4.09) | |||||||||
| COC | Not reported | 0.66 (0.09, 4.78) | |||||||||
| Ref | Not reported | -- | |||||||||
| Kiddugavu [24] | 2003 | Uganda | N=5117 women in Rakai community-based HIV prevention trial | 25 | Inj-DMPA%% | 16 | 0.84 (0.41, 1.72)$$ | Non-hormonal or no method, excluding condoms | 10 | 31.2 (median) | |
| OCs | 12 | 1.12 (0.48, 2.56) $$ |
OCP = oral contraceptive pills, type not specified; COC = combined oral contraceptive pills; DMPA = injectable depo medroxyprogesterone acetate; Net-En = injectable norethisterone enanthate
Inverse probability of treatment weighted marginal structural model
Original analysis [16] presented pooled estimate for all injectables; subsequent reply presented results separately for DMPA users [15];
Authors note that injectable category includes only DMPA or generic alternative;
Authors note that the injectable group includes “mainly Depo-Provera users”;
Women using no method were excluded from all analyses;
Women using condoms or non-hormonal methods were considered as separate exposure categories and therefore were not included in the reference group;
Authors present effect estimates for women in both a 6 and 24 month cohort. Given high loss to follow up in the 24 month cohort (32%), we include only the estimate from the 6 month cohort in pooled analyses. However, since the authors do not present the number of sero-conversions or mean duration of follow up for the 6 month cohort, we include these indicators here;
Incidence Rate Ratio;
Authors note that condom use was analyzed as a separate covariate and was not used to determine the reference group. As a result, women using only condoms would likely be classified in the reference group as a “no method” user.
TABLE 2.
Exposures assessed in studies included in primary pooled analyses of the association of hormonal contraception and HIV
| First author and citation | Exposures | Number of HIV seroconversions | Effect estimate (adjusted hazard ratio, unless otherwise noted) | IPTW-MSM estimate |
|---|---|---|---|---|
| Crook [47] | Injectable depot medroxyprogesterone acetate | 146+ | 1.45 (1.09–1.93) | 1.49 (1.06–2.09) |
| Injectable norethisterone enanthate | 69 | 1.20(0.84–1.69) | 1.31 (0.86–1.99) | |
| Oral contraceptive pills, type not specified | 50 | 0.90 (0.63–1.26) | 1.00 (0.62–1.61) | |
| Reference | 117 | -- | -- | |
| McCoy [18] | Injectable depot medroxyprogesterone acetate | 63 | 1.22 (0.84–1.74) | Not reported |
| Injectable norethisterone enanthate | 17 | 1.15 (0.58-0.58–1.95) | Not reported | |
| Oral contraceptive pills, type not specified | 61 | 0.84 (0.57–1.22) | 0.86 (0.32–1.78) | |
| Combined oral contraceptive pills | 44 | 0.80 (0.53–1.19) | Not reported | |
| Reference | 108 | -- | -- | |
| Morrison [17] | Injectable depot medroxyprogesterone acetate | 270 (total) | 1.27 (0.93–1.73) | 1.28 (0.92–1.78) |
| Injectable norethisterone enanthate | 270 (total) | 0.87 (0.60–1.25) | 0.92 (0.64–1.32) | |
| Combined oral contraceptive pills | 270 (total) | 0.88 (0.49–1.30) | 0.84 (0.51–1.39) | |
| Reference | 270 (total) | -- | -- | |
| Heffron [16] | Injectable form, type not specified | 10 | 2.05 (1.04–4.04) | 2.19 (1.01–4.74)* |
| Injectable depot medroxyprogesterone acetate^ | Not reported | 3.93 (1.38–11.22) | Not reported | |
| Oral contraceptive pills, type not specified | 3 | 1.80 (0.55–5.82) | 1.63 (0.57–5.66) | |
| Reference | 60 | -- | -- | |
| Wand [45] | Injectable depot medroxyprogesterone acetate** | Not reported | 2.02 (1.37–3.00) | -- |
| Oral contraceptive pills, type not specified | Not reported | 0.95 (0.62–1.46) | -- | |
| Reference | Not reported | -- | -- | |
| Morrison [17, 20] | Injectable depot medroxyprogesterone acetate | 87 | 1.25 (0.89–1.78) | 1.48 (1.02–2.15) |
| Combined oral contraceptive pills | 71 | 0.99 (0.69–1.42) | 1.19 (0.80–1.76) | |
| Reference | 58 | -- | -- | |
| Reid [44] | Injectable form, type not specified | Not reported | 0.94 (0.46–1.92) | -- |
| Oral contraceptive pills, type not specified | Not reported | 0.91 (0.45–1.83) | -- | |
| Reference | Not reported | -- | -- | |
| Baeten [39] | Injectable depot medroxyprogesterone acetate | 79 | 1.73 (1.28–2.34) | -- |
| Oral contraceptive pills, type not specified | 38 | 1.46 (1.00–2.13) | -- | |
| Reference | 118 | -- | -- | |
| Kleinschmidt [40] | Injectable depot medroxyprogesterone acetate | 1 | 0.46 (0.06–3.79) | -- |
| Injectable norethisterone enanthate | 10 | 1.76 (0.64–4.84) | -- | |
| Reference | 12 | -- | -- | |
| Myer [19] # | Injectable depot medroxyprogesterone acetate | Not reported | 0.75 (0.33–1.68) | -- |
| Injectable norethisterone enanthate | Not reported | 1.60 (0.63–4.09) | -- | |
| Combined oral contraceptive pills | Not reported | 0.66 (0.09–4.78) | -- | |
| Reference | Not reported | -- | -- | |
| Kiddugavu [26] | Injectable depot medroxyprogesterone acetate^^ | 16 | 0.84 (0.41–1.72) || | -- |
| Oral contraceptive pills, type not specified | 12 | 1.12 (0.48–2.56) || | -- | |
| Reference | Not reported | -- | -- |
IPTW-MSM – inverse probability of treatment-weighted marginal structural model.
This estimate was not used in pooled analysis since it was not specific to depot medroxyprogesterone.
Original analysis [16] presented pooled estimates for all injectable hormonal contraception; subsequently, results were presented separately for users of injectable depot medroxyprogesterone acetate. [15]
Wand and colleagues [45] noted that the injectable category includes only depot medroxyprogesterone acetate or a generic alternative.
Investigators presented effect estimates for women in both 6-month and 24-month cohorts; with the high loss to follow-up in the 24month cohort (32%), we included only the estimate from the 6-month cohort in pooled analyses; however, since the investigators did not present the number of seroconversions or mean duration of follow-up for the 6-month cohort, we include these indicators here.
Kiddugavu and colleagues [26] noted that the injectable group includes mainly users of depot medroxyprogesterone acetate.
Incidence rate ratio.
Funnel plots for studies assessing injectables and OCPs were symmetrical, suggesting no major evidence of publication bias (Appendix, Figure 1A and 1B).
DMPA-HIV
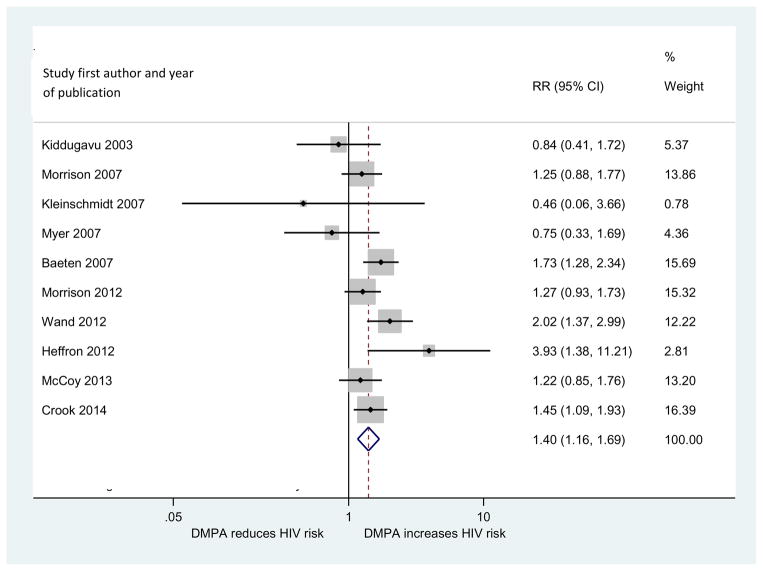
Ten articles examined the DMPA-HIV association. In pooled analyses, DMPA use was associated with an elevated risk of HIV acquisition as compared to use of non-hormonal or no methods [pooled relative risk (RR) =1.40, 95% CI: 1.16, 1.69] (Figure 2). An influence analysis revealed that no single study was driving results. The pooled effect estimate across the two studies that used IPTW-MSMs was comparable to the overall estimate [pooled relative risk (RR)=1.41, 95% CI: 1.15, 1.72] (Table 3).
FIGURE 2.
Forest plot of primary analysis of DMPA-HIV relationship
TABLE 3.
Comparison of results for primary, subgroup, and sensitivity analyses of the DMPA-HIV relationship using random effects models*
| Number of studies | I2 statistic (95% Confidence Interval) | Pooled HR (95% Confidence Interval) | Studies included | ||
|---|---|---|---|---|---|
| Primary analysis | 10 | 42.5% (0%, 72.5%) | 1.40 (1.16, 1.69) | 16, 18, 19, 24, 37, 38, 39, 40, 45, 47 | |
|
| |||||
| IPTW-MSM analysis# | 3 | 0% (0%, 58.2%) | 1.41 (1.15, 1.72) | 17, 18, 47 | |
|
| |||||
| Subgroup analysis | |||||
|
| |||||
| Higher risk women | 2 | 54.0% (0%, 88.7%) | -- | 16, 37 | |
|
|
|||||
| Women in the general population | 8 | 27.3% (0%, 67.3%) | 1.31 (1.10, 1.57) | 18, 19, 24, 38, 39, 40, 45, 47 | |
|
|
|||||
| Prospective cohort | 3 | 36.7% (0%, 79.9%) | 1.44 (1.04, 2.01) | 37, 39, 40 | |
|
|
|||||
| Sample from RCT | 7 | 51.1% (0%, 79.3%) | -- | 16, 18, 19, 24, 38, 45, 47 | |
|
| |||||
| Sensitivity analysis | |||||
|
| |||||
| Reference group includes women using non-hormonal or no methods^ | 9 | 40.8% (0%, 72.7%) | 1.44 (1.20, 1.73) | 16, 18, 19, 37, 38, 39, 40, 45, 47 | |
|
| |||||
| Inter-survey interval ≤ 3 months% | 8 | 36.1% (0%, 71.7%) | 1.48 (1.24, 1.77) | 16, 18, 19, 37, 39, 40, 45, 47 | |
All pooled analyses were limited to published, prospective studies that assessed incident HIV infection where the exposure category was predominantly (or exclusively) DMPA, the comparison group was comprised of women using non-hormonal or no contraceptive method (including condom users, unless noted), the model was adjusted for potential confounders of the HC-HIV relationship, including condom use and age, and no more than 30% of the study population was lost to follow up.
Two additional studies [16, 19] present estimates derived using IPTW-MSMs; however they were for injectables and not specific to DMPA and are therefore not included here.
One study in which condom users were explicitly excluded from the reference group [24] was excluded.
In subgroup analyses, the pooled relative risk among the three prospective cohort studies was 1.44 (95% CI: 1.04, 2.01). A high level of between-study heterogeneity (I2=51.1%, 95% CI: 0%, 79.3%) among the seven secondary analyses of cohorts from RCTs precluded calculating a pooled estimate among this subgroup (Table 3).
The eight studies conducted among women in the general population had a lower amount of heterogeneity (I2=27.3%, 95% CI: 0%, 67.3%) than the primary analysis (42.5%, 95% CI: 0%, 72.5%). The pooled estimate suggested a moderate increase in risk of HIV acquisition [pooled relative risk=1.31, 95% CI: 1.10, 1.57]. Individual study-level estimates were higher in the two studies with high-risk women (HR=1.73 [95%CI:1.28, 2.34] among CSWs (37) and 3.93 [95% CI: 1.37, 11.2] among women in SD partnerships(16)) (Table 3). However, a high level of heterogeneity (I2= 54%, 95% CI: 0%, 88.7%) between these two studies contraindicated pooling estimates.
In an analysis restricted to the nine studies in which the reference group included women using condoms (in addition to other methods or no method), the pooled effect estimate did not change substantively from the primary analysis (pooled RR = 1.44, 95% CI: 1.20, 1.73). An analysis restricted to the eight studies in which the inter-survey interval did not exceed three months revealed a pooled effect estimate that was slightly larger than our primary analysis (pooled RR=1.48, 95% CI: 1.24, 1.76) (Table 3).
COC/OCP-HIV
Ten studies presented estimates of the COC/OCP-HIV relationship. There was no elevated risk of HIV acquisition among COC/OCP users as compared to those using non-hormonal or no methods (pooled relative risk = 1.00, 95% CI: 0.86, 1.16) (Table 4) and our influence analysis revealed that no one study was driving these results. There was minimal evidence of between study heterogeneity (I2=0%, 95% CI: 0%, 48.6%). The pooled estimate among five studies using IPTW-MSMs was similar to the primary pooled result (pooled RR= 1.03, 95%CI: 0.81, 1.32) (Table 4). A subgroup analysis of the two studies conducted among high risk women revealed an elevated risk of HIV acquisition among COC/OCP users (pooled RR= 1.49, 95%CI: 1.04, 2.13) (Table 4).
TABLE 4.
Comparison of results for primary and subgroup analyses of the COC-HIV and NetEn-HIV relationship using random effects models*
| Number of studies | I2 statistic (95% Confidence Interval) | Pooled HR (95% Confidence Interval) | Studies included | ||
|---|---|---|---|---|---|
| Primary analysis – COCs | 10 | 0% (0%, 48.6%) | 1.00 (0.86, 1.16) | 16, 18, 19, 24, 37, 38, 40, 44, 45, 47 | |
|
| |||||
| MSM-IPTW analysis – COCs | 5 | 0% (0%, 55.2%) | 1.03 (0.81, 1.32) | 16, 17, 18, 19, 47 | |
|
| |||||
| Subgroup analysis – COCs | |||||
|
| |||||
| Higher risk women | 2 | 0% (0%, 0%) | 1.49 (1.04, 2.13) | 16, 37 | |
|
|
|||||
| Women in the general population | 8 | 0% (0%, 0%) | 0.92 (0.78, 1.18) | 18, 19, 24, 38, 40, 44, 45, 47 | |
|
|
|||||
| Prospective cohort | 2 | 52% (0%, 88.3%) | -- | 37, 40 | |
|
|
|||||
| Sample from RCT | 8 | 0% (0%, 0%) | 0.91 (0.75, 1.10) | 16, 18, 19, 24, 38, 44, 45, 47 | |
|
| |||||
| Sensitivity analysis – COCs | |||||
|
| |||||
| Reference group includes women using non-hormonal or no methods# | 8 | 0% (0%, 64.8%) | 1.00 (0.85, 1.17) | 16, 18, 19, 37, 38, 40, 45, 47 | |
|
| |||||
| Inter-survey interval ≤ 3 months% | 8 | 0% (0%, 64.3%) | 1.00 (0.86, 1.16) | 16, 18, 19, 37, 40, 44, 45, 47 | |
|
| |||||
| Primary analysis – NetEN | 5 | 0% (0%, 74.6%) | 1.10 (0.88, 1.37) | 18, 19, 38, 39, 47 | |
|
| |||||
| IPTW-MSM analysis – NetEn | 2 | 36% (0%, 78.1%) | 1.08 (0.77, 1.52) | 18, 47 | |
All pooled analyses were limited to published, prospective studies that assessed incident HIV infection where the exposure category was predominantly (or exclusively) COCs/NetEn, the comparison group was comprised of women using non-hormonal or no contraceptive method (including condom users, unless noted), the model was adjusted for potential confounders of the HC-HIV relationship, including condom use and age, and no more than 30% of the study population was lost to follow up.
NetEn-HIV
Analysis of the five studies that presented estimates on the Net-En-HIV relationship revealed no elevated risk of HIV acquisition (pooled RR=1.10; 95% CI: 0.88, 1.37) (Table 4) and minimal heterogeneity (I2=0%, 95% CI: 0%, 74.6%). Similar results were observed for the two studies estimated using IPTW-MSMs (pooled RR=1.08, 95% CI: 0.78, 1.52) (Table 4). An influence analysis was non-significant and subgroup analyses were not possible given the small number of studies.
DISCUSSION
Our meta-analysis found that among observational studies with similarly and precisely defined exposures, adjustment for key confounders, minimal selection bias, and sound analytic approaches, there is evidence of a small but increased risk of HIV acquisition associated with DMPA use. Consistent with an earlier meta-analysis on OCPs (48), no elevated risk was observed for OCP/COC users in the general population. Further, there was no elevated risk among Net-En users; however, the few studies contributing to this analysis precludes making any definitive statements on its association with HIV.
The results from this analysis, particularly for DMPA, should be used as an input parameter in ongoing modeling studies quantifying the tradeoffs associated with removing injectables from the contraceptive method mix. For example, Butler et al. (6) used both a hypothetical (RR=1.2) and a single study (OR=2.19)(16) estimate to predict changes in the numbers of HIV and maternal deaths following reductions in injectable HC use. Their findings suggest that, except in southern Africa where both HIV incidence and injectable use are high, the effect of removing HC on the number of maternal and HIV related deaths is sensitive to the effect estimate chosen. Given these results, it is possible that an increased risk of the magnitude found in our study (RR=1.4), particularly for women in the general population, would not merit complete withdrawal of DMPA as maternal mortality would still exceed HIV related deaths in most settings, particularly if women did not immediately have access to and uptake alternate, effective contraceptive options in the absence of DMPA, one of the assumption in Butler et al.’s models. Moving forward, we encourage Butler et al. (6) and others (5, 7) to apply our estimates and more fully explore regional/geographic and subpopulation differences so that context-specific contraceptive policy can be developed.
Our analysis also offers insight into potential sources of heterogeneity in results. Studies among women in the general population, which constitute the majority in our analysis, provide estimates of the average population level effect of HC on women’s risk of HIV acquisition. In contrast, those conducted among high risk women, of which there were two in our analysis, provide estimates of the effect of HC conditioned on a high likelihood of HIV exposure. For the millions of HC users worldwide, most of whom are not in serodiscordant or other high risk partnerships, this distinction is critical. While the elevated risks for DMPA and COC/OCP users reported in the two studies with CSWs (37) and women in SD partnerships(16) may warrant consideration of changing contraceptive guidelines for these populations, it would be premature to do so based on two studies. Further, it is critical that their results not be inadvertently generalized to women in the general population, which our study found had a more modest increase in risk that may only warrant a policy change in specific local contexts.
A priori, we established a strict set of inclusion criteria for our meta-analysis. Although this left us with fewer studies, and less power in our planned secondary analyses or to explore heterogeneity through meta-regression, it ensured that only comparable estimates were combined. Contrary to the perception that this literature is too diverse for meta-analysis, we did not uncover levels of heterogeneity that would preclude pooling estimates in most analyses. One notable exception is that although they contribute to the primary pooled analyses, we were unable to present a separate pooled estimate among the subset of studies conducted as secondary analyses of randomized controlled trials. The heterogeneity statistic for this group (I2=51.1%, 95% CI: 0%, 79.3%) rests on the border between “moderate” and “substantial” according to current Cochrane guidance(49). Whereas the prospective cohort studies were all designed specifically to answer this research question, the trials had divergent research objectives that may be reflected in the higher level of heterogeneity. Given this, a very conservative application of our findings would be to use the pooled RR and CI from only the prospective cohort studies. However, the strengths of the randomized trials, notably their large sample sizes, frequent assessment of contraceptive method use and switching, and efforts to ensure high retention, are compelling. Regardless, in the absence of another prospective cohort study or data from the proposed RCT on HC-HIV(50), the results of which would not be available for several years, other HIV prevention trials represent the primary source of data with which to explore this important question in the near future(51).
Our study findings should be interpreted in the context of several limitations. First, meta-analyses of observational studies, like observational studies themselves, are inherently more prone to concerns about bias and are not able to address whether the association between HC and HIV is causal (14). There has been extensive discussion about whether studies to date have sufficiently addressed the potential confounding effects of misreported condom use (23, 52), particularly since many study populations were drawn from HIV prevention trials where condom use is strongly encouraged and women may feel pressure to report socially desirable behaviors(53, 54). However, recent modeling studies suggest that the practical effects of condom misreporting may be overstated. For example, Smith et al. (55) demonstrate that only a substantial amount of condom use underreporting by non-hormonal contraceptive users, an unlikely scenario, could explain the elevated effect estimate observed in the recent Heffron et al. study (HR=2.19 for all injectables and HR=3.93 for DMPA specifically). Further, our own work with biomarkers of unprotected sex has demonstrated that misreporting of condom use is not statistically different between women using HC and those using other methods, and therefore may not bias effect estimates to the extent suggested(56). Note that even a randomized controlled trial will likely not be able to overcome many of the measurement challenges inherent to studying this question (5, 57). Likewise, the limitations of the original studies remain limitations of our analysis. For example, none of the studies prospectively assessed acute HIV infection, which would strengthen our confidence in the timing of exposure to HC and women’s subsequent acquisition of HIV.
A second limitation is that, despite our efforts to ensure systematic inclusion of all studies that assessed the HC-HIV relationship and explore publication bias using funnel plots, as with all meta-analyses, our results may be biased if only studies with significant results have been published. However, here, publication bias is less likely because over the past two decades, a null finding was equally compelling in terms of advancing the debate. Regardless, if studies that found positive and significant effects of HC on women’s risk of HIV acquisition were more likely to be published, that would imply that our findings represent an overestimate of the true association between HC and HIV.
Although our study findings echo what was previously presented qualitatively in two systematic reviews(8–10) (ie, there is evidence of a moderate increase in risk of HIV for injectable users, potentially isolated to high risk women), this study is the first to quantitatively summarize existing evidence, particularly for DMPA, and offer a series of weighted, pooled estimates of effect and their variances, by precise HC method type, for all studies published through May 2014. Since we approached data extraction and definitions of study quality independently from the other reviews, our study also contributes another perspective on the methodological rigor of the existing body of evidence.
Given concerns about the observational evidence collected to date, efforts are currently underway to fund a randomized trial on the HC-HIV relationship. Some might argue that the moderate increase in risk found in our study for DMPA users, who would comprise one of the intervention arms, might violate the principal of equipoise required for a trial (58). Importantly, also of concern is whether, given the methodological challenges inherent to studying this question (57), the randomized trial will offer evidence superior to that which currently exists, especially when also considering the personal and financial investments required for a trial(1). Our pooled estimates can immediately inform contraceptive policy, without waiting several years for trial data. In addition, our findings highlight an immediate need to refocus secondary analyses on CSWs and women in serodiscordant partnerships, because evidence for these high risk women is limited but suggests an elevated risk. Meanwhile, basic science research must continue to definitely document the biological mechanisms underlying the observed association documented here(59). Finally, it is the public health imperative to continue to promote a wider array of existing methods and develop and promote long-term reversible contraceptive options for women worldwide.
Supplementary Material
Acknowledgments
This analysis was done without a dedicated funding source; however, SIM is supported by the National Institute of Mental Health (Rockville, MD, USA), award number K01MH094246. The content of this report is solely the responsibility of the authors and does not necessarily represent the offi cial views of the National Institute of Mental Health or the National Institutes of Health. We thank Chelsea Polis for her input on the research protocol and helpful comments on an early version of the manuscript.
Footnotes
The WHO used an unpublished version of the systematic review later published by Polis and Curtis (8), which was subsequently updated and published in October 2014 (10).
When analyses on the same study population were published in multiple articles and all articles met inclusion criteria, we selected only the most comprehensive or recent paper to include in pooled analyses. See Appendix Table 1 for details.
Although some authors did not explicitly describe the OCP under study as either combined or progestin-only method, use of POPs is less common in sub-Saharan Africa, and typically restricted to postpartum, breastfeeding women. Thus, we assumed that OCP categories would be comprised predominantly of COC users, and combine those studies that offer estimates for COCs specifically or OCPs generally in our analysis, to produce pooled effect estimates that represent the COC-HIV relationship. Four studies [18, 19, 38, 40] did present separate COC and POP estimates, and we use the COC estimate in pooled analyses.
Contributors
LJR, SIM, and NSP conceptualized the study and developed the research protocol. LJR and KS identified articles for full-text review, and LJR, SIM, and KS extracted data from all eligible studies. LJR conducted all statistical analyses with input from SIM and NSP. All authors contributed to manuscript preparation.
Conflicts of interest
All authors have no financial or personal conflicts to disclose.
References
- 1.Gollub E, Stein Z. Living with uncertainty: acting in the best interests of women. AIDS research and treatment. 2012:524936. doi: 10.1155/2012/524936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Affairs UNDoEaS. World Contraceptive Use 2011. 2012 Jan 24;2011 Available from: http://www.un.org/esa/population/publications/contraceptive2011/wallchart_front.pdf. [Google Scholar]
- 3.Singh S, Darroch JE, Ashford LS, Vlassoff M. Adding it up: The costs and benefits of investing in family planning and maternal and newborn health. New York: Guttmacher Institute and United Nations Population Fund; 2009. [Google Scholar]
- 4.Hormonal contraception and HIV: technical statement. Geneva, Switzerland: WHO; 2012. [PubMed] [Google Scholar]
- 5.Jain AK. Hormonal contraception and HIV acquisition risk: implications for individual users and public policies. Contraception. 2012;86(6):645–52. doi: 10.1016/j.contraception.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Butler AR, Smith JA, Polis CB, Gregson S, Stanton D, Hallett TB. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. Aids. 2013;27(1):105–13. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez MI, Reeves MF, Caughey AB. Evaluating the competing risks of HIV acquisition and maternal mortality in Africa: a decision analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2012;119(9):1067–73. doi: 10.1111/j.1471-0528.2012.03402.x. [DOI] [PubMed] [Google Scholar]
- 8.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. The Lancet Infectious Diseases. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 9.Morrison CS, Turner AN, Jones LB. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Practice & Research Clinical Obstetrics & Gynaecology. 2009;23(2):263–84. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–90. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Greenland S. Can meta-analysis be salvaged? American journal of epidemiology. 1994;140(9):783–7. doi: 10.1093/oxfordjournals.aje.a117326. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA : the journal of the American Medical Association. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Systematic reviews in health care: meta-analysis in context. 2. London: BMJ Publishing Group; 2001. [Google Scholar]
- 15.Heffron R, Rees H, Mugo N, Baeten JM. Authors Reply. Lancet Infect Dis. 2012;12:510–1. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet Infectious Diseases. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison CS, Chen P-L, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. Aids. 2010;24(11):1778–81. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison CS, Skoler-Karpoff S, Kwok C, Chen P-L, van de Wijgert J, Gehret-Plagianos M, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. Aids. 2012;26(4):497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 19.McCoy SI, Zheng W, Montgomery ET, Blanchard K, van der Straten A, de Bruyn G, et al. Oral and injectable contraception use and risk of HIV acquisition among women in sub-Saharan Africa. Aids. 2013;27(6):1001–9. doi: 10.1097/QAD.0b013e32835da401. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology (Cambridge, Mass) 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Gray RH. Use of hormonal contraceptives and risk of HIV-1 transmission. Lancet Infect Dis. 2012;12(7):507. doi: 10.1016/S1473-3099(12)70112-3. author reply 10-1. [DOI] [PubMed] [Google Scholar]
- 24.Kiddugavu M, Makumbi F, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, et al. Hormonal contraceptive use and HIV-1 infection in a population based cohort in Rakai, Uganda. Aids. 2003;17:233–240. doi: 10.1097/00002030-200301240-00014. [DOI] [PubMed] [Google Scholar]
- 25.Hernan MA. The hazards of hazard ratios. Epidemiology (Cambridge, Mass) 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2008. [Google Scholar]
- 27.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss J, Gakinya MN, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. Journal of Infectious Diseases. 1991;162(2):233–9. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Saracco A, Musicco M, Nicolosi A, Angarano G, Arici C, Gavezzeni G, et al. Man-to-woman sexual transmission of HIV: Longitudinal study of 343 steady partners of infected men. JAIDS. 1993;6:497–502. [PubMed] [Google Scholar]
- 29.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. Aids. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A. Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. Aids. 1994;8:1585–91. doi: 10.1097/00002030-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Sinei SK, Fortney JA, Kigondu CS, Feldblum PJ, Kuyoh M, Allen MY, et al. Contraceptive use and HIV infection in Kenyan family planning clinic attenders. International Journal of STD & AIDS. 1996;7:65. doi: 10.1258/0956462961917104. [DOI] [PubMed] [Google Scholar]
- 32.Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T. Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;12(5):500–7. doi: 10.1097/00042560-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 33.Kilmarx PH, Limpakarnjanarat K, Mastro TD, Saisorn S, Kaewkungwal J, Korattana S, et al. HIV-1 seroconversion in a prospective study of female sex workers in northern Thailand: continued high incidence among brothel-based women. Aids. 1998;1998(12):1889–98. doi: 10.1097/00002030-199814000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Martin HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. The Journal of Infectious Diseases. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 35.Kapiga S, Lyamuya EF, Lwihula GK, Hunter DJ. The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. Aids. 1998;12:75–84. doi: 10.1097/00002030-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Lavreys L, Baeten JM, Martin HL, Jr, Overbaugh J, Mandaliya K, Ndinya-Achola J, et al. Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. Aids. 2004;18(4):695. doi: 10.1097/00002030-200403050-00017. [DOI] [PubMed] [Google Scholar]
- 37.Baeten JM, Benki S, Chohan V, Lavreys L, McClelland RS, Mandaliya K, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women Aids. 2007;21:1771–7. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 38.Myer L, Denny L, Wright TC, Kuhn L. Prospective study of hormonal contraception and women’s risk of HIV infection in South Africa. International Journal of Epidemiology. 2006;36(1):166–74. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 39.Kleinschmidt I, Rees H, Delany-Moretlwe S, Smith D, Dinat N, Nkala B, et al. Injectable progestin contraceptive use and risk of HIV infection in a South African family planning cohort. Contraception. 2007;75:461–7. doi: 10.1016/j.contraception.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, et al. Hormonal contraception and the risk of HIV acquisition. Aids. 2007;21(1):85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 41.Kumwenda NI, Kumwenda J, Kafulafula G, Makanani B, Taulo F, Nkhoma C, et al. HIV-1 incidence among women of reproductive age in Malawi. International Journal of STD & AIDS. 2008;19(5):339–41. doi: 10.1258/ijsa.2007.007165. [DOI] [PubMed] [Google Scholar]
- 42.Watson-Jones D, Baisley K, Weiss HA, Tanton C, Changalucha J, Everett D, et al. Risk factors for HIV incidence in women participating in an HSV suppressive treatment trial in Tanzania. Aids. 2009;23(3):415–22. doi: 10.1097/QAD.0b013e32831ef523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldblum PJ, Lie C-C, Weaver MA, Van Damme L, Halpern V, Adeiga A, et al. Baseline Factors Associated With Incident HIV and STI in Four Microbicide Trials. Sexually Transmitted Diseases. 2010:1. [PubMed] [Google Scholar]
- 44.Reid SE, Dai JY, Wang J, Sichalwe BN, Akpomiemie G, Cowan FM, et al. Pregnancy, Contraceptive Use, and HIV Acquisition in HPTN 039: Relevance for HIV Prevention Trials Among African Women. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009:1. doi: 10.1097/QAI.0b013e3181bc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. Aids. 2012;26(3):375–80. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 46.Lutalo T, Musoke R, Kong X, Makumbi F, Serwadda D, Nalugoda F, et al. Effects of hormonal contraceptive use on HIV acquisition and transmission among HIV-discordant couples. Aids. 2013;27 (Suppl 1):S27–34. doi: 10.1097/QAD.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 47.Crook AM, Ford D, Gafos M, Hayes R, Kamali A, Kapiga S, et al. Injectable and oral contraceptives and risk of HIV acquisition in women: an analysis of data from the MDP301 trial. Human Reproduction. 2014 doi: 10.1093/humrep/deu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang CC, Reilly M, Kreiss JK. Risk of HIV infection in oral contraceptive pill users: a meta-analysis. Journal of acquired immune deficiency syndromes (1999) 1999;21(1):51–8. doi: 10.1097/00126334-199905010-00007. [DOI] [PubMed] [Google Scholar]
- 49.Higgins J, GS, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 50.Cates W. Research on hormonal contraception and HIV. Lancet. 2014;383(9914):303–4. doi: 10.1016/S0140-6736(14)60097-0. [DOI] [PubMed] [Google Scholar]
- 51.Polis CB, Westreich D, Balkus JE, Heffron R. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. Aids. 2013;27 (Suppl 1):S35–43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz SR, Pettifor A, Stuart GS, Cohen MS. Hormonal contraception and HIV: the methods have confused the message. Aids. 2013;27 (Suppl 1):S45–53. doi: 10.1097/QAD.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 53.Gallo MF, Behets FM, Steiner MJ, Thomsen SC, Ombidi W, Luchters S, et al. Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya--PSA analysis. Int J STD AIDS. 2007;18(1):33–8. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 54.Warner P. Concerns regarding design, analysis, and interpretation of the morrison study on hormonal contraceptive use and acquisition of cervical infections. Sex Transm Dis. 2005;32(10):644. doi: 10.1097/01.olq.0000175374.98875.33. author reply 5. [DOI] [PubMed] [Google Scholar]
- 55.Smith JA, Butler AR, Polis CB, Gregson S, Stanton D, Hallett T. Programmatic implications: balancing maternal mortality and HIV risk. 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Altanta, GA, USA. 2013. [Google Scholar]
- 56.McCoy SI, Ralph LJ, Padian NS, Minnis AM. Are Hormonal Contraceptive Users More Likely to Misreport Unprotected Sex? Evidence From a Biomarker Validation Study in Zimbabwe. AIDS and behavior. 2014 doi: 10.1007/s10461-014-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ralph LJ, McCoy SI, Hallett T, Padian N. Next steps for research on hormonal contraception and HIV. Lancet. 2013;382(9903):1467–9. doi: 10.1016/S0140-6736(13)61420-8. [DOI] [PubMed] [Google Scholar]
- 58.Jones HE. Time to focus on improving the contraceptive method mix in high HIV prevalence settings and let go of unanswerable questions. Contraception. 2014;90(4):357–9. doi: 10.1016/j.contraception.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Murphy K, Irvin SC, Herold BC. Research gaps in defining the biological link between HIV risk and hormonal contraception. American journal of reproductive immunology (New York, NY: 1989) 2014;72(2):228–35. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.