Abstract
Background
Cardiac disease is a major cause of death in muscular dystrophies. The use of feasible and reproducible echocardiographic measures of cardiac function is critical to advance the field of therapeutics for dystrophic cardiomyopathy.
Methods
Participants aged 8 to 18 years with genetically confirmed Duchenne (DMD), Becker (BMD) or limb girdle (LGMD) muscular dystrophies were enrolled at five centers and a standardized echocardiogram (echo) was performed. Measures of systolic and diastolic function and speckle tracking echo (STE) derived cardiac strain were reviewed independently by two central readers. Furthermore, echo measures from DMD participants were compared to retrospective aged matched controls from a single site to assess measures of myocardial function.
Results
48 participants (mean age of 13.3±2.7 years) were enrolled. Shortening fraction (SF%) had a greater inter-observer correlation (intra-class correlation coefficient; ICC=0.63) compared to ejection fraction (EF%; ICC=0.49). One reader could only measure EF% in 53% of participants. Myocardial performance index (MPI) measured by pulse wave Doppler and tissue Doppler imaging showed similar ICCs (0.55 and 0.54). STE showed a high ICC (0.96). Focusing on DMD participants (n=33), significantly increased mitral A wave velocities, lower E/A ratios and lower TDI mitral lateral E’ velocities were observed compared to age matched controls. STE demonstrated subclinical myocardial dysfunction with decreased average circumferential and longitudinal strain in 3 distinct subgroups: DMD participants with normal SF%; DMD participants with age <13 years; and DMD participants with MPI<0.40 compared to controls.
Conclusions
In a muscular dystrophy cohort, assessment of cardiac function is feasible and reproducible using SF%, diastolic measures and MPI. Cardiac strain measures identified early myocardial disease in DMD.
Keywords: muscular dystrophy, echocardiography, cardiac strain, cardiomyopathy
Introduction
Cardiomyopathy causes significant morbidity and mortality in multiple forms of muscular dystrophy (MD) affecting children, including Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), and subtypes of autosomal recessive limb-girdle muscular dystrophy (LGMD2).[1] The prevalence of cardiomyopathy increases with age and is currently under diagnosed in DMD.[2, 3] Therefore, it is increasingly important to study cardiac disease in muscular dystrophies to determine the best diagnostic and treatment modalities.
Adequately powered pharmaceutical studies for the treatment of MD require the collection of reproducible data across multiple clinical sites due to the rarity of these diseases. Several studies have researched cardiac endpoints; however comparisons across studies are hindered by different study designs and functional measures, which include percent shortening fraction (SF%), percent ejection fraction (EF%), mitral inflow velocities, myocardial performance index (MPI), and myocardial strain.[4-25] These measures are also affected by technical limitations in MD patients including scoliosis, barrel chest deformities with lung hyperinflation and limited mobility.
The purpose of this study was to assess the feasibility and reproducibility of non-invasive echocardiography-based functional cardiac measures in a multi-center cohort of MD participants and determine which measures can detect early subclinical changes in myocardial function. The Cooperative International Neuromuscular Research Group (CINRG) is a coalition of academic clinical centers dedicated to MD research. CINRG has validated skeletal muscle functional measures for multi-site DMD studies.[26] The results from this study will help direct selection of cardiac measurements for future clinical trials and enhance consistency within the field of cardiomyopathies in patients with MD.
Methods
This was a multicenter, prospective study investigating different echo parameters with participants enrolling at five institutions in the CINRG network, all also part of the Clinical and Translational Science Award (CTSA) network (www.ctsacentral.org). The study was approved by the Institutional Review Board at each institution. Written informed consent and assent were obtained from all participants and their parents or legal guardians. The study was registered with ClinicalTrials.gov: NCT01066455. Participants had a confirmed genetic diagnosis of DMD, BMD, LGMD2C-2F or LGMD2I. Participants were excluded if they had a history of a congenital cardiac defect or other cardiac disease unrelated to MD. Retrospective controls for DMD participants were patients referred to the cardiology clinic at one of the five participating CINRG clinical sites (Children's National Health System) for complaints of cardiac murmur or chest pain and were determined to have a normal cardiac evaluation with normal conventional echo parameters. Race/ethnicity were self-reported and not required for controls.
Methods used to determine the feasibility and reproducibility of non-invasive echocardiography-based functional cardiac measures: A central sonographer travelled to each participating CINRG site to review and train the site personnel on a centralized protocol using standard imaging planes and recording three beat loops saved in DICOM format. Echos were performed with participants in the supine position on an exam table or seated in a power wheelchair if transfer to an exam table was not possible. These digital loops were interpreted by two pediatric cardiologists and one pediatric cardiology fellow. There were two readers at the Washington, DC site (CFS and SJG – fellow) and one reader at the Pittsburgh, PA site (FMM).
All measurements and calculations including SF%, EF% using single plane modified Simpson's protocol, wall stress, velocity of circumferential shortening (Vcf), rate corrected Vcf (Vcfc), myocardial performance index [MPI; includes measures of ejection time (ET), isovolumic contraction time (IVCT) and isovolumic relaxation time (IVRT)] using both pulse wave Doppler (PWD) and tissue Doppler imaging (TDI), mitral inflow and left ventricular peak E wave velocities were made according to standards of the American Society of Echocardiography.[27] Each reader (CFS and FMM) measured 2D and Doppler values over three cardiac cycles (or the maximum feasible when imaging was limited quality) and the average was used for analysis. In order to assess intra-observer variability, 47 echos were reassessed by the same observer (CFS) after a period of at least 2 weeks. Inter-observer variability was assessed by having a different reader (FMM) perform all the measures on the conventional echo images independently.
Methods used to determine early subclinical myocardial changes: Cardiac strain was measured using speckle tracking echocardiography (STE) on the subset of DMD participants from all five participating CINRG centers and historical controls from a single institution (Children's National Health System), using a proprietary software (Syngo Velocity Vector Imaging, Siemens Medical Solutions, Ultrasound Division, Mountain View, California). STE analysis was performed by two readers (CFS and SJG). For STE, endocardial tracings of the left ventricle were manually performed on the apical 4 chamber view (for longitudinal measurements) and the parasternal short-axis view at the level of mid-papillary muscles (for circumferential measurements). A single cardiac beat with the best appearing image quality was utilized. Tracking was automatically performed by the software and the analysis was accepted as satisfactory only after visual inspection. Endocardial tracing and automatic tracking were performed twice on each beat and the average of the two measurements was recorded for each variable. If tracking was suboptimal, the endocardial border was retraced until either satisfactory tracking was accomplished or 5 minutes had passed, in which case the view was excluded from analysis. Measured deformation parameters included average peak systolic longitudinal strain (LS; %), average peak systolic longitudinal strain rate (LSR; 1/sec), average peak systolic circumferential strain (CS; %), and average peak systolic circumferential strain rate (CSR; 1/sec). Segmental data were not analyzed. Mathematically, all of these parameters are negative (indicating shortening) and a smaller absolute value indicates worse ventricular function. In order to assess intra-observer variability for STE, a randomly selected set of eleven echos (6 DMD, 5 controls) were reassessed by the same observer (SJG) after a period of at least 2 weeks. Inter-observer variability was assessed by having a different observer (CFS) perform STE measurements on all possible DMD echos using the same beat as the original observer.
Statistical analysis
Descriptive measurements including demographic characteristics (age, ethnicity and race), blood pressure (BP), heart rate (HR) and body mass index (BMI) were summarized as mean ± SD, range or frequencies appropriate for each data type. Age, BP and HR were compared between the overall MD group and the DMD controls using t-test analysis while BMI required log-transformed data for t-test analysis. Both the number of participants and the distribution of diagnosis (DMD, BMD and LGMD) were compared between medication use (glucocorticoids, cardiac medications, Coenzyme Q10) with exact chi-squared analysis. Mean age in the different medications use group was compared with a t-test. Race and ethnicity were not consistently captured for the controls and hence were not compared to the MD patients.
Nineteen continuous echo parameters were summarized as mean ± SD and ranges for each reader. Agreements between echo measurements were assessed by calculation of the intra-class correlation coefficient (ICC) both between the two readers (inter-observer) and between repeated measurements from Reader 1 (intra-observer). Bland Altman plots were produced showing the mean bias and 95% limits of agreement. EF% and SF% were compared using a t-test while MPI required log transformed data for t-test analysis. Left ventricular EF%, SF% and MPI measurements were also dichotomized between normal and abnormal based on the following criteria: EF% (normal ≥55, abnormal <55), SF% (normal ≥28, abnormal <28), TDI & PWD MPI (normal<0.40, abnormal ≥0.40).[28, 29] These dichotomized variables were then compared pairwise to evaluate agreement in normal/abnormal designation using a McNemar test.
Continuous measurements from STE, along with basic demographic characteristics, were summarized by mean ± SD and compared between DMD cases and controls using t-tests. Subgroup analyses were performed in the same manner on those who have a normal SF or EF value, those under the age of 13 years, and those with an abnormal MPI value (<0.40).
All analyses used Stata V13 (College Station, TX) and a p-value of <0.05 was considered statistically significant. No adjustments for multiple tests were done due to the observational focus of this manuscript.
Results
Patient Characteristics
Information for all participants (MD and retrospective controls) is included in Table 1. There were 52 MD participants consented from five sites and 48 completed the study. There were 33 controls without MD used for subset analysis of DMD participants. MD participants had a genetically confirmed diagnosis of DMD (73%), BMD (23%) or LGMD (4%). The mean age for the MD cohort was 13.3±2.7 years, with a range of 8.4 to 17.7 years and the mean age for the control cohort was 12.8±2.8 years, with a range of 8.7 to 17.9 years. Information on other patient characteristics including demographics, vital signs, BMI and past and current medications (glucocorticoids, cardiac medications, and coenzyme Q10) for the MD cohort are shown in Table 1.
Table 1.
Participant characteristics
| Characteristic | Muscular Dystrophy Cohort | Control for DMD Cohort | P values | |||
|---|---|---|---|---|---|---|
| DMD | BMD | LGMD | All | |||
| No. (%) or Mean ± SD | No. (%) or Mean ± SD | No. (%) or Mean ± SD | No. (%) or Mean ± SD | No. (%) of Mean ± SD | ||
| Number | 35 (73%) | 11 (23%) | 2 (4%) | 48 (100%) | 33 (100%) | |
| Age, y | 13 ± 3 | 14 ± 3 | 14 ± 2 | 13 ± 3 | 13 ± 3 | 0.43 |
| Ethnicity | ||||||
| Hispanic | 5 (14%) | 0 (0%) | 1 (50%) | 6 (13%) | N/A | |
| Non-Hispanic | 30 (86%) | 11 (100%) | 1 (50%) | 42 (88%) | N/A | |
| Race | ||||||
| Caucasian | 29 (83%) | 10 (91%) | 1 (50%) | 40 (83%) | N/A | |
| Asian | 2 (6%) | 0 (0%) | 0 (0%) | 2 (4%) | N/A | |
| African American | 3 (9%) | 1 (9%) | 1 (50%) | 5 (10%) | N/A | |
| Other | 1 (3%) | 0 (0%) | 0 (0%) | 1 (2%) | N/A | |
| Vital signs | ||||||
| SBP (mmHg) | 109 ± 13 | 111 ±9 | 95 ± 3 | 109 ± 12 | 115 ± 10 | 0.02 |
| DBP (mmHg) | 65 ± 8 | 64 ± 9 | 60 ± 5 | 65 ± 7 | 64 ± 7 | 0.76 |
| Heart Rate (bpm) | 92 ± 14 | 79 ± 12 | 93 ± 1 | 89 ± 15 | 67 ± 13 | <0.001 |
| BMI (kg/m2) | 24 ± 7 | 20 ± 3 | 18 ± 2 | 23 ± 7 | 22 ± 6 | 0.68 |
| Glucocorticoid Use | 0.003 | |||||
| Past users | 6 (17%) | 0 (0%) | 1 (50%) | 7 (15%) | N/A | |
| Current user | 24 (69%) | 4 (36%) | 1 (50%) | 29 (61%) | N/A | |
| Never used | 5 (14%) | 7 (64%) | 0 (0%) | 12 (25%) | N/A | |
| Cardiac Medication Use | 0.76 | |||||
| Past users | 1 (3%) | 0 (0%) | 0 (0%) | 1 (2%) | N/A | |
| Current user | 13 (37%) | 4 (36%) | 0 (0%) | 17 (36%) | N/A | |
| Never used | 21 (60%) | 7 (64%) | 2 (100%) | 30 (63%) | N/A | |
| CoQ10 Use | 0.99 | |||||
| Past users | 3 (9%) | 2 (18%) | 0 (0%) | 5 (10%) | N/A | |
| Current user | 8 (23%) | 1 (9%) | 0 (0%) | 9 (19%) | N/A | |
| Never used | 24 (69%) | 8 (73%) | 2 (100%) | 34 (71%) | N/A | |
P-values are comparisons between the controls (N=33) and the entire MD cohort (N=48) for characteristics available in the entire cohort and are between MD diagnoses for characteristics only applicable to the MD group.
Abbreviations: DMD – Duchenne muscular dystrophy, BMD – Becker muscular dystrophy, LGMD – Limb girdle muscular dystrophy, SD – standard deviation, y – years, BMI – Body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, mmHg – millimeters of mercury, bpm – beats per minute, N/A – not applicable
Feasibility
Based on image quality, only one study could not be read by both readers (CFS and FMM) and some measures were not obtained in all studies by both readers. For EF%, Reader 1 was only able to confidently trace the endocardial border in 25/48 (52%) and Reader 2 in 43/48 (90%). For Reader 1, the average age of participants where EF% could not be measured was 14.0±2.7 years compared to 12.7±2.6 years for studies with measured EF% (p<0.08). There were 6 participants that could not transfer out of wheelchair and Reader 1 was unable to measure the EF% in 4 of these participants.
Reproducibility
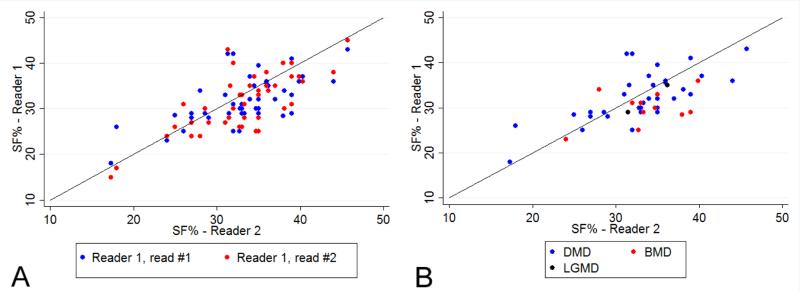
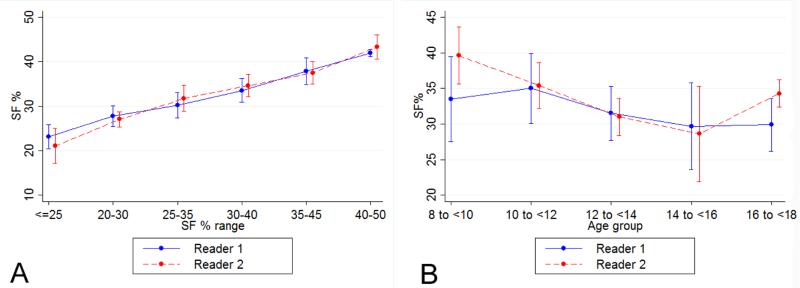
The means, ranges and inter-observer ICCs for all echo measures from Readers 1 and 2 are presented in Table 2. Using SF%, 40 (85%) participants showed normal systolic function and 7 (15%) participants had decreased systolic function. M-mode measures of LVIDd and LVIDs used for SF% had two of the three highest ICC levels for all conventional echo measures. While there was no single case in which EF% was normal and SF% was abnormal, there were six cases with decreased EF% values and normal SF% values. SF% measurements also showed high agreement between repeated measurements by the same reader (intra-observer ICC=0.73). We did not observe any pattern of differences in SF% values between two readers (Figure 1A) or by diagnosis (Figure 1B). The variability of SF% remained consistent over the range of observed magnitudes (Figure 2A) and over the age range of participants in this study (Figure 2B), although the two readers showed some differences in range for the youngest age group.
Table 2.
Echo measures for Reader 1 and 2 and inter-observer correlations between the two readers
| Cardiac measure | Reader 1 | Reader 2 | Reader 1 and 2 | ||||
|---|---|---|---|---|---|---|---|
| Conventional | N | Mean ± SD | Range | N | Mean ± SD | Range | Inter-observer ICC (95% CI)* |
| LVIDd (mm) | 47 | 4.5 ± 0.6 | 3.3 – 5.9 | 47 | 4.4 ± 0.6 | 3.2 – 5.8 | 0.88 (0.80 – 0.93) |
| LVIDs (mm) | 47 | 3.0 ± 0.6 | 2.2 – 4.9 | 47 | 3.0 ± 0.6 | 2.1 – 4.6 | 0.89 (0.82 – 0.94) |
| SF% | 47 | 33 ± 6 | 17 – 46 | 47 | 32 ± 5 | 18 – 43 | 0.63 (0.42 – 0.77) |
| EF% | 25 | 61 ± 8 | 44 – 79 | 43 | 54 ± 8 | 24 – 64 | 0.49 (0.19 – 0.70) |
| WS (g/cm2) | 46 | 56 ± 16 | 20 – 112 | 47 | 61 ± 19 | 27 – 116 | 0.38 (0.12 – 0.59) |
| VCF (circ/s) | 46 | 1.3 ± 0.3 | 0.7 – 2.1 | 46 | 1.2 ± 0.3 | 0.6 – 1.8 | 0.31 (0.05 – 0.54) |
| VCFc (circ/s) | 46 | 1.1 ± 0.2 | 0.6 – 1.5 | 46 | 1.0 ± 0.2 | 0.5 – 1.4 | 0.35 (0.07 – 0.58) |
| PWD | |||||||
| ET (ms) | 47 | 256 ± 26 | 200 – 310 | 46 | 250 ± 20 | 210 – 290 | 0.71 (0.50 – 0.83) |
| IVRT (ms) | 47 | 51 ± 11 | 30 – 80 | 46 | 53 ± 10 | 33 – 77 | 0.43 (0.16 – 0.63) |
| IVCT (ms) | 47 | 49 ± 10 | 30 – 80 | 46 | 49 ± 11 | 30 – 75 | 0.41 (0.14 – 0.62) |
| MPI | 46 | 0.39 ± 0.09 | 0.26 – 0.67 | 46 | 0.41 ± 0.08 | 0.28 – 0.60 | 0.55 (0.27 – 0.73) |
| MV E wave (cm/s) | 46 | 86 ± 15 | 52 – 124 | 46 | 91 ± 14 | 63 – 123 | 0.82 (0.53 – 0.92) |
| MV A wave (cm/s) | 46 | 49 ± 10 | 27 – 78 | 46 | 54 ± 10 | 36 – 75 | 0.63 (0.17 – 0.82) |
| TDI | |||||||
| ET (ms) | 46 | 266 ± 25 | 210 – 330 | 46 | 266 ± 22 | 210 – 313 | 0.92 (0.85 – 0.95) |
| IVRT (ms) | 46 | 49 ± 9 | 30 – 70 | 46 | 47 ± 12 | 30 – 70 | 0.61 (0.40 – 0.76) |
| IVCT (ms) | 46 | 52 ± 11 | 30 – 80 | 46 | 48 ± 11 | 20 – 73 | 0.57 (0.30 – 0.74) |
| MPI | 46 | 0.39 ± 0.09 | 0.26 – 0.63 | 46 | 0.36 ± 0.09 | 0.23 – 0.60 | 0.54 (0.28 – 0.83) |
| Septal LV peak E’ Vel (cm/s) | 46 | 12 ± 3 | 3 – 17 | 46 | 14 ± 3 | 7 – 19 | 0.61 (0.02 – 0.83) |
| Lat LV peak E’ Vel (cm/s) | 46 | 15 ± 5 | 0.9 – 32 | 46 | 18 ± 5 | 7 – 34 | 0.72 (0.12 – 0.89) |
| STE | |||||||
| LS (%) | 30 | −14 ± 4 | −21 ± 7 | 30 | −15 ± 4 | −23 ± 7 | 0.90 (0.77 – 0.95) |
| CS (%) | 28 | −20 ± 5 | −33 ± 11 | 28 | −20 ± 5 | −31 ± 12 | 0.96 (0.92 – 0.98) |
| LSR (1/s) | 30 | −1.1 ± 0.4 | −2.4 ± 0.5 | 30 | −1.3 ± 0.5 | −2.7 ± 0.5 | 0.64 (0.35 – 0.82) |
| CSR (1/s) | 28 | −1.9 ± 0.5 | −2.7 ± 0.9 | 28 | −2.1 ± 0.7 | −3.4 ± 0.8 | 0.78 (0.47 – 0.91) |
ICC (Interclass correlation) uses lesser value in cases of discrepant N values.
SD – standard deviation, CI – confidence interval, LVIDd – left ventricular internal diameter in diastole, LVIDs – left ventricular internal diameter in systole, SF% - percent shortening fraction, EF% - percent ejection fraction, WS – wall stress, ET – ejection time, IVRT – isovolumic relaxation time, PWD – pulse wave Doppler, TDI – tissue Doppler imaging, IVCT – isovolumic contraction time, VCF – velocity of circumferential fiber shortening, VCFc – velocity of circumferential fiber shortening corrected for heart rate, MPI – myocardial performance index, LV – left ventricle, Vel – velocity, Lat – lateral, MV – mitral valve, E- mitral peak early filling wave, A- mitral late diastolic filling wave, E’- mitral early diastolic annular velocity, STE – Speckle tracking echo, LS – average longitudinal strain, CS – average circumferential strain, LSR – average longitudinal strain rate, CSR – average circumferential strain rate.
Figure 1.
Agreement of percent shortening fraction (SF%) measures within and between readers (Panel A: shows agreement within the same reader (blue is measure #1 and red is measure #2) and Panel B shows agreement between readers separated by diagnosis (blue = DMD, red = BMD, black = LGMD); for both panels, the closer the values are to the identity line, the smaller the discrepancy between the two reads.
Figure 2.
Assessment of variability in percent shortening fraction (SF%) measurements across SF% magnitudes (Panel A; a rolling average with standard deviation (SD) bars across the range of observed SF% values) and across different patient ages (Panel B; average with SD bars shown by 2 year intervals) with reader#1 in blue and reader #2 in red.
Early subclinical myocardial involvement
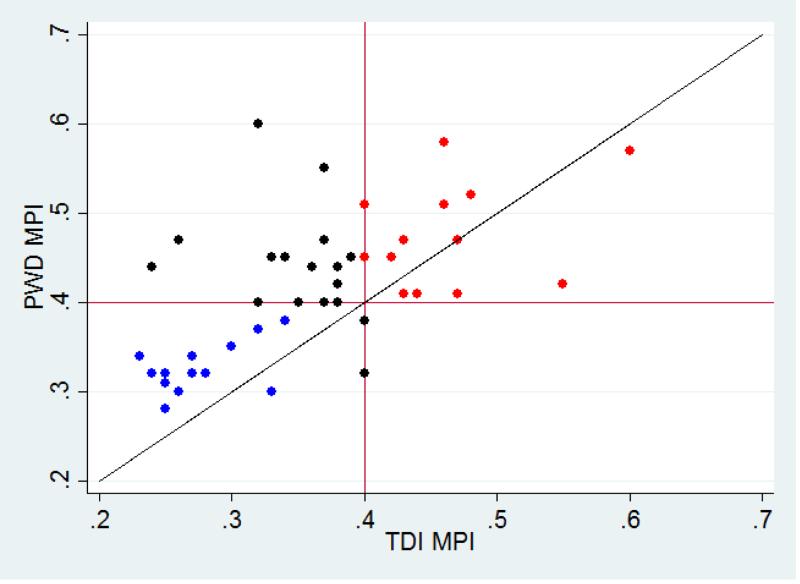
Both TDI and PWD methods were used to determine MPI as a measure of subclinical myocardial disease. Between the two readers, the MPI measured via PWD had a similar ICC compared to the MPI measured via TDI (Table 2). Looking at specific components of the MPI measure (IVCT, IVRT and ET), the PWD MPI measures consistently had lower correlations compared to the TDI MPI measures and the ICC for ET measured in TDI tracings showed the highest correlation of all measures. In our MD cohort, PWD and TDI derived MPI values only demonstrated agreement in 29/46 (63%) of the studies when using MPI=0.40 as a cutoff between normal and abnormal (Figure 3). In cases where SF% ≥28, PWD MPI was ≥0.40 in 23/38 (61%) and TDI MPI was ≥0.40 in only 14/38 (37%). Thus, TDI MPI showed a lower rate of defining subclinical disease compared to PWD MPI when the SF% was normal. To better understand the differences between PWD and TDI measures of MPI, we performed a sub analysis on the 15 cases in the upper left quadrant of Figure 3 with TDI MPI <0.40 and PWD MPI ≥0.40. In these cases, TDI measured significantly longer ETs (267±19 via TDI vs. 239±17 via PWD; p<0.001) and shorter IVRT's (48±8 via TDI vs. 52±9 via PWD; p<0.001), leading to a lower calculated TDI MPI. There were no significant differences in IVCT measurements. For ET and IVRT measures, a higher ICC was observed for each using TDI compared to PWD (see Table 2).
Figure 3.
Agreement between MPI via PWD and TDI. Blue dots represent participants deemed normal by both PWD and TDI; Red dots represent participants deemed abnormal by both PWD and TDI; Black dots represent participants where PWD and TDI MPI disagreed.
Speckle tracking echocardiography (STE) was a second technique used to determine subclinical myocardial disease. Only DMD participants were included in this analysis due to the often earlier onset of cardiomyopathy in DMD compared to BMD and LGMD. In this subset of 33 DMD participants (2 participant echos did not upload into Syngo), satisfactory longitudinal tracking was obtained in 30 (91%) participants and satisfactory circumferential tracking was obtained in 28 (85%) participants. Mean frame rate for the apical four chamber view in DMD echos was 64±13 frames/sec (fps) and 53±12 fps in controls. Mean frame rate for parasternal short axis view in DMD echos was 72±18 fps and 56±10 fps in controls. Comparisons of conventional and STE data between DMD cases and controls are shown in Table 3. While both groups demonstrated mean SF% and EF% within the normal ranges, controls were significantly higher as would be expected (p<0.001). Participants with DMD had significantly decreased E/A ratio compared to controls (p<0.001). Average LS, LSR, CS and CSR were significantly decreased (less negative) in participants with DMD compared to controls (p<0.001). Other measurements, in particular the TDI MPI, were not significantly different between the DMD group and controls. Conventional echocardiography measurements demonstrated normal cardiac systolic function (SF%≥28) in 26 of the 33 (79%) participants with DMD. This subgroup with SF%≥28 showed significantly decreased average LS, CS and CSR as compared to controls (Table 4). Seventeen of the 33 (52%) participants with DMD were younger than 13 years of age (range 7.7 to 12.8 years). These younger participants also showed decreased average LS, CS and CSR as compared to controls (17 of 33; 52%) in the same age group (Table 4). Twenty-two of the 33 (67%) participants had a TDI MPI <0.40 and also demonstrated significantly decreased average LS, CS and CSR compared to controls (Table 4). Average LS and CS demonstrated high inter-observer correlations with ICCs of 0.94 and 0.96, respectively.
Table 3.
Comparison of conventional and speckle tracking echocardiography data in participants with DMD and controls.
| Measure | DMD (n = 33, unless noted) Mean (SD) | Controls (n = 33) Mean (SD) | P value |
|---|---|---|---|
| SF% | 33 (5) | 38 (4) | <0.001 |
| EF% | 58 (6) | 63 (5) | 0.001 |
| MPI (TDI) | .36 (.07) | .35 (.06) | 0.49 |
| MV E wave velocity (cm/s) | 94 (13) | 100 (14) | 0.15 |
| MV A wave velocity (cm/s) | 58 (12) | 48 (14) | 0.005 |
| MV E/A ratio | 1.7 (.3) | 2.2 (.6) | <0.001 |
| Septal E’ velocity (cm/s) | 14 (3) | 14 (3) | 0.67 |
| Lateral E’ velocity (cm/s) | 17 (5) | 20 (3) | 0.014 |
| Septal E/E’ ratio | 7 (2) | 7 (2) | 0.79 |
| Lateral E/E’ ratio | 6 (2) | 5 (1) | 0.09 |
| Average LV Longitudinal Strain (%) | −14.1 (3.5) (n=30) | −17.7 (3.4) | <0.001 |
| Average LV Circumferential Strain (%) | −19.8 (4.8) (n=27) | −25.7 (3.5) | <0.001 |
| Average LV Longitudinal SR (1/s) | −1.1 (.4) (n=30) | −1.3 (.3) | 0.033 |
| Average LV Circumferential SR (1/s) | −1.8 (.6) (n=27) | −2.4 (.5) | <0.001 |
Abbreviations: DMD – Duchenne muscular dystrophy, SD – standard deviation, SF% - percent shortening fraction, EF% - percent ejection fraction, MPI – myocardial performance index, TDI – tissue Doppler imaging, MV – mitral valve, E- mitral peak early filling wave, A- mitral late diastolic filling wave, E’- mitral early diastolic annular velocity, LV – left ventricle, SR – strain rate
Table 4.
Comparison of speckle tracking-derived deformation parameters between controls and three subgroups of participants with DMD: 1) those with SF%>28 2) those younger than 13 years and 3) those with TDI MPI <0.40.
| Group | SF%>28 | Age<13 years | MPI<0.40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | DMD, (n=26) | Controls (n=28) | P value | DMD, (n=17) | Controls (n=17) | P value | DMD, (n=22) | Controls (n=25) | P value |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| LV Shortening Fraction (%) | 34 (4) | 38 (4) | <.001 | 34 (4) | 38 (4) | .015 | 32 (5) | 38 (4) | <.001 |
| Average LV Longitudinal Strain (%) | −14.3 (3.6) | −17.8 (3.0) | <.001 | −15.3 (3.6) | −17.6 (2.3) | .033 | −14.5 (3.4) | −17.4 (3.0) | .003 |
| Average LV Circumferential Strain (%) | −20.2 (4.6) | −25.5 (3.5) | <.001 | −19.3 (3.9) | −25.9 (3.5) | <.001 | −20.7 (5.1) | −25.7 (3.2) | <.001 |
| Average LV Longitudinal Strain Rate (1/s) | −1.2 (.4) | −1.3 (.3) | .16 | −1.2 (.3) | −1.3 (.2) | .07 | −1.1 (.3) | −1.3 (.3) | .045 |
| Average LV Circumferential Strain Rate (1/s) | −1.9 (.5) | −2.4 (.5) | .001 | −1.9 (.5) | −2.3 (.5) | .013 | −1.9 (.5) | −2.4 (.5) | .004 |
Abbreviations: DMD – Duchenne muscular dystrophy, SF% - percent shortening fraction, TDI – tissue Doppler imaging, MPI – myocardial performance index, SD – standard deviation, LV - left ventricle, NS - not significant, SD - standard deviation
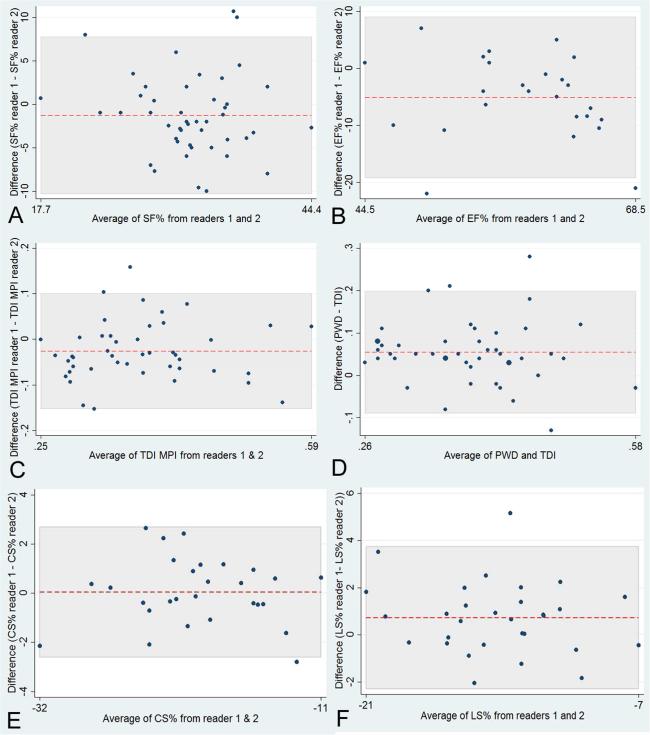
Bland Altman analysis was performed on SF%, EF%, MPI and STE (Figure 4). Overall, these plots demonstrated no remarkable differences in fixed or proportional biases between the two readers for the analyzed measures.
Figure 4.
Bland-Altman plots demonstrate variability of inter-observer measures for: percent shortening fraction (SF%, Panel A); percent ejection fraction (EF%, Panel B); myocardial performance index (MPI, inter-observer differences in Panel C and inter-method (PWD vs. TDI) in Panel D); average circumferential (CS%, Panel E) and longitudinal strain (LS%, Panel F). The red dashed line represents the mean bias and the shaded area indicates the area between the limits of agreement.
Discussion
As clinical therapies for skeletal and respiratory dysfunction in MD patients improve, cardiac disease is becoming an increasingly important factor in morbidity and mortality. The investigation of innovative therapies that may have their greatest benefit if implemented early in the disease course will depend on cardiac measures that can be compared across sites and studies. The purpose of this study was two-fold: 1) to determine the feasibility and reproducibility of echocardiographic functional cardiac measures using independent central readers and a central sonographer who trained site sonographers; 2) identify echocardiographic measures of early subclinical changes in myocardial function.
Systolic function is a commonly used measure clinically and in research studies; however, methods of measurement can vary greatly. In this study, we showed that SF% is the preferred measure in our MD cohort. SF% could be reproducibly measured by two independent readers. Using M-mode imaging, the measures of LVIDs and LVIDd had two of the three highest correlation values (ICC=0.88 and 0.89 respectively). The ICC for SF% was greater than EF% (0.63 vs. 0.49). SF% measures also did not significantly vary with age or magnitude of measure. SF% was also more feasible in the study population. One reader could only confidently trace the endocardial border for calculation of EF% in 53% of participants, which reflected difficulties in performing 2D imaging in the apical 4 chamber position in patients with MD due to body positioning and lung interference. This difficulty was also identified in a previous study by Markham et al.[6] Our data provides strong support for the use of SF% as the best measure of cardiac systolic function in MD. In contrast, EF% was difficult to obtain in many participants, especially older ones with more advanced disease who were unable to transfer from chairs, and was less reproducible across readers. A single plane EF% measurement was also chosen to improve the likelihood of obtaining sufficient images; however, this has known limitations and did not improve the measures.[30, 31]
We also looked for early subclinical markers of myocardial dysfunction since most young children with MD have normal systolic function. A decrease in diastolic function often predates the decline in systolic function and may provide a useful early marker of cardiac disease in children.[32] We found significantly increased mitral A wave velocities and decreased mitral TDI lateral peak E wave velocities in participants with DMD compared to controls. Shabanian et al. also found increased mitral A wave velocities, decreased mitral E wave velocities, and decreased TDI lateral peak E wave velocities in patients with DMD.[22] Furthermore, we observed a good correlation between readers for PWD mitral E wave measures (ICC=0.82), PWD mitral A wave velocities (ICC=0.63) and TDI lateral LV peak E’ velocities (ICC=0.72). These results demonstrate that conventional measures of diastolic function are easy to obtain and can detect early changes in myocardial function during longitudinal studies in young children with DMD.
Another echo measure designed to identify early myocardial disease is the MPI. MPI incorporates measures of systolic function (IVCT and ET) and diastolic function (IVRT) and is independent of heart rate, ventricular geometry and preload/ afterload.[33] Previous studies showed that MPI was increased in participants with MD who demonstrated normal cardiac function by systolic measures.[11, 22, 34, 35] We therefore looked at the utility of MPI as an early marker of cardiac dysfunction in our DMD cohort. PWD MPI detected subclinical disease in more subjects than TDI. However, when comparing the measurements of MPI between PWD and TDI, it is clear that TDI measurement components have higher correlations between readers. Based on our ICC measures, the TDI MPI is more reproducible between readers and is the preferred method. Also, Bland Altman analysis of PWD versus TDI MPI measures shows overall PWD measures are approximately 0.05 units greater than TDI measures (Figure 4, Panel D). Shabanian et al. also studied MPI measures in participants with DMD using both conventional PWD and TDI and found higher MPI values using PWD compared to TDI. [22] Thus, it is worth considering using a lower threshold for TDI MPI than for PWD MPI to diagnose subclinical cardiac disease in patients with DMD.
Myocardial strain has emerged as a promising echocardiographic measure of subclinical myocardial function. Myocardial strain assesses the change in myocardial fiber length as compared to its original length in the plane in which it is measured.[36] Unlike EF% and SF%, which are limited to one plane, strain can be measured in three planes, providing a more in-depth determination of cardiac function. Although strain imaging can be measured using TDI or STE, STE is superior since it is independent of angle and only measures active contraction, avoiding the tethering effect of non-contractile tissue. In recent years, several studies have supported the utility of STE to measure cardiac strain in clinical practice with excellent reproducibility.[37-40]
We analyzed myocardial strain measured by STE in a subset of participants with DMD since this diagnosis is usually associated with earlier onset of cardiomyopathy. We found that average LS and CS measurements were significantly decreased in participants with DMD compared to normal controls. STE also had very high inter-observer ICC values; this correlation may have been augmented by measuring the exact same cardiac beat by the two independent observers. When limiting the analyses to participants with normal systolic function, those less than 13 years of age or those with a normal TDI MPI, each time STE demonstrated significantly decreased average LS and CS values. Therefore, the measurement of strain by STE detected loss of myocardial function while other conventional echo measures remained normal, permitting the study of cardiac involvement in MD prior to the development of systolic dysfunction.
Our findings are consistent with other recent studies of cardiac strain in patients with DMD. Ryan et al. (2013) compared DMD participants less than 8 years old with aged matched normal controls and found that despite normal SF% values, average CS at the mid-chamber level was significantly decreased in the DMD participants (−19.8±4.2% vs. −21.7±3.8%, p<0.01). Inter-observer and intra-observer ICC were 0.88 and 0.91 respectively.[24] Our current study confirmed these findings by demonstrating an average CS of -19.3±4% in participants with DMD younger than 13 years of age. These results demonstrate the usefulness of strain measures to detect subclinical disease in the DMD population.
This study has some limitations that reflect constraints of current echocardiography practice and the disease process. Echos were obtained at different centers with varying image quality. Six patients were unable to transfer to a supine position for echo imaging and remained in their power chairs for the study, contributing to poor acoustic windows often seen in MD. In apical 4 chamber imaging, it could be difficult to accurately trace endocardial borders for EF. Some imaging had relatively low frame rates which decreased resolution even further. A primary goal of this study was to assess the effects of these limitations as they impact therapeutic research protocols to discover treatment options for cardiac involvement in MD. The study can, therefore, aid in identifying which echo measures are most robust for clinical research.
In conclusion, this study supports the utility of SF% as the most feasible and reproducible conventional echo measure of systolic function in MD. Diastolic measures of mitral A velocity, lower E/A ratio, lower mitral lateral E' velocity TDI and MPI values provided assessment of preclinical myocardial disease in patients with MD. STE derived myocardial strain detected the presence of subclinical myocardial dysfunction in DMD participants with normal systolic function on conventional echocardiography. Lower average strain values were also seen in subgroups of younger participants and those with normal TDI MPI. Use of these feasible and reproducible cardiac measures for future clinical trials in muscular dystrophy will facilitate the assessment of potential therapeutic agents.
Acknowledgements
University of Pittsburgh: This study was supported by an administrative supplement (3UL1RR024153-04S5) to the Clinical Translational Science Award held by the University of Pittsburgh (5ULRR024153-03). We acknowledge the support provided by the Director of the University of Pittsburgh Clinical and Translational Science Institute, Steven Reis, MD, and his staff. The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government.
Texas Children's Hospital: The BCM GCRC grant was funded by: National Institutes of Health, M01-RR00188, General Clinical Research Center.
Washington University: This publication was also made possible by Grant No. UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
University of California at Davis: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Children's National Health System: This publication was supported by Award Number UL1TR000075 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spurney CF. Cardiac Complications in Neuromuscular Disorders. In: Bertorini TE, editor. Neuromuscular Disorders: Treatment and Management. Elsevier; Philadelphia, PA: 2011. pp. 33–50. [Google Scholar]
- 2.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–7. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 3.Spurney C, Shimizu R, Morgenroth LP, Kolski H, Gordish-Dressman H, Clemens PR. Cooperative international neuromuscular research group duchenne natural history study demonstrates insufficient diagnosis and treatment of cardiomyopathy in duchenne muscular dystrophy. Muscle Nerve. 2014;50:250–6. doi: 10.1002/mus.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, et al. Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J. 2006;70:991–4. doi: 10.1253/circj.70.991. [DOI] [PubMed] [Google Scholar]
- 5.Kirchmann C, Kececioglu D, Korinthenberg R, Dittrich S. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatr Cardiol. 2005;26:66–72. doi: 10.1007/s00246-004-0689-2. [DOI] [PubMed] [Google Scholar]
- 6.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–70. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Markham LW, Spicer RL, Khoury PR, Wong BL, Mathews KD, Cripe LH. Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatr Cardiol. 2005;26:768–71. doi: 10.1007/s00246-005-0909-4. [DOI] [PubMed] [Google Scholar]
- 8.Ramaciotti C, Heistein LC, Coursey M, Lemler MS, Eapen RS, Iannaccone ST, et al. Left ventricular function and response to enalapril in patients with duchenne muscular dystrophy during the second decade of life. Am J Cardiol. 2006;98:825–7. doi: 10.1016/j.amjcard.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Thomas TO, Morgan TM, Burnette WB, Markham LW. Correlation of heart rate and cardiac dysfunction in Duchenne muscular dystrophy. Pediatr Cardiol. 2012;33:1175–9. doi: 10.1007/s00246-012-0281-0. [DOI] [PubMed] [Google Scholar]
- 10.Gulati S, Saxena A, Kumar V, Kalra V. Duchenne muscular dystrophy: prevalence and patterns of cardiac involvement. Indian J Pediatr. 2005;72:389–93. doi: 10.1007/BF02731732. [DOI] [PubMed] [Google Scholar]
- 11.Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation. 2005;112:2799–804. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 12.Kwon SW, Kang SW, Kim JY, Choi EY, Yoon YW, Park YM, et al. Outcomes of cardiac involvement in patients with late-stage Duchenne muscular dystrophy under management in the pulmonary rehabilitation center of a tertiary referral hospital. Cardiology. 2012;121:186–93. doi: 10.1159/000336810. [DOI] [PubMed] [Google Scholar]
- 13.Ogata H, Ishikawa Y, Minami R. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol. 2009;53:72–8. doi: 10.1016/j.jjcc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Viollet L, Thrush PT, Flanigan KM, Mendell JR, Allen HD. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol. 2012;110:98–102. doi: 10.1016/j.amjcard.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Houde S, Filiatrault M, Fournier A, Dube J, D'Arcy S, Berube D, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol. 2008;38:200–6. doi: 10.1016/j.pediatrneurol.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar RW, Allen HD, Ray WC, Alvarez CE, Kissel JT, Pestronk A, et al. Analysis of dystrophin deletion mutations predicts age of cardiomyopathy onset in becker muscular dystrophy. Circ Cardiovasc Genet. 2009;2:544–51. doi: 10.1161/CIRCGENETICS.109.867242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am J Cardiol. 2003;91:769–72. doi: 10.1016/s0002-9149(02)03429-x. [DOI] [PubMed] [Google Scholar]
- 18.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–7. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 19.Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years' follow-up. Am Heart J. 2007;154:596–602. doi: 10.1016/j.ahj.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes J, Margossian R, Darras BT, Colan SD, Jenkins KJ, Geva T, et al. Safety and efficacy of carvedilol therapy for patients with dilated cardiomyopathy secondary to muscular dystrophy. Pediatr Cardiol. 2008;29:343–51. doi: 10.1007/s00246-007-9113-z. [DOI] [PubMed] [Google Scholar]
- 21.van Bockel EA, Lind JS, Zijlstra JG, Wijkstra PJ, Meijer PM, van den Berg MP, et al. Cardiac assessment of patients with late stage Duchenne muscular dystrophy. Neth Heart J. 2009;17:232–7. doi: 10.1007/BF03086253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shabanian R, Aboozari M, Kiani A, Seifirad S, Zamani G, Nahalimoghaddam A, et al. Myocardial performance index and atrial ejection force in patients with Duchenne's muscular dystrophy. Echocardiography. 2011;28:1088–94. doi: 10.1111/j.1540-8175.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- 23.Kwon HW, Kwon BS, Kim GB, Chae JH, Park JD, Bae EJ, et al. The effect of enalapril and carvedilol on left ventricular dysfunction in middle childhood and adolescent patients with muscular dystrophy. Korean Circ J. 2012;42:184–91. doi: 10.4070/kcj.2012.42.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan TD, Taylor MD, Mazur W, Cripe LH, Pratt J, King EC, et al. Abnormal Circumferential Strain is Present in Young Duchenne Muscular Dystrophy Patients. Pediatr Cardiol. 2013 doi: 10.1007/s00246-012-0622-z. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto T, Tanaka H, Matsumoto K, Lee T, Awano H, Yagi M, et al. Utility of transmural myocardial strain profile for prediction of early left ventricular dysfunction in patients with duchenne muscular dystrophy. Am J Cardiol. 2013;111:902–7. doi: 10.1016/j.amjcard.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Mayhew JE, Florence JM, Mayhew TP, Henricson EK, Leshner RT, McCarter RJ, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve. 2007;35:36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 27.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–95. doi: 10.1016/j.echo.2010.03.019. quiz 576-7. [DOI] [PubMed] [Google Scholar]
- 28.Eidem BW, Tei C, O'Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr. 1998;11:849–56. doi: 10.1016/s0894-7317(98)70004-5. [DOI] [PubMed] [Google Scholar]
- 29.Eidem BW, Sapp BG, Suarez CR, Cetta F. Usefulness of the myocardial performance index for early detection of anthracycline-induced cardiotoxicity in children. Am J Cardiol. 2001;87:1120–2. A9. doi: 10.1016/s0002-9149(01)01476-x. [DOI] [PubMed] [Google Scholar]
- 30.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 31.St John Sutton M, Otterstat JE, Plappert T, Parker A, Sekarski D, Keane MG, et al. Quantitation of left ventricular volumes and ejection fraction in post-infarction patients from biplane and single plane two-dimensional echocardiograms. A prospective longitudinal study of 371 patients. Eur Heart J. 1998;19:808–16. doi: 10.1053/euhj.1997.0852. [DOI] [PubMed] [Google Scholar]
- 32.Frommelt PC. Echocardiographic measures of diastolic function in pediatric heart disease. Curr Opin Cardiol. 2006;21:194–9. doi: 10.1097/01.hco.0000221580.63996.93. [DOI] [PubMed] [Google Scholar]
- 33.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 34.Gurrala RR, Alla VM, Aronow WS, Shankar JS, Angamutta MK, Lanka K, Sr., et al. Occult left ventricular dysfunction diagnosed by myocardial performance index in patients with limb girdle muscle dystrophy: A case control study. Int J Angiol. 2007;16:139–42. doi: 10.1055/s-0031-1278268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, et al. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr. 2008;21:1049–54. doi: 10.1016/j.echo.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401–13. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Frank LH, Fenton KE, Sable CA, Levy RJ, Berger JT. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med. 2012;13:259–64. doi: 10.1097/PCC.0b013e3182288445. [DOI] [PubMed] [Google Scholar]
- 38.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, et al. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiogr. 2012;25:401–10. e1. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Blanc J, Stos B, de Montalembert M, Bonnet D, Boudjemline Y. Right ventricular systolic strain is altered in children with sickle cell disease. J Am Soc Echocardiogr. 2012;25:511–7. doi: 10.1016/j.echo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Cheung YF, Hong WJ, Chan GC, Wong SJ, Ha SY. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96:1137–41. doi: 10.1136/hrt.2010.194118. [DOI] [PubMed] [Google Scholar]