Abstract
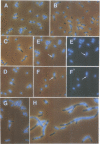
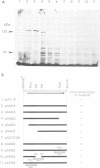
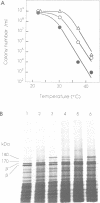
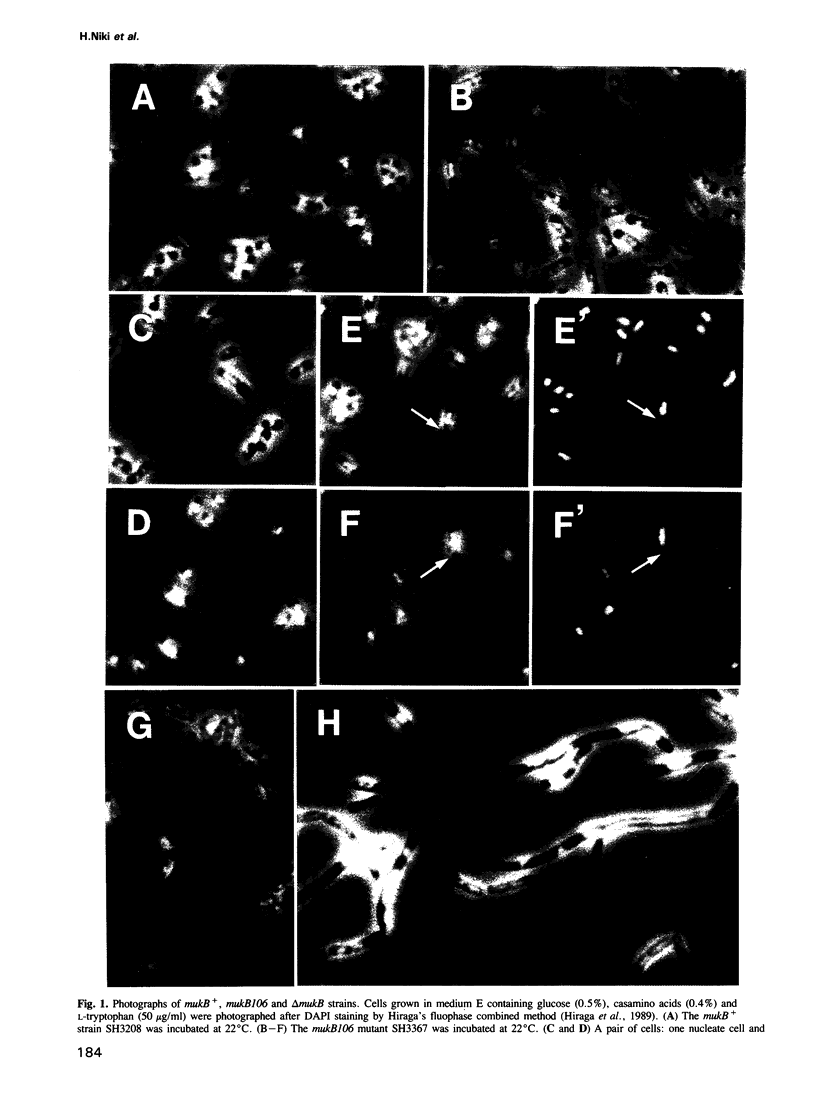
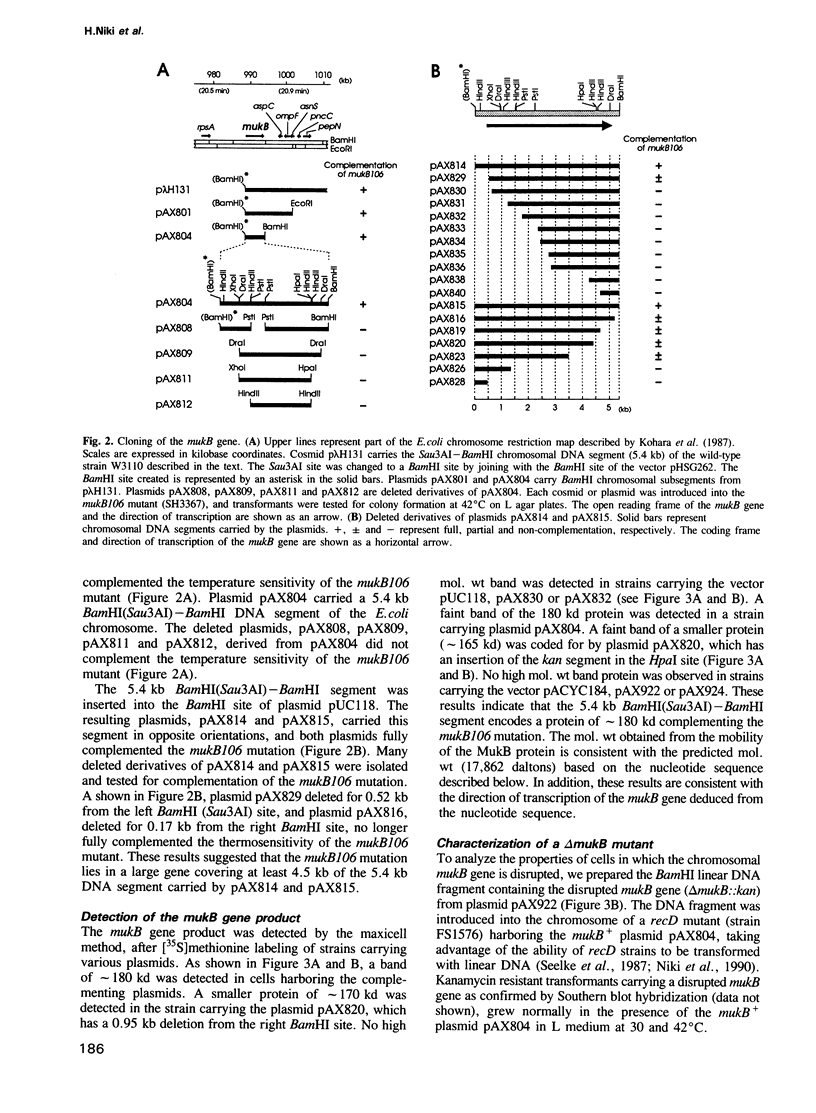
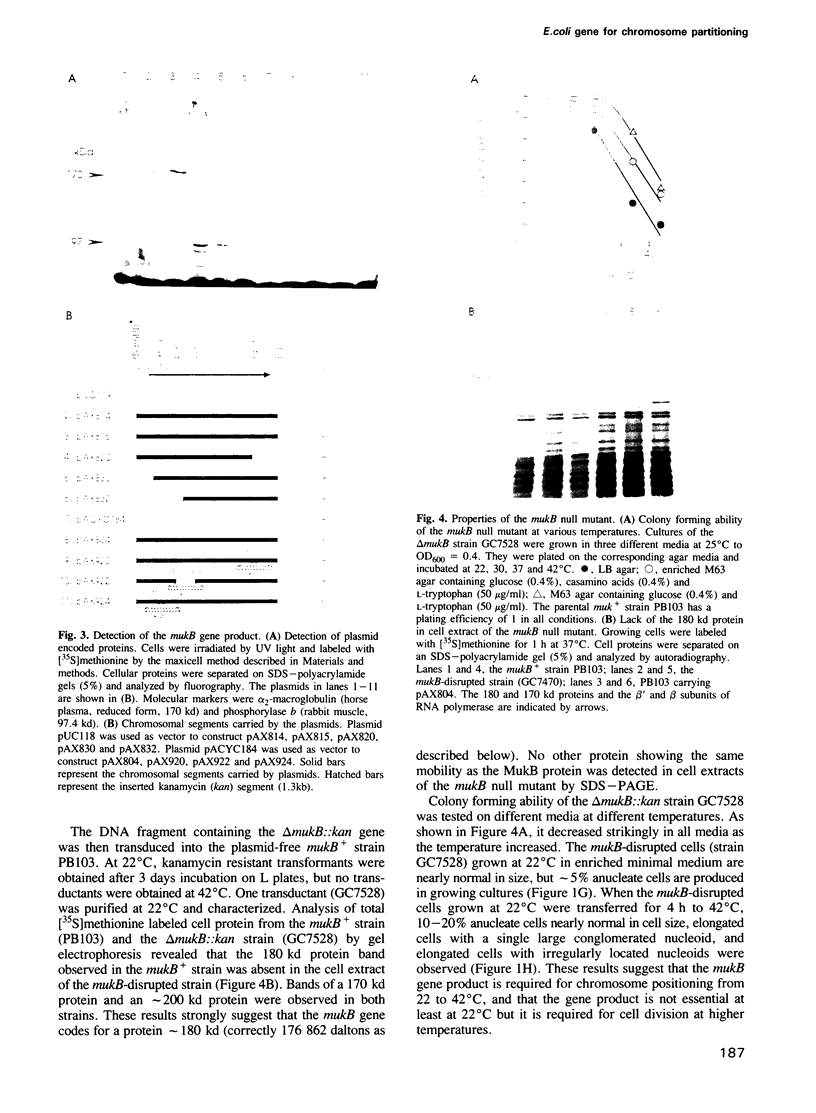
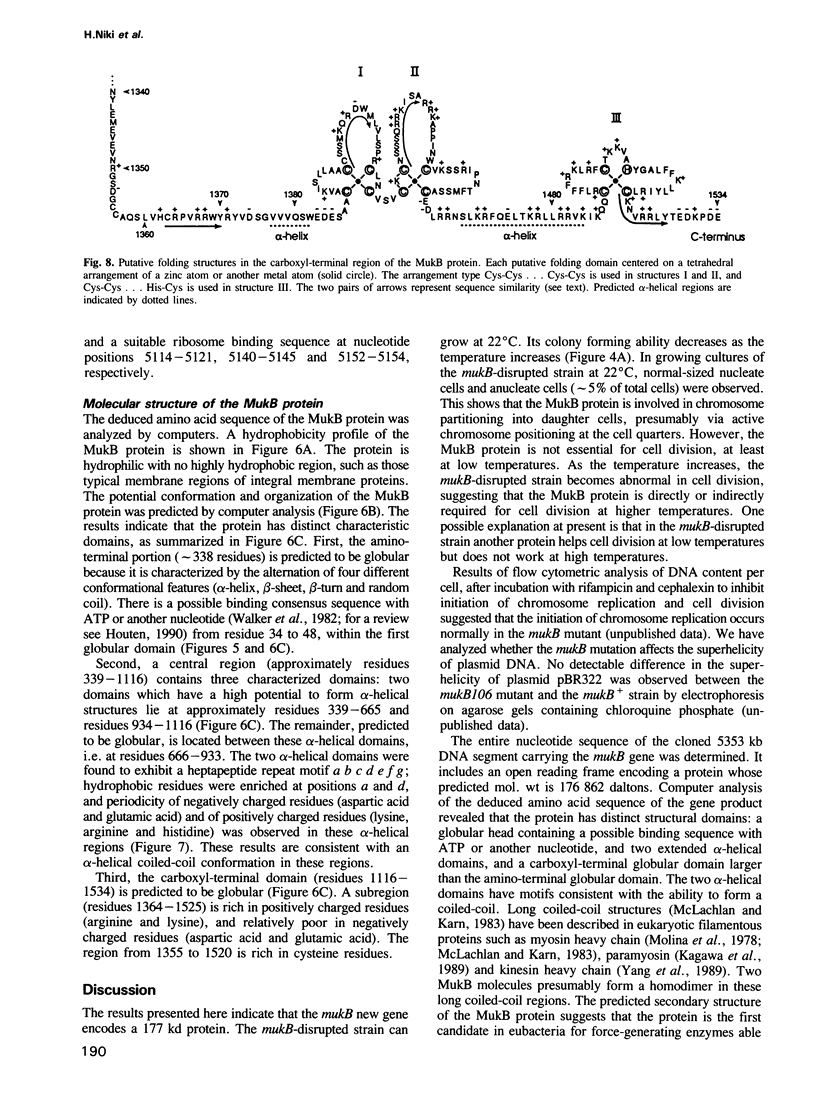
An Escherichia coli temperature sensitive mutant which produces spontaneously normal size anucleate cells at low temperature was isolated. The mutant is defective in a previously undescribed gene, named mukB, located at 21 min on the chromosome. The mukB gene codes for a large protein (approximately 180 kd). A 1534 amino acid protein (176,826 daltons) was deduced from the nucleotide sequence of the mukB gene. Computer analysis revealed that the predicted MukB protein has distinct domains: an amino-terminal globular domain containing a nucleotide binding sequence, a central region containing two alpha-helical coiled-coil domains and one globular domain, and a carboxyl-terminal globular domain which is rich in Cys, Arg and Lys. A 180 kd protein detected in wild-type cell extracts by electrophoresis is absent in mukB null mutants. Although the null mutants are not lethal at low temperature, the absence of MukB leads to aberrant chromosome partitioning. At high temperature the mukB null mutants cannot form colonies and many nucleoids are distributed irregularly along elongated cells. We conclude that the MukB protein is required for chromosome partitioning in E. coli.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Pollard T. D. Binding of myosin I to membrane lipids. Nature. 1989 Aug 17;340(6234):565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. Flow cytometry: illuminating microbiology. New Biol. 1990 Feb;2(2):119–125. [PubMed] [Google Scholar]
- Brady G., Jantzen H. M., Bernard H. U., Brown R., Schütz G., Hashimoto-Gotoh T. New cosmid vectors developed for eukaryotic DNA cloning. Gene. 1984 Feb;27(2):223–232. doi: 10.1016/0378-1119(84)90143-4. [DOI] [PubMed] [Google Scholar]
- Casaregola S., Norris V., Goldberg M., Holland I. B. Identification of a 180 kD protein in Escherichia coli related to a yeast heavy-chain myosin. Mol Microbiol. 1990 Mar;4(3):505–511. doi: 10.1111/j.1365-2958.1990.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Jaffé A., Bouloc P., Robin A. Cyclic AMP and cell division in Escherichia coli. J Bacteriol. 1988 Jan;170(1):65–70. doi: 10.1128/jb.170.1.65-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Chromosome partition in Escherichia coli requires postreplication protein synthesis. J Bacteriol. 1989 Oct;171(10):5405–5409. doi: 10.1128/jb.171.10.5405-5409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Niki H., Ichinose C., Mori H. Positioning of replicated chromosomes in Escherichia coli. J Bacteriol. 1990 Jan;172(1):31–39. doi: 10.1128/jb.172.1.31-39.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Partitioning of nucleoids. Res Microbiol. 1990 Jan;141(1):50–56. doi: 10.1016/0923-2508(90)90097-a. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J Bacteriol. 1986 Jan;165(1):66–71. doi: 10.1128/jb.165.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé A., Ogura T., Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985 Sep;163(3):841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Korn E. D., Hammer J. A., 3rd The heavy chain of Acanthamoeba myosin IB is a fusion of myosin-like and non-myosin-like sequences. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6720–6724. doi: 10.1073/pnas.84.19.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H., Gengyo K., McLachlan A. D., Brenner S., Karn J. Paramyosin gene (unc-15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence and models for thick filament structure. J Mol Biol. 1989 May 20;207(2):311–333. doi: 10.1016/0022-2836(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Titus M. A., De Lozanne A., Spudich J. A. Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 1989 Mar;8(3):923–932. doi: 10.1002/j.1460-2075.1989.tb03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M. I., Kropp K. E., Gulick J., Robbins J. The sequence of an embryonic myosin heavy chain gene and isolation of its corresponding cDNA. J Biol Chem. 1987 May 15;262(14):6478–6488. [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Médigue C., Bouché J. P., Hénaut A., Danchin A. Mapping of sequenced genes (700 kbp) in the restriction map of the Escherichia coli chromosome. Mol Microbiol. 1990 Feb;4(2):169–187. doi: 10.1111/j.1365-2958.1990.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Niki H., Ogura T., Hiraga S. Linear multimer formation of plasmid DNA in Escherichia coli hopE (recD) mutants. Mol Gen Genet. 1990 Oct;224(1):1–9. doi: 10.1007/BF00259444. [DOI] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Nuclear segregation in Bacillus subtilis. Nature. 1974 Jul 19;250(463):252–254. doi: 10.1038/250252a0. [DOI] [PubMed] [Google Scholar]
- Seelke R., Kline B., Aleff R., Porter R. D., Shields M. S. Mutations in the recD gene of Escherichia coli that raise the copy number of certain plasmids. J Bacteriol. 1987 Oct;169(10):4841–4844. doi: 10.1128/jb.169.10.4841-4844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., von Meyenburg K., Hansen F. G., Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988 Feb;170(2):852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatko B. E., Croft R., Moran L. S., Wilson G. G. Cloning and analysis of the HaeIII and HaeII methyltransferase genes. Gene. 1988 Dec 25;74(1):45–50. doi: 10.1016/0378-1119(88)90248-x. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., Kobayashi I., Thaler D., Stahl M. M. Direction of travel of RecBC recombinase through bacteriophage lambda DNA. Genetics. 1986 Jun;113(2):215–227. doi: 10.1093/genetics/113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vale R. D., Goldstein L. S. One motor, many tails: an expanding repertoire of force-generating enzymes. Cell. 1990 Mar 23;60(6):883–885. doi: 10.1016/0092-8674(90)90334-b. [DOI] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts F. Z., Shiels G., Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987 Nov;6(11):3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Kambe-Honjoh H., Nagai K., Tamura G. Early replicative intermediates of Escherichia coli chromosome isolated from a membrane complex. EMBO J. 1986 Apr;5(4):787–791. doi: 10.1002/j.1460-2075.1986.tb04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M., Nagai K., Tamura G. Asymmetric replication of an oriC plasmid in Escherichia coli. Mol Gen Genet. 1986 Aug;204(2):214–220. doi: 10.1007/BF00425501. [DOI] [PubMed] [Google Scholar]
- de Boer P. A., Crossley R. E., Rothfield L. I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989 Feb 24;56(4):641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]