Abstract
Context
Fatigue is the most common symptom in oncology patients during chemotherapy (CTX). Little is known about the predictors of interindividual variability in initial levels and trajectories of morning fatigue severity in these patients.
Objectives
An evaluation was done to determine which demographic, clinical, and symptom characteristics were associated with initial levels as well as the trajectories of morning fatigue and to compare findings with our companion paper on evening fatigue.
Methods
A sample of outpatients with breast, gastrointestinal, gynecological, and lung cancer (N=586) completed demographic and symptom questionnaires a total of six times over two cycles of CTX. Fatigue severity was evaluated using the Lee Fatigue Scale. Hierarchical linear modeling (HLM) was used to answer the study objectives.
Results
A large amount of interindividual variability was found in the morning fatigue trajectories. A piecewise model fit the data best. Patients with higher body mass index (BMI), who did not exercise regularly, with a lower functional status, and who had higher levels of state anxiety, sleep disturbance and depressive symptoms, reported higher levels of morning fatigue at enrollment. Variations in the trajectories of morning fatigue were predicted by the patients’ ethnicity and younger age.
Conclusion
The modifiable risk factors that were associated with only morning fatigue were BMI, exercise, and state anxiety. Modifiable risk factors that were associated with both morning and evening fatigue included functional status, depressive symptoms, and sleep disturbance. Using this information, clinicians can identify patients at higher risk for more severe morning fatigue and evening fatigue, provide individualized patient education, and tailor interventions to address the modifiable risk factors.
Keywords: morning fatigue, evening fatigue, cancer, oncology, chemotherapy, hierarchical linear modeling, symptom trajectories, diurnal variations
Introduction
Fatigue is reported by over 80% of patients during cancer treatment.1 Fatigue during cancer treatment is different from tiredness; it is disproportionate to daily activity; and it is not relieved by rest.2 Patients undergoing chemotherapy (CTX) identify fatigue as their most disturbing symptom because it limits their activities and decreases their quality of life (QOL).3-6
In those studies that evaluated changes in fatigue severity during CTX,7-10 fatigue levels increased from enrollment through the course of treatment. However, none of these studies evaluated for changes in fatigue severity in relationship to specific time points within a CTX cycle (e.g., acute effects of CTX, mid-cycle, recovery period).
Additional work has identified demographic, clinical, and symptom characteristics that were associated with changes in mean daily fatigue severity scores in patients undergoing CTX.5,11-15 For example, patients who were younger,5,11 female,5 unmarried,11 and with more education13 reported higher fatigue severity. In other studies, patients who were employed,16 had a higher stage of disease at diagnosis,12 and those who reported more comorbidities12,17 had higher fatigue severity scores during CTX. Across these studies,5,11-17 inconsistencies were found in the predictors, which may be related to the timing of the fatigue assessments; the failure to examine demographic, clinical and symptom characteristics in the same patients longitudinally; and/or the failure to evaluate diurnal variations in fatigue severity.
Diurnal Variations in Fatigue Severity
In healthy people, it is common to awake in the morning feeling rested and with an increased sense of energy.18 Fatigue severity gradually increases during the course of a day, with higher levels of fatigue reported in the evening.19 In oncology patients, previous work by our research team identified diurnal variations in fatigue severity in patients with breast16 and prostate16 cancer who underwent radiation therapy (RT). Using hierarchical linear modeling (HLM), younger age, higher body mass index (BMI), and higher levels of sleep disturbance prior to RT were associated with higher levels of morning fatigue in the patients with breast cancer.16 In contrast, age and sleep disturbance predicted initial levels as well as changes over time in both morning and evening fatigue in patients with prostate cancer.20 Only two studies were found that examined diurnal variations in morning and evening fatigue in oncology patients who underwent CTX.21,22 While the authors of the CTX studies suggested that morning and evening fatigue were distinct symptoms,21,22 neither study evaluated for predictors of diurnal variations in fatigue severity associated with CTX.
In our companion paper,23 we identified demographic, clinical, and symptom characteristics that predicted initial levels as well as the trajectories of evening fatigue. Using HLM, patients who were White, diagnosed with breast, gynecological (GYN), and lung cancer, and who had more years of education, child care responsibilities, lower functional status, and higher levels of sleep disturbance and depression reported higher levels of evening fatigue at enrollment. In this study, we evaluated the trajectories of morning fatigue in the same patients. Specifically, in a sample of outpatients with breast, gastrointestinal (GI), GYN, and lung cancer who were receiving two cycles of CTX, the purposes of this study were: to evaluate for variations in morning fatigue severity and to determine which demographic, clinical, and symptom characteristics predicted initial levels as well as the trajectories of morning fatigue. In the discussion section of this paper, we compare the findings from the morning and evening fatigue23 studies.
Methods
Patients and Settings
Details of the methods used in this study are published in the companion paper.23 In brief, patients were eligible to participate if they were 18 years of age or older; had a diagnosis of breast, GI, GYN, or lung cancer; had received CTX within the preceding four weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent.
Instruments
Patients completed a demographic questionnaire, the Karnofsky Performance Status (KPS) Scale,24 and the Self-Administered Comorbidity Questionnaire (SCQ).25 Concurrent symptoms were evaluated using the Spielberger State-Trait Anxiety Inventories (STAI-S and STAI-T),26 the Center for Epidemiologic Studies-Depression Scale (CES-D),27 and the General Sleep Disturbance Scale (GSDS).28 In addition, they reported whether or not they experienced pain.
Fatigue was evaluated using the Lee Fatigue Scale (LFS), which assesses physical fatigue and energy.29 Total fatigue and energy scores were calculated and can range from 0 to 10. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate LFS questionnaires, patients were asked to rate each item based on how they felt within 30 minutes of awakening (i.e., morning fatigue and morning energy) and prior to going to bed (i.e., evening fatigue and evening energy). The LFS has well established validity and reliability.20
Study Procedures
The study was approved by the Institutional Review Board at each of the study sites. Written informed consent was obtained from all patients. Depending on the length of their CTX cycles (14 days, 21 days, or 28 days), patients completed study questionnaires in their homes a total of six times over two cycles of CTX.
Statistical Analyses
Descriptive statistics and frequency distributions were generated on the sample characteristics and symptom severity scores at enrollment using the Statistical Package for the Social Sciences (SPSS) v. 22.30
HLM based on full maximum likelihood estimation was performed using software developed by Raudenbush and Bryk.31 The specific HLM methods are described in detail in the companion paper.23 A piecewise model strategy was employed to evaluate the pattern of change in morning fatigue over time because the six assessments encompass two cycles of CTX.32 Then, interindividual differences in the piecewise trajectories of morning fatigue were examined by modeling the individual change parameters (i.e., intercept and slope parameters) as a function of proposed predictors. Table 1 lists the potential predictors that were developed based on a review of the literature on fatigue in oncology patients undergoing CTX. The most parsimonious model was constructed using the list of potential predictors. A P-value of <0.05 indicated statistical significance.
Table 1.
Potential Predictors of Intercept, Piecewise 1 and Piecewise 2 Linear and Quadratic Components for Morning Fatigue
| Potential Predictors | Intercept | Piecewise 1 Linear Component |
Piecewise 1 Quadratic Component |
Piecewise 2 Linear Component |
Piecewise 2 Quadratic Component |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age | ◆ | ◆ | ◆ | ||
| Sex | ◆ | ||||
| Ethnicity | ◆ | ◆ | ◆ | ||
| Education | |||||
| Marital status | ◆ | ||||
| Live alones | ◆ | ||||
| Employment status | ◆ | ||||
| Child care responsibilities | ◆ | ||||
| Clinical Characteristics | |||||
| Body mass index (kg/m2) | ◆ | ||||
| Hemoglobin (gm/dL) at enrollment | |||||
| Karnofsky Performance Status Scale score | ◆ | ||||
| Exercise on a regular basis | ◆ | ||||
| Self-administered Comorbidity Questionnaire score | ◆ | ||||
| Cancer diagnosis (breast, gastrointestinal, gynecological, lung) | |||||
| Time since cancer diagnosis | ◆ | ◆ | |||
| Any prior cancer treatments | ◆ | ||||
| Number prior cancer treatments | ◆ | ||||
| Type of prior cancer treatments | |||||
| Chemotherapy cycle length | |||||
| Number of metastatic sites including lymph node involvement | ◆ | ◆ | |||
| Number of metastatic sites excluding lymph node involvement | |||||
| Symptom Characteristics | |||||
| Lee Fatigue Scale: Morning Fatigue score at enrollment | ◆ | ◆ | ◆ | ||
| Lee Fatigue Scale: Evening Fatigue score at enrollment | ◆ | ◆ | |||
| Lee Fatigue Scale: Morning Energy score at enrollment | ◆ | ||||
| Lee Fatigue Scale: Evening Energy score at enrollment | ◆ | ||||
| Center for Epidemiological Studies-Depression Scale score at enrollment |
◆ | ||||
| General Sleep Disturbance Scale score at enrollment | ◆ | ||||
| Trait Anxiety score at enrollment | ◆ | ||||
| State Anxiety score at enrollment | ◆ | ||||
| Pain present at enrollment | ◆ |
◆= From exploratory analysis had a t-value of ≥2.0.
Abbreviations: gm/dL = grams per deciliter; kg/m2 = kilogram per meters squared.
Results
Sample Characteristics
The demographic, clinical, and symptom characteristics of the sample (N=586) are presented in Table 2. The sample was predominately female (80%), White (69%), had a mean age of 57 years, was well educated (mean of 16 years), currently not working for pay (66%), partnered (68%), and did not have child care responsibilities (77%). The patients were on average 2.5 years (median = 0.49 years) since their cancer diagnosis, primarily being treated with 21-day CTX cycles (55.3%), and had an average of one metastatic site.
Table 2.
Demographic, Clinical, and Symptom Characteristics of the Patients (n=586)
| Demographic Characteristics | |
| Age (years; mean (SD)) | 57.2 (11.9) |
| Gender (% female (n)) | 80.0 (469) |
| Ethnicity (% (n)) | |
| White | 69.3 (406) |
| Black Non-Hispanic | 7.0 (41) |
| Asian/Pacific Islander | 12.8 (75) |
| Hispanic/Mixed/Other | 10.9 (64) |
| Education (years; mean (SD)) | 16.3 (3.0) |
| Married or partnered (% yes (n)) | 67.7 (397) |
| Lives alone (% yes (n)) | 19.8 (116) |
| Currently employed (% yes (n)) | 34.3 (201) |
| Child care responsibilities (% yes (n)) | 22.9 (134) |
| Income (% yes (n)) | |
| Less than $30,000 | 18.7 (98) |
| $30,000 to <$70,000 | 17.8 (104) |
| $70,000 to < $100,000 | 15.2 (89) |
| More than $100,000 | 39.9 (234) |
| Clinical Characteristics | |
| Self-administered Comorbidity Questionnaire score (mean (SD)) | 5.6 (3.0) |
| Body mass index (kg/m2; mean (SD)) | 26.3 (5.8) |
| Hemoglobin (gm/dL; mean (SD)) | 11.7 (1.4) |
| Karnofsky Performance Status score (mean (SD)) | 80.6 (11.8) |
| Exercise on a regular basis (% yes (n)) | 70.5 (413) |
| Specific comorbidities reported (% yes (n)) | |
| High blood pressure | 31.4 (184) |
| Back pain | 27.0 (158) |
| Depression | 20.5 (120) |
| Osteoarthritis | 13.7 (80) |
| Anemia or blood disease | 12.1 (71) |
| Lung disease | 9.9 (58) |
| Diabetes | 8.7 (51) |
| Liver disease | 6.8 (40) |
| Heart disease | 5.5 (32) |
| Rheumatoid arthritis | 4.1 (24) |
| Ulcer or stomach disease | 4.1 (24) |
| Kidney disease | 1.2 (7) |
| Cancer diagnosis (% yes (n)) | |
| Breast | 42.8 (251) |
| Gastrointestinal | 26.8 (157) |
| Gynecological | 20.3 (119) |
| Lung | 10.1 (59) |
| Time since cancer diagnosis (years; mean (SD)) | 2.5 (4.4) |
| Time since cancer diagnosis (years; median) | 0.49 |
| Any prior cancer treatments (% yes (n)) | 82.4 (483) |
| Number prior cancer treatments (mean (SD)) | 1.9 (1.6) |
| Type of prior cancer treatment (% yes (n)) | |
| No prior treatment | 17.6 (103) |
| Only surgery, chemotherapy, or RT | 40.4 (237) |
| Surgery and chemotherapy, or surgery and RT, or chemotherapy and RT | 23.0 (135) |
| Surgery and chemotherapy and RT | 17.7 (104) |
| Chemotherapy cycle length (% (n)) | |
| 14 days | 36.0 (211) |
| 21 days | 55.3 (324) |
| 28 days | 8.7 (51) |
| Number of metastatic sites including lymph node involvement (mean (SD)) | 1.4 (1.3) |
| Number of metastatic sites excluding lymph node involvement (mean (SD)) | 0.9 (1.2) |
| Symptom Characteristics at Enrollment | |
| Lee Fatigue Scale: evening fatigue score (mean (SD)) | 5.3 (2.1) |
| Lee Fatigue Scale: morning fatigue score (mean (SD)) | 3.1 (2.2) |
| Lee Fatigue Scale: evening energy score (mean (SD)) | 3.5 (1.9) |
| Lee Fatigue Scale: morning energy score (mean (SD)) | 4.5 (2.2) |
| Center for Epidemiological Studies-Depression Scale score (mean (SD)) | 12.6 (9.4) |
| General Sleep Disturbance Scale score (mean (SD)) | 52.2 (19.4) |
| Trait Anxiety score (mean (SD)) | 35.0 (10.4) |
| State Anxiety score (mean (SD)) | 33.2 (12.1) |
| Pain present (% yes (n)) | 73.5 (431) |
Abbreviations: gm/dL = grams per deciliter; kg/m2 = kilograms per meters squared; SD = standard deviation; RT = radiation therapy.
Changes in Morning Fatigue Severity Over Time
The initial HLM analysis examined how morning fatigue scores changed within the two cycles of CTX. The linear and quadratic trend within each of the CTX cycles was significant (P<0.001).
The estimates for the initial piecewise model are presented in Table 3. Since the model was unconditional (i.e., no covariates), the intercept represents the average morning fatigue severity score at enrollment (i.e., 3.06 on a scale of 0 to 10). The estimated linear piecewise rates of change were 1.136 and 0.487 for piecewise linear 1 (PW1) and piecewise linear 2 (PW2), respectively. The estimated quadratic piecewise rates of change were -0.579 and -0.140 for piecewise quadratic 1 (PW12) and piecewise quadratic 2 (PW22), respectively. The combination of each coefficient determines the curves for the two piecewise components for changes in morning fatigue scores over time.
Table 3.
Hierarchical Linear Model for Morning Fatigue
| Morning Fatigue | Coefficient (SE) | |
|---|---|---|
| Unconditional Model | Final Model | |
| Fixed effects | ||
| Intercept | 3.057 (.095)+ | 3.048 (.076)+ |
| Piecewise 1 – linear rate of change | 1.136 (.142)+ | 1.137 (.141)+ |
| Piecewise 1 – quadratic rate of change | −0.579 (.069)+ | −0.579 (.068)+ |
| Piecewise 2 – linear rate of change | 0.487 (.092)+ | 0.491 (.091)+ |
| Piecewise 2 – quadratic rate of change | −0.140 (.030)+ | −0.141 (.030)+ |
| Time invariant covariates | ||
| Intercept | ||
| Body mass index | 0.023 (.010)* | |
| Exercise | −0.405 (.132)* | |
| Functional status | −0.028 (.006)+ | |
| State anxiety | 0.028 (.007)** | |
| Depressive symptoms | 0.033 (.010)** | |
| Sleep disturbance | 0.038 (.004)+ | |
| Piecewise 1 – linear rate of change | ||
| Age | −0.033 (.011)* | |
| Piecewise 1 – quadratic rate of change | ||
| Age | −0.021 (.006)+ | |
| Piecewise 2 – linear rate of change | ||
| Age | −0.019 (008)* | |
| Ethnicitya | ||
| Black versus White | −1.132 (.299)+ | |
| Asian versus White | 0.012 (.227) | |
| Hispanic versus White | 0.008 (.252) | |
| Piecewise 2 – quadratic rate of change | ||
| Age | 0.004 (.003) | |
| Ethnicitya | ||
| Black versus White | 0.339 (.107)* | |
| Asian versus White | 0.024 (.081) | |
| Hispanic versus White | −0.032 (.089) | |
| Variance components | ||
| In intercept | 3.556+ | 1.661+ |
| Goodness-of-fit deviance (parameters estimated) | 11858.550 (7)** | 11432.973 (23)* |
| Model comparison χ2 (df) | 21.626 (6)* | |
Ethnicity – represented by three “dummy” coded variables significantly improved the model fit (p=0.002)
p<.05,
p<.001,
p<.0001
Abbreviations: df = degrees of freedom; SE = standard error
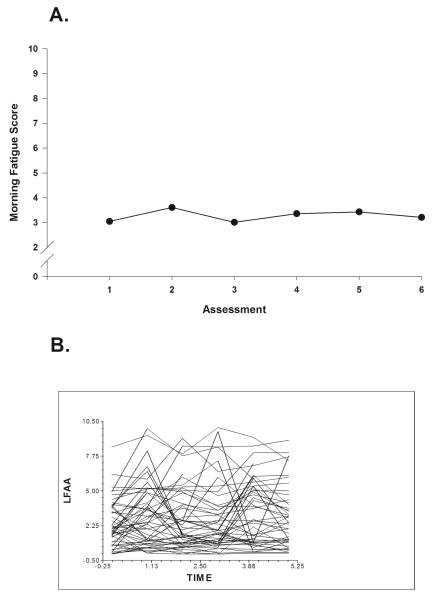
Fig. 1A displays the mean morning fatigue scores over two cycles of CTX from the unconditional model. Morning fatigue severity rose and declined with a distinct peak at assessment 2, decreased at assessment 3, and increased through assessment 5. A spaghetti plot of a random sample of 20% of the sample’s data demonstrates the large amount of interindividual variability in the severity of morning fatigue (Fig. 1B). These results supported additional analyses of predictors of interindividual variability in initial levels of, as well as in the trajectories of, morning fatigue severity.
Fig. 1.
A. Piecewise model of mean morning fatigue scores for six assessment points over two cycles of chemotherapy (CTX).
B. Spaghetti plots of individual morning fatigue trajectory for a random sample of 20% of total sample (n=117) over two cycles of CTX. The assessment points are based on individual patient CTX cycles. LFAA = Lee Fatigue Scale Morning (severity score).
Interindividual Differences in Initial Levels and Trajectories of Morning Fatigue
As shown in the final model (Table 3), no demographic characteristics predicted interindividual differences in the initial levels (i.e., intercept) of morning fatigue. The clinical characteristics that predicted interindividual differences in the initial levels of morning fatigue were BMI, exercise, and functional status. The severity of state anxiety, depressive symptoms, and sleep disturbance at enrollment were the symptom characteristics that predicted interindividual differences in the intercept for morning fatigue.
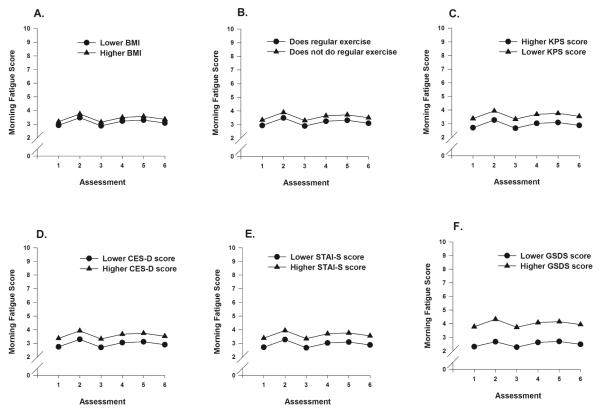
To illustrate the effects of each of the clinical characteristics, Figs. 2A through 2C display the adjusted change curves for morning fatigue that were estimated based on differences in BMI (lower BMI/higher BMI calculated as one standard deviation (SD) above and below the mean), exercise (does or does not exercise on a regular basis), and functional status (lower KPS/higher KPS score calculated as one SD above and below the mean KPS score). Figs. 2D through 2F display the adjusted change curves for morning fatigue that were estimated based on the differences in depressive symptoms (lower CES-D/higher CES-D calculated as one SD above and below the mean CES-D score), state anxiety (lower STAI-S/higher STAI-S calculated as one SD above and below the mean STAI-S score), and sleep disturbance (lower GSDS/higher GSDS calculated as one SD above and below the mean GSDS score).
Figs 2A-F.
Figs. 2A-C display the adjusted change curves for morning fatigue that were estimated based on differences in body mass index (BMI) (lower BMI/higher BMI calculated as one standard deviation (SD) above and below the mean), exercise (does or does not exercise on a regular basis), and functional status (lower Karnofsky Performance Status (KPS) score/higher KPS score calculated as one SD above and below the mean KPS score). Figs. 2D-F display the adjusted change curves for morning fatigue that were estimated based on the differences in depressive symptoms (lower Center for Epidemiological Studies-Depression (CES-D) score/higher CES-D score calculated as one SD above and below the mean CES-D score), state anxiety (lower Speilberger State Anxiety Inventory (STAI-S) score/higher STAI-S score calculated as one SD above and below the mean STAI-S score), and sleep disturbance (lower General Sleep Disturbance Scale (GSDS) score/higher GSDS calculated as one SD above and below the mean GSDS score).
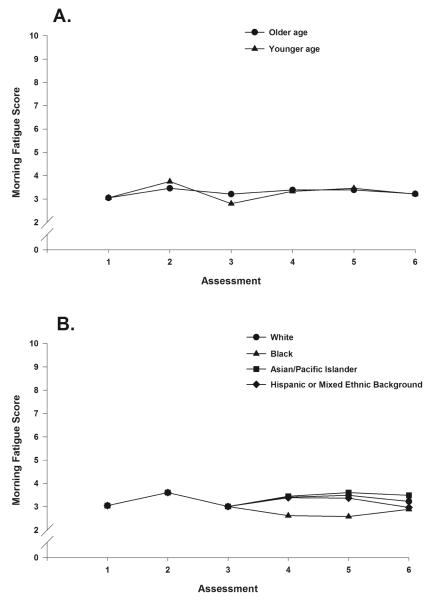
Two demographic characteristics, age and ethnicity, predicted changes in morning fatigue over time (i.e., slope). In Figs. 3A and 3B, the effects of age (older/younger age calculated as one SD above and below the mean age) and ethnicity (White, Black, Asian/Pacific Islander, and Hispanic or mixed ethnic background) on the trajectories of morning fatigue are plotted.
Figs. 3A, 3B.
Effects of age (older/younger age calculated as one standard deviation above and below the mean age) and ethnicity (White, Black, Asian/Pacific Islander, and Hispanic or mixed ethnic background) on the trajectories of morning fatigue.
Discussion
This study is the first to evaluate for and determine predictors of interindividual variability in morning fatigue severity in oncology patients undergoing two cycles of CTX. While typical morning fatigue scores prior to the next dose of CTX (i.e., 3.06) were just below the clinically meaningful cut-off score of 3.2, there was substantial interindividual variability in these scores (Fig. 1B). In addition, using piecewise modeling, cyclic variations in morning fatigue severity were identified (during each cycle, the severity of morning fatigue increased following the administration of CTX and then decreased in the week following CTX). These results indicate a sample-wide change in morning fatigue severity over time. However, they do not indicate that all of the patients’ morning fatigue severity scores changed at the same rate over time. The variance components (Table 3) suggest that considerable interindividual variability existed in the trajectories of morning fatigue. Taken together, these findings suggest that an evaluation of changes in morning fatigue severity over the course of multiple cycles of CTX warrant the use of more sophisticated statistical techniques like HLM.
A direct comparison of our findings regarding the trajectories of morning fatigue over the two cycles of CTX is not possible because no studies were identified that used the same fatigue measure; the same assessment time points; and HLM as the analysis method. The piecewise model of changes over time in morning fatigue during the administration of CTX contrasts with our previous findings regarding changes in morning fatigue severity in patients with breast16 and prostate20 cancer who underwent RT. While a quadratic trajectory was observed for changes in morning fatigue in both the patients with breast and prostate cancer, these two groups had distinct trajectories. In the patients with prostate cancer, morning fatigue severity scores increased gradually from the beginning to the end of RT and then returned to pre-treatment levels after the completion of RT.20 In contrast, the trajectory of morning fatigue in the patients with breast cancer decreased gradually over the course of RT and then plateaued at the completion of treatment.16 These between-treatment (CTX versus RT) and between-diagnosis differences in the trajectories of morning fatigue warrant additional investigation. Because patients in the current study were not enrolled prior to their first dose of CTX and followed to the completion of and post CTX treatment, one cannot determine if the severity of morning fatigue increased over each cycle of CTX or when these scores returned to pre-treatment levels.
A comparison of the trajectories of morning and evening fatigue23 during CTX in this sample of patients identified that both types of fatigue exhibited a piecewise model of change. However, across the six assessments, morning fatigue severity scores were above the clinically meaningful cut-off for five of the six assessments (assessments 2, 3, 4, 5, and 6), compared to only two of the assessments for evening fatigue (assessments 2 and 5). Taken together, these findings suggest that morning and evening fatigue are distinct but related symptoms.
Similar to the findings for evening fatigue,23 the severity of morning fatigue (3.1) during CTX was somewhat higher than that reported by patients with breast (2.9) and prostate (1.8) cancer prior to and at the completion of RT.16,20 These findings suggest that the severity of morning fatigue can vary across different cancer treatments. In prior studies,33-35 patients undergoing CTX reported higher fatigue severity scores compared to those undergoing RT. Patients may experience inflammatory responses differently with CTX compared to RT, which may contribute to higher morning and evening fatigue severity during CTX. However, the patients with breast cancer at the completion of RT had similar morning and evening fatigue severity scores.16 The similar scores suggest that RT may have cumulative effects comparable to CTX. Future studies will need to determine the mechanisms that underlie differences in fatigue severity across various cancer treatments.
The HLM analysis provided insights into which demographic, clinical, and symptom characteristics were associated with more severe morning fatigue. As summarized in Table 4, common as well as unique characteristics were associated with initial levels as well as the trajectories of morning and evening23 fatigue. The remainder of this discussion will compare and contrast these findings.
Table 4.
Comparison of Intercept and Slope Predictors of Morning and Evening Fatigue During Chemotherapy
| Characteristic | Morning Fatigue | Evening Fatigue |
|---|---|---|
| Intercept Predictors | ||
| Non-White ethnicity | ◆ | |
| Education | ◆ | |
| Child care responsibilities | ◆ | |
| Body mass index | ◆ | |
| Exercise | ◆ | |
| Functional status | ◆ | ◆ |
| Cancer diagnosis | ◆ | |
| State anxiety at enrollment | ◆ | |
| Depressive symptoms at enrollment | ◆ | ◆ |
| Sleep disturbance at enrollment | ◆ | ◆ |
| Slope Predictors | ||
| Age | ◆ | |
| Ethnicity | ◆ | |
| Evening fatigue at enrollment | ◆ | |
Common Predictors of Morning and Evening Fatigue
The common predictors of morning and evening fatigue severity were ethnicity, functional status, depressive symptoms, and sleep disturbance. While ethnicity was associated with both morning and evening fatigue severity, it predicted different components of the trajectory. For morning fatigue, ethnicity predicted changes in the severity of the symptom over time (i.e., slope). The trajectories of morning fatigue severity during the first CTX cycle (assessments 1, 2, and 3) were the same for all of the ethnic groups. However, during the second cycle (assessments 4, 5, and 6), the trajectories of morning fatigue severity differed by ethnic group (Fig. 3B). Whites, Asian/Pacific Islanders, and Hispanics or individuals with mixed ethnic backgrounds reported higher morning fatigue severity scores during the second CTX cycle than did patients of Black ethnicity. For evening fatigue, ethnicity predicted initial severity (i.e. intercept) but not changes in the severity scores of the symptom over time.23 Patients who were White reported higher evening fatigue severity scores at enrollment that persisted over the two CTX cycles. In prior studies of patients receiving CTX, there are inconsistent associations between ethnicity and mean daily fatigue scores.7,8,15,36-38 In addition, definitive conclusions about the asssociation of ethnicity and morning and evening fatigue cannot be drawn given the paucity of studies that evaluated diurnal variations in fatigue severity.
For both morning and evening fatigue,23 lower functional status was associated with higher fatigue severity scores at enrollment. In contrast, functional status was not a predictor of either morning or evening fatigue in patients with breast16 or prostate cancer20 who received RT. This inconsistency may be related to the lower functional status of the patients in this study (mean KPS score of 80.6) compared to the patients in the RT studies (mean KPS scores of 87.7 and 95.7, respectively). When morning and evening fatigue scores were predicted for patients with a KPS score of one SD below the mean (i.e., 68.8), these predicted scores (3.4 for morning fatigue and 5.7 for evening fatigue), are above the clinically meaningful cut-off scores for both symptoms. These findings suggest that patients with lower functional status are at higher risk for clinically meaningful levels of morning and evening fatigue that persist across two cycles of CTX. Initial, as well as changes in, functional status scores are often used to assess the impact of both cancer and its treatment. In addition, lower KPS scores are associated with decreases in QOL and survival.39 Of note, patients with a KPS score of 68.8 are unable to work outside the home, need occasional assistance with personal care,24 and require careful assessment of further functional decline.15,40 The use of a simple measure, like the KPS score, to assess functional status will provide early recognition of patients who are at higher risk for more severe morning and evening fatigue. Interventions to improve functional status have the potential to decrease the severity of both morning and evening fatigue severity and improve patients’ QOL.
Depressive symptom scores were associated with both morning and evening fatigue23 severity during CTX. In our previous studies, depressive symptoms were associated with higher levels of evening fatigue prior to the initiation of RT in patients with breast cancer16 and with higher levels of morning fatigue prior to the initiation of RT as well as the trajectory of morning fatigue during RT in patients with prostate cancer.20 One of the diagnostic criteria for depression is morning fatigue,41 which supports the consistent positive association between depressive symptoms and morning fatigue severity in patients with prostate cancer undergoing RT as well as the patients in this study. Compared to the patients with prostate cancer (CES-D score of 5.9),20 patients in this study (CES-D score of 12.6) had higher depressive symptom scores that were associated with both morning and evening fatigue severity. This finding suggests that the associations between depressive symptoms and morning and evening fatigue may depend on the severity of the depressive symptoms. However, if this hypothesis was correct, then the depressive symptom score of the patients with breast cancer who underwent RT (CES-D score of 12.0),16 which was similar to the CES-D score for this sample, should have been associated with both morning and evening fatigue severity. These inconsistent findings suggest that additional demographic (e.g., gender) or clinical characteristics (e.g., treatment with RT or CTX) or timing of the measures may influence the associations between depressive symptoms and morning and evening fatigue severity. Another possible explanation for the inconsistent findings may be the effect of variations in cytokine genes on the associations between depressive symptoms and morning and evening fatigue.42-44 For example, variations in the genes for tumor necrosis factor alpha (TNFA) were associated with morning fatigue (single nucleotide polymorphism [SNP] rs3093662) and evening fatigue (SNP rs2229094)43 and with depressive symptoms (SNPs rs2229094 and rs1800629).44
Consistent with our findings for evening fatigue in this sample,23 as well as our findings for morning fatigue in patients with breast cancer16 and both morning and evening fatigue in patients with prostate cancer20 who underwent RT, sleep disturbances predicted initial levels of morning fatigue. Of note, this sample’s mean sleep disturbance score at enrollment (52.2) was above the clinically meaningful cut-off score of 43.28 When morning and evening fatigue scores were predicted for patients who were one SD above the mean GSDS score (71.6) predicted scores for morning (3.8) and evening (5.8) fatigue were above the clinically meaningful cut-off scores for both symptoms. Taken together, the consistent findings across our studies5,16,20,23,28,45 and other cross-sectional studies8,46-49 provide strong evidence of the positive association between sleep disturbance and fatigue severity. Additional research is warranted to determine which symptom is the driver.
Unique Predictors of Morning Fatigue
The unique clinical characteristics that predicted morning but not evening fatigue severity were: age, BMI, exercise, and state anxiety. Age predicted the trajectory of morning fatigue (i.e., slope). Compared to older patients, younger patients exhibited a slightly higher peak in morning fatigue severity at assessment 2, with a decline at assessment 3 (Fig. 3A). However, a similar pattern was not found for the second CTX cycle. These age differences in the trajectory of morning fatigue may be explained by a “response shift” that alters older patients’ perception of symptoms.50,51 Another potential explanation for the differences in the trajectory of morning fatigue could be age-related changes in the hypothalamic-adrenal-pituitary axis that may mediate the severity of fatigue.52 While younger age was associated with higher morning fatigue scores in patients with breast16 and prostate20 cancer prior to the initiation of RT, age did not predict the trajectory of fatigue during RT.
Patients with a higher BMI reported higher levels of morning fatigue prior to their next dose of CTX (Fig. 2A). This finding is consistent with our previous report in patients with breast cancer at the initiation of RT.16 A BMI of one SD above the mean for patients in this study (32.1 kg/m2) and for patients with breast cancer undergoing RT (34.7 kg/m2) were above the clinical cut-off for obesity (≥30 kg/m2).53 The inflammatory responses associated with obesity may be one of the underlying mechanisms for an increase in morning fatigue.54-56
The lack of regular exercise was associated with a small increase in morning but not evening23 fatigue severity at enrollment (Fig. 2B). A direct comparison of the effects of exercise on morning and evening fatigue is not possible because no studies were identified that evaluated this association. However exercise has been shown to improve fatigue in oncology patients.57-59 In addition, regular exercise is known to regulate circadian rhythms,60 which become progressively disrupted during CTX.61 Exercise-induced maintenance or improvements in circadian rhythm may result in decreases in sleep disturbance and morning fatigue.58,59,62,63 While the association between altered circadian rhythms and morning fatigue severity has not been studied in oncology patients, it was found to be associated with more severe morning fatigue in mothers with an infant in the ICU64 and in women with HIV disease.65
Higher levels of state anxiety were associated with higher levels of morning fatigue at enrollment but not with morning or evening fatigue in our RT studies16,20 or with evening fatigue in this study.23 This finding is inconsistent with previous reports that found positive associations between anxiety and mean daily fatigue scores.7,35,66 Patients in this study reported state anxiety scores that were above the clinically meaningful cut-off scores.26 One possible explanation why anxiety was associated with only morning fatigue is that increased anxiety is associated with sleep disturbance in oncology patients.47,55,67 While morning fatigue was not evaluated in these studies, patients with higher levels of anxiety reported lower levels of morning energy47,55 and higher levels of sleep disturbance.67 Taken together, our findings suggest that additional research is warranted on the associations among morning fatigue, anxiety, sleep disturbance, and regular exercise.
Unique Predictors of Evening Fatigue
Child care responsibilities and level of education were the demographic characteristics that were associated with only the severity of evening fatigue during CTX.23 This finding is consistent with previous research in healthy individuals68 that found that individuals that cared for children had higher levels of evening fatigue. While higher levels of education were associated with higher levels of evening fatigue severity in our study,23 associations between education and fatigue severity are inconsistent.16,20,69 Further research is needed to clarify the association between education and evening fatigue severity.
A diagnosis of breast, GYN, and lung cancer, compared to patients with GI cancer, were associated with higher levels of evening23 but not morning fatigue. This finding contrasts with prior studies with patients with mixed cancer diagnoses that identified patients with lung cancer as having higher levels of fatigue compared to other diagnoses.12,15,17 A better understanding of the common and distinct molecular mechanisms that underlie the development of morning and evening fatigue may provide insights into how the unique and common predictors of diurnal variations in fatigue severity are interrelated. Across our two companion papers, common as well as unique characteristics were identified that were associated with initial levels as well as the trajectories of morning and evening fatigue. Taken together, these findings suggest that morning and evening fatigue are distinct but related symptoms.
Comparisons Between Oncology Patients and Healthy Individuals in Diurnal Variations in Fatigue
Compared to healthy individuals,70-72 oncology patients experience more severe mean daily fatigue that is not associated with activity. This sample of oncology patients experienced clinically meaningful elevations in morning fatigue at more assessment points during their CTX cycle than evening fatigue. In contrast, healthy individuals often reported a feeling of tiredness in the evening that was associated with daily activities.72-74 These findings suggest that additional research is warranted to evaluate for the differences in both the phenotypic and molecular characteristics that are associated with diurnal variations in fatigue in healthy individuals and patients undergoing cancer treatments.
While prior studies have not differentiated between the occurrence of morning and evening fatigue in the general population, diurnal variations were found in patients with sarcoidosis,75 HIV disease,65 and inflammatory disorders.76 These results suggest that some of the underlying mechanisms for morning and evening fatigue may be different. In addition, assessment of diurnal variations in fatigue severity and interventions targeted at the modifiable risk factors for these two distinct but related symptoms may decrease fatigue severity and improve the QOL.
Limitations and Strengths
Several limitations and strengths need to be acknowledged. Because patients were recruited at various points in their CTX treatment, changes in fatigue severity from the initiation of CTX cannot be evaluated. The majority of the sample had at least four years of college education, which is not representative of the United States population (only 27% have four years of college77)). Patients rated their experience of morning fatigue over the last week. Daily assessments of morning fatigue may provide more insights into the variability of morning fatigue during a CTX cycle.78 However, this large, representative sample of oncology outpatients undergoing CTX, the evaluation of morning fatigue across two cycles of CTX, and the use of HLM to identify predictors of morning fatigue are major strengths of this study. Additionally, this study is the first to compare predictors of initial levels as well as trajectories of morning and evening fatigue in order to describe specific risk profiles for these two distinct but related symptoms.
Clinical Implications
The findings reported in this paper and our companion paper have important clinical implications. Assessment of diurnal variations in fatigue severity, as well as associated risk factors, need to be incorporated into clinical practice. These assessments may allow clinicians to focus interventions on one or both of these symptoms. Several modifiable risk factors for more severe morning fatigue (BMI, regular exercise, functional status) were identified. The prevalence of obesity in our sample (20%) contributes to the importance of BMI as a modifiable risk factor to improve the severity of morning fatigue. Regular exercise may provide more benefit than calorie restriction alone for weight loss. Weight loss and exercise interventions may decrease the severity of morning fatigue, which in turn may improve patients’ functional status.6,57 Interventions that improve functional status have the potential to decrease both morning and evening fatigue severity. Early assessments and initiation of treatment for state anxiety, sleep disturbances, and depressive symptoms may decrease the severity of both morning and evening fatigue and improve patients’ QOL.
Future Research
Longitudinal studies are needed that evaluate morning and evening fatigue severity before, during, and after CTX. Studies of the underlying mechanisms that contribute to diurnal variations in fatigue severity (e.g., genetic variations, alterations in stress responses) are needed to clarify the associations between and among the various characteristics that predict morning and evening fatigue severity. Additional research that examines the underlying mechanisms of morning and evening fatigue severity would clarify the specific risk profiles for each symptom so that tailored interventions can be developed and tested. Given the associations among depressive symptoms, sleep disturbance, morning fatigue and evening fatigue,10,16,20,22,46,56 future longitudinal studies need to determine which symptom influences the trajectory of the other symptoms. Statistical techniques like parallel process growth modeling or classification and regression tree analysis could be used to provide insights into which symptom to target for an intervention in order to reduce the other symptoms.
Acknowledgments
This study was funded by the National Cancer Institute (NCI)(CA134900). This study was partially supported by a graduate assistantship through the New York University College of Nursing. Dr. Miaskowski is supported by a grant from the American Cancer Society and NCI (CA168960).
Footnotes
Disclosures The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henry DH, Viswanathan HN, Elkin EP, et al. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee KA, Lentz MJ, Taylor DL, Mitchell ES, Woods NF. Fatigue as a response to environmental demands in women’s lives. Image J Nurs Sch. 1994;26:149–154. doi: 10.1111/j.1547-5069.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 4.Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 6.Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(Suppl 11):2124–2130. doi: 10.1002/cncr.28058. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Grem JL, Visovsky C, Marunda HA, Yurkovich JM. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum. 2010;37:E359–369. doi: 10.1188/10.ONF.E359-E369. [DOI] [PubMed] [Google Scholar]
- 8.Berger AM, Lockhart K, Agrawal S. Variability of patterns of fatigue and quality of life over time based on different breast cancer adjuvant chemotherapy regimens. Oncol Nurs Forum. 2009;36:563–570. doi: 10.1188/09.ONF.563-570. [DOI] [PubMed] [Google Scholar]
- 9.Hallqvist A, Bergman B, Nyman J. Health related quality of life in locally advanced NSCLC treated with high dose radiotherapy and concurrent chemotherapy or cetuximab--pooled results from two prospective clinical trials. Radiother Oncol. 2012;104:39–44. doi: 10.1016/j.radonc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Trudel-Fitzgerald C, Savard J, Ivers H. Evolution of cancer-related symptoms over an 18-month period. J Pain Symptom Manage. 2013;45:1007–1018. doi: 10.1016/j.jpainsymman.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 12.Fisch MJ, Zhao F, O’Mara AM, et al. Predictors of significant worsening of patient-reported fatigue over a 1-month timeframe in ambulatory patients with common solid tumors. Cancer. 2014;120:442–450. doi: 10.1002/cncr.28437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang HP, Chen ML, Liang J, Miaskowski C. Changes in and predictors of severity of fatigue in women with breast cancer: a longitudinal study. Int J Nurs Stud. 2014;51:582–592. doi: 10.1016/j.ijnurstu.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brant JM, Beck SL, Dudley WN, et al. Symptom trajectories during chemotherapy in outpatients with lung cancer colorectal cancer, or lymphoma. Eur J Oncol Nurs. 2011;15:470–477. doi: 10.1016/j.ejon.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Dimsdale JE, Ancoli-Israel S, Elsmore TF, Gruen W. Taking fatigue seriously: I. Variations in fatigue sampled repeatedly in healthy controls. J Med Eng Technol. 2003;27:218–222. doi: 10.1080/0309190031000075354. [DOI] [PubMed] [Google Scholar]
- 19.Dodge R. Circadian rhythms and fatigue: a discrimination of their effects on performance. Aviat Space Environ Med. 1982;53:1131–1137. [PubMed] [Google Scholar]
- 20.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48:247–252. doi: 10.1176/appi.psy.48.3.247. [DOI] [PubMed] [Google Scholar]
- 22.Jim HS, Small B, Faul LA, et al. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42:321–333. doi: 10.1007/s12160-011-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright F, Melkus GD, Hammer M, et al. Trajectories of evening fatigue in oncology outpatients receiving chemotherapy. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2015.02.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnofsky D. Performance scale. Plenum Press; New York, NY: 1977. [Google Scholar]
- 25.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y) Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 27.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 28.Dhruva A, Paul SM, Cooper BA, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2012;44:215–228. doi: 10.1016/j.jpainsymman.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 30.IBM . IBM SPSS Statistics for Windows, Version 22. IBM Corp.; Armonk, NY: 2013. [Google Scholar]
- 31.Raudenbush SW, Bryk A. Hierarchical linear models: Applications and data analysis methods. 2nd ed Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 32.Osborne C, Berger LM, Magnuson K. Family structure transitions and changes in maternal resources and well-being. Demography. 2012;49:23–47. doi: 10.1007/s13524-011-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofso K, Miaskowski C, Bjordal K, Cooper BA, Rustoen T. Previous chemotherapy influences the symptom experience and quality of life of women with breast cancer prior to radiation therapy. Cancer Nurs. 2012;35:167–177. doi: 10.1097/NCC.0b013e31821f5eb5. [DOI] [PubMed] [Google Scholar]
- 35.Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates and interventions. Eur J Cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 36.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000;27:1443–1448. [PubMed] [Google Scholar]
- 37.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 38.Stone P, Richardson A, Ream E, et al. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11:971–975. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 39.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol. 2008;31:424–430. doi: 10.1097/COC.0b013e31816d915f. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- 42.Bower JE, Ganz PA, Irwin MR, et al. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–1661. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhruva A, Aouizerat BE, Cooper B, et al. Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biol Res Nurs. 2015;17:175–184. doi: 10.1177/1099800414534313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn LB, Aouizerat BE, Langford DJ, et al. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. Eur J Oncol Nurs. 2013;17:346–353. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miaskowski C, Paul SM, Cooper BA, et al. Predictors of the trajectories of self-reported sleep disturbance in men with prostate cancer during and following radiation therapy. Sleep. 2011;34:171–179. doi: 10.1093/sleep/34.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22:2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18:105–114. doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 48.Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35:237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. J Pain Symptom Manage. 2009;37:341–351. doi: 10.1016/j.jpainsymman.2008.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritchie C, Dunn LB, Paul SM, et al. Differences in the symptom experience of older oncology outpatients. J Pain Symptom Manage. 2014;47:697–709. doi: 10.1016/j.jpainsymman.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Soc Personal Psychol Compass. 2008;2:223–244. [Google Scholar]
- 53.Centers for Disease Control and Prevention [Accessed September 11, 2014];Assessing your weight. 2014 Available at: http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- 54.O’Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berger AM, Hertzog M, Geary CR, Fischer P, Farr L. Circadian rhythms, symptoms, physical functioning, and body mass index in breast cancer survivors. J Cancer Surviv. 2012;6:305–314. doi: 10.1007/s11764-012-0218-x. [DOI] [PubMed] [Google Scholar]
- 56.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra SI, Scherer RW, Snyder C, et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomlinson D, Diorio C, Beyene J, Sung L. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93:675–686. doi: 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 60.Davis MP, Goforth HW. Long-term and short-term effects of insomnia in cancer and effective interventions. Cancer J. 2014;20:330–344. doi: 10.1097/PPO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 61.Savard J, Liu L, Natarajan L, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32:1155–1160. doi: 10.1093/sleep/32.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Courneya KS, Segal RJ, Mackey JR, et al. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144:361–369. doi: 10.1007/s10549-014-2883-0. [DOI] [PubMed] [Google Scholar]
- 63.Ratcliff CG, Lam CY, Arun B, Valero V, Cohen L. Ecological momentary assessment of sleep, symptoms, and mood during chemotherapy for breast cancer. Psychooncology. 2014;23:1220–1228. doi: 10.1002/pon.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SY, Lee KA, Aycock D, Decker M. Circadian activity rhythms for mothers with an infant in ICU. Front Neurol. 2010;1:155. doi: 10.3389/fneur.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 66.Dragomir BI, Fodoreanu L. Correlations between state anxiety and quality of life in metastatic breast cancer patients. Rev Med Chir Soc Med Nat Iasi. 2013;117:610–615. [PubMed] [Google Scholar]
- 67.Van Onselen C, Cooper BA, Lee K, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Support Care Cancer. 2012;20:2611–2619. doi: 10.1007/s00520-012-1381-3. [DOI] [PubMed] [Google Scholar]
- 68.Bakker M, van der Beek AJ, Hendriksen IJ, Bruinvels DJ, van Poppel MN. Predictive factors of postpartum fatigue: a prospective cohort study among working women. J Psychosom Res. 2014;77:385–390. doi: 10.1016/j.jpsychores.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Von Ah DM, Kang DH, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31:134–144. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- 70.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 71.de Raaf PJ, de Klerk C, Timman R, Hinz A, van der Rijt CC. Differences in fatigue experiences among patients with advanced cancer, cancer survivors, and the general population. J Pain Symptom Manage. 2012;44:823–830. doi: 10.1016/j.jpainsymman.2011.12.279. [DOI] [PubMed] [Google Scholar]
- 72.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care. 1996;5(2 Suppl):8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 73.Solomon L, Nisenbaum R, Reyes M, Papanicolaou DA, Reeves WC. Functional status of persons with chronic fatigue syndrome in the Wichita, Kansas, population. Health Qual Life Outcomes. 2003;1:48. doi: 10.1186/1477-7525-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van’t Leven M, Zielhuis GA, van der Meer JW, Verbeek AL, Bleijenberg G. Fatigue and chronic fatigue syndrome-like complaints in the general population. Eur J Public Health. 2010;20:251–257. doi: 10.1093/eurpub/ckp113. [DOI] [PubMed] [Google Scholar]
- 75.De Kleijn WP, Drent M, Vermunt JK, Shigemitsu H, De Vries J. Types of fatigue in sarcoidosis patients. J Psychosom Res. 2011;71:416–422. doi: 10.1016/j.jpsychores.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 76.van Oers ML, Bossema ER, Thoolen BJ, et al. Variability of fatigue during the day in patients with primary Sjogren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:715–721. [PubMed] [Google Scholar]
- 77.United States Census Bureau . Educational attainment in the United States: 2013. U.S. Census Burear; Washington, DC: 2013. [Google Scholar]
- 78.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]