Abstract
Background
1.5 million children under 12 months of age are exposed to general anesthesia annually in the United States alone. Human and especially animal studies provide evidence that exposure to general anesthesia during the early postnatal period may lead to long-term neurocognitive abnormalities via poorly understood mechanisms. We investigated whether an immature stress response system and γ-aminobutyric acid (GABA) type A receptor activities are involved in mediating these abnormalities.
Methods
Sprague-Dawley rats at postnatal days 4, 5 or 6 were anesthetized with 2.1% sevoflurane for 6 hrs; maternally separated and house reared rats served as controls.
Results
Sevoflurane anesthesia markedly increased corticosterone levels in rat pups of both genders. In adulthood, these rats responded to stress with heightened secretion of corticosterone and a greater increase in corticosterone levels in males versus females. Only male rats, previously exposed to neonatal sevoflurane, had a higher frequency of miniature inhibitory postsynaptic currents in CA1 neurons, spent a shorter time in open arms of the elevated plus maze (EPM) and exhibited impaired prepulse inhibition (PPI) of startle. Pretreatment of male rats prior to sevoflurane with the Na+-K+-2Cl− cotransporter inhibitor, bumetanide, or the mineralocorticoid receptor antagonist, RU28318, normalized endocrine responses to stress and the EPM behavior in adulthood, while only those pretreated with bumetanide exhibited normalized PPI of startle responses. Neither bumetanide nor RU28318 altered the effect of sevoflurane on synaptic activity.
Conclusions
Sevoflurane-enhanced neuronal excitation and elevated corticosteroid levels at the time of anesthesia contribute to the mechanisms initiating neonatal sevoflurane-induced long-term endocrine and neurobehavioral abnormalities.
Keywords: Sevoflurane, Corticosterone, Neonatal, Brain, Endocrine, GABA
1. Introduction
More than 1 in 4 children are exposed to general anesthesia in their first year of life. Long-term developmental effects of general anesthesia, traditionally considered safe and reversible upon anesthetic withdrawal, are increasingly recognized as a potentially important health concern (Servick, 2014). Data from numerous laboratories demonstrate that animals who were anesthetized during their neurodevelopmental window of vulnerability to anesthetic agents, which in rodents covers approximately the first two postnatal weeks, exhibit not only acute abnormalities, but also develop neurobehavioral alterations, which become more prominent in adulthood (Stratmann, 2011). Studies of children, who had medical procedures during the first years of life that required general anesthesia, also report cognitive and neurological deficiencies (Sanders et al., 2013). The underlying mechanisms of developmental effects of general anesthetics and the spectrum of neonatal anesthesia-induced abnormalities are poorly understood. Because the main molecular targets involved in mediating general anesthesia are in the central nervous system (Franks, 2008), it is not surprising that studies of the developmental effects of neonatal anesthesia have been focused on neural effects of anesthetics (Stratmann, 2011; Sanders et al., 2013).
We recently reported that in neonatal rats the adverse developmental effects of sevoflurane, the general anesthetic of choice in pediatric anesthesia, whose anesthetic actions include enhancement of γ-aminobutyric acid (GABA) type A receptor (GABAAR) activity, were associated with paradoxical hyperexcitatory electroencephalographic patterns and a prominent increase in serum levels of the mineralocorticosteroid hormone, aldosterone, at the time of anesthesia (Edwards et al., 2010; Cao et al., 2012; Seubert et al., 2013). Secretion of aldosterone by the adrenal cortex is mainly regulated by the renin-angiotensin system. However, this mineralocorticoid is also secreted together with the main stress hormone, corticosterone, in response to activation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis by stressful stimuli. Furthermore, aldosterone’s mineralocorticoid receptors are the primary mediators of corticosterone action in the brain (Ulrich-Lai and Herman, 2009; Kubzansky and Adler, 2010).
The known properties of the corticosteroid-based stress response system and GABAergic signaling during development support the plausibility of their involvement in mediating the adverse effects of neonatal anesthesia. Both systems play important regulatory roles during the early postnatal period and both undergo critical transformations in their developmental effects during the age period that coincides with the brain transformation from being highly susceptible to relatively resistant to the developmental effects of general anesthetics. The first two postnatal weeks in rodents are characterized by high vulnerability to both general anesthetics and to excess glucocorticosteroids. After the first two weeks of life, this susceptibility dramatically diminishes (Brunson et al., 2005; Joëls and Baram, 2009; Oomen et al., 2010; Li et al., 2013). Stressful conditions during early life, such as prolonged and repeated maternal separation result in profound behavioral abnormalities and exacerbated endocrine responses to stress later in life (Mitev et al., 2003). Adult male rodents may exhibit greater abnormalities induced by maternal separation (Patchev et al., 1997; Mitev et al., 2003).
The ontogenetic transition in GABA action from depolarizing/stimulatory to inhibitory occurs during the second postnatal week in rat cortex and hippocampus (Khazipov et al., 2004; Glykys et al., 2009) with an earlier shift in females in comparison to their male counterparts (Galanopoulou, 2008). The GABAAR-mediated depolarization may be sufficient to initiate calcium influxes through activation of N-methyl-D-aspartate receptors and voltage-operated Ca++ channels leading to activation of signaling pathways that control many developmental processes (Khazipov et al., 2004; Glykys et al., 2009; Tyzio et al., 2014). Excessive GABAAR-mediated depolarization is linked to developmental disorders, such as autism spectrum disorders (Tyzio et al., 2014).
GABAAR-mediated inhibition of the LHPA axis activity, enhanced by neurosteroids (such as allopregnanolone, which is secreted in response to activation of the LHPA axis by stress), is an important mechanism of adaption to stress (Mody and Maguire, 2012; Crowley et al., 2014). Because in immature neurons GABAAR-mediated signaling can cause activation (Gao et al., 2001; Li et al., 2013), there is a possibility that the GABA-based mechanism of adaptation to stress in mature brain may lead to opposite, stress-exacerbating effects in neonatal brain. Consequently, agents that enhance GABAAR activity, such as general anesthetics, may imitate the effects of stress in neonates.
In this study, we tested whether a single exposure of neonatal rats to sevoflurane would induce developmental abnormalities similar to those associated with the known developmental effects of stress from prolonged and repeated maternal separation, e.g., abnormalities not only in the brain, but also in the endocrine system, and whether such abnormalities could be alleviated by pretreatment with the NKCC1 inhibitor, bumetanide, and/or the mineralocorticoid, receptor antagonist, RU28318.
2. Methods and Materials
2.1. Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee (Gainesville, FL). Sprague-Dawley rats were studied. Animals were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (23–24 °C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched groups of two for the rest of the study. To control for litter variability, we used several pups from different litters for each treatment condition. Multiple sets of animals were used in the experiments. The data reported in this study was collected from 116 rats.
2.2. Treatment groups
Five experimental/treatment groups were studied. P4, P5, or P6 rat pups of both genders kept in a temperature-controlled chamber (+37 ºC) with a continuous supply of oxygen (1.5 L/min) during anesthesia – 6% sevoflurane for 3 min for anesthesia induction and then 2.1% sevoflurane for 357 min for anesthesia maintenance (group 1). The control animals were separated from the dams for 6 hrs in a temperature-controlled chamber with a continuous supply of oxygen without exposure to sevoflurane (group 2). Gas monitoring was performed using a calibrated Datex side stream analyzer (Datex-Ohmeda, Helsinki, Finland), which sampled from the interior of the animal chamber. According to Orliaguet et al. (2001) 2.1% sevoflurane lies near 0.6 minimum alveolar concentration for P4–P6 rats. At the dose of 2.1% sevoflurane the pups did not exhibit a righting reflex. Previously we have shown that blood glucose and gas levels after anesthesia with 2.1% sevoflurane were in the normal range, while higher doses of sevoflurane (e.g. 2.9%) may cause respiratory depression in spontaneously breathing rats (Edwards et al., 2010). In order to control for maternal separation an additional cohort of animals of both genders were subjected to animal facility rearing only (group 3).
To study the role of GABAAR-mediated depolarization/activation and corticosterone at the time of anesthesia on the long-term developmental effects of sevoflurane, a subgroup of P4, P5 or P6 male rats received a single injection of the NKCC1 inhibitor, bumetanide (1.82 mg/kg, intraperitoneally) (group 4), or the mineralocorticoid receptor antagonist RU28318 (10 mg/kg, intraperitoneally) (group 5), 15 min prior to initiation of anesthesia with sevoflurane. Bumetanide in this concentration/dose range is widely used as most selective of currently available inhibitors of NKCC1 (Dzhala et al., 2005; Tyzio et al., 2014). We previously demonstrated that bumetanide at this dose alleviated the developmental effects of sevoflurane and propofol in neonatal rats (Edwards et al., 2010; Cao et al., 2012; Seubert et al., 2013; Tan et al., 2014; Willis et al., 2015). The dose for RU28318 was originally chosen based on its depressant effect of acute stress in rats (Yuen et al., 2009). Subsequently, we demonstrated that bumetanide and RU28318 at these doses depressed propofol-induced electroencephalographic seizures in neonatal rats (Willis et al., 2015). In order to control for the injections in groups 4 and 5, groups 1 and 2 received equal volumes of intraperitoneal saline or DMSO. Previously we have shown that neither saline nor DMSO at these volumes caused any obvious physiological responses (Tan et al., 2014; Willis et al., 2015). The effects of bumetanide and RU28318 (groups 4 and 5) were studied in male rats only because our previous study of propofol (Tan et al., 2014) and preliminary data on the side effects of sevoflurane indicate that female rats are relatively resistant to the long-term developmental effects of the anesthetics, at least based on physiological and behavioral parameters that we measured.
All rats were sequentially evaluated in the elevated plus maze (EPM) at ~P60, and prepulse inhibition (PPI) of the acoustic startle response at ~P80 (non-invasive procedures). To assess responsiveness of the LHPA axis to stress, serum corticosterone levels were measured at P120 (minimally invasive procedure). Subsequently, the rats from each treatment group were used for cell electrophysiology (terminal procedure). The serum levels of corticosterone at the time of anesthesia at P4, P5 or P6 were measured in separate groups of rats. These rats were not used in any other experiments since the blood sampling was a terminal procedure.
2.3. Measurement of serum corticosterone
Serum corticosterone was measured using commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI). In order to assess acute changes in serum levels of corticosterone, trunk blood samples were collected from P4, P5 or P6 rats after sacrifice by decapitation immediately after completion of 6 h sevoflurane anesthesia (group 1) or maternal separation only (group 2).
To study long-term effects of sevoflurane anesthesia at P4, P5 or P6 on endocrine responses to stress, serum levels of corticosterone were measured in blood samples collected from the P120 rats 5 min after exposure to physical restraint for 30 min. Physical restraint was administered using rodent holders (Kent scientific Corporation, Torrington, CT). Blood sampling was done using the “tail clip” method. Specifically, the distal 0.5 mm of the tail was removed using a sterile scalpel blade, and blood was allowed to drain directly into a microcentrifuge tube.
2.4. Slice electrophysiology
Brain hippocampal slices were prepared from >P125 rats. The brain was removed after decapitation and was placed into ice-cold sucrose buffer containing (in mM): 254 sucrose,10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4, saturated with 95% O2/5% CO2, at pH 7.4, 300 mOsm. Transverse hippocampal slices (300 μM thick) were cut with a VT 1000S microtome (Leica, Deerfield, IL). Slices were transferred immediately into a holding chamber and were incubated at 32 to 33°C for a 30-min recovery period in a mixture of 50% sucrose saline and 50% artificial cerebrospinal fluid (aCSF) containing (in mM): 128 NaCl, 10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4. Slices were then placed on a nylon mesh, submerged in normal aCSF bubbled continuously with 95% O2/5% CO2, and maintained at room temperature (~21–24°C) until whole-cell patch-clamp recording, typically within 0.5 to 5 h.
Slices were transferred to a submersion-type recording chamber (Warner Instruments, Hamden, CT) on a Burleigh Gibraltar fixed-stage system (Burleigh Instruments, Fisher, NY), secured beneath a nylon harp, and perfused with aCSF heated to 30 to 33°C with an inline heater (Warner SC-20) at a rate of 2 to 3 mL per min. CA1 pyramidal cells and interneurons were identified visually by using a microscope (Leica DM LFS, Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) equipped with a 40× water-immersion objective coupled with an infrared differential interference contrast camera system. Whole-cell patch-clamp recordings from pyramidal CA1 neurons were established using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Membrane current and potential signals were digitized and analyzed with Digidata 1322A and pClamp 10.0 systems (Molecular Devices, Sunnyvale, CA). Patch pipettes of ≈5 MΩ were pulled with a P-1000 puller (Sutter Instruments, Novato, CA). The pipette solution had the following composition (in mM) unless otherwise stated: 140 KCl, 0.1 CaCl2, 5 EGTA, 10 HEPES, 4 ATP-Mg2+, 0.4 GTP-2Na+, 1 QX314 (Lidocaine N-ethyl bromide), pH 7.2, 290 mOsm. The diffusion potential (liquid junction potential) was 4 mV, calculated by Clampex software. QX314 was added to the pipette solution to block the GABABR-mediated currents and to prevent the generation of Na+-dependent action potentials. Under these conditions, total miniature postsynaptic currents (mPSCs) were acquired in aCSF containing TTX (1 μM) at a holding potential of −70 mV. To record miniature inhibitory postsynaptic currents (mIPSCs), glutamate receptor antagonists DNQX (6,7-dinitroquinoxaline-2,3-dione, 20 μM) and AP5 (DL-2-amino-5-phosphonovaleric acid, 20 μM) were added to aCSF. Drugs were administered by bath application. Synaptic currents were collected for 5 min for each experimental condition. Access resistance (<25 MΩ) was regularly monitored during recordings, and cells were rejected if resistance changed >15% during the experiment. If the access resistance increased during the course of the experiment and caused significant reductions in the synaptic current amplitudes, efforts were made to improve access (such as applying additional suction or slight positive pressure); if this failed, the experiment was discontinued. Spontaneously occurring synaptic currents were filtered at 2 kHz and were digitized at 10 kHz using Digidata 1322A. Offline data analysis was performed using the MiniAnalysis software (version 6.0.7; Synaptosoft, Decatur, GA). Synaptic currents were screened automatically using an amplitude threshold of 10 pA. Events were then visually screened to ensure that the analysis was not distorted by changes in the noise level or by membrane fluctuations. If the background noise increased during the recording, the data from that cell were discarded.
2.5. Assessment of behavior in the elevated plus maze (EPM)
The EPM (EB Instruments, Pinellas Park, FL) was used. The maze consists of two opposing open (50 × 10 × 0.5 cm) and two enclosed (50 × 10 × 45 cm) arms elevated 75 cm above the floor, with a 0.5-cm edge on the open arms. Testing occurred during the light phase of the dark–light cycle. Animals were placed in the center square facing an open arm and were allowed to explore the maze for 5 min, at which time they were removed from the apparatus. During EPM testing, each rat’s behavior was recorded using BIO-EPM 3C video tracking software (EB Instruments, Pinellas Park, FL). The percentage of time spent in the open and enclosed arms, and the total distance traveled during 5 min of recording as an index of the locomotor activity were compared. If a fall occurred, the animal was removed from the study. The EPM trials were performed at ~P60.
2.6. Measurements of the acoustic startle response and PPI of startle
The PPI of startle tests were performed in young adulthood at ~P80 to assess sensorimotor gating. PPI of startle tests were performed using the SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory (Cao et al., 2012; Seubert et al., 2013; Tan et al., 2014). Testing occurred during the light phase of the dark–light cycle. At the beginning of every testing session, each animal was placed in the cylindrical animal enclosure and was then exposed to a 75-dB white noise background for a 5-min acclimation period. The acclimation period was followed by a test session consisting of five different trials: a 120-dB 40-ms pulse only; a 120-dB 40-ms pulse preceded by a prepulse of a 20-ms duration at 5, 10, and 15 dB above background; and a no-stimulus trial of background noise. The delay between the onset of the prepulse and the onset of the pulse was 100 ms. The trials were presented in pseudorandom order with variable inter-trial intervals averaging 15 s. The first four trials and last three trials consisted of 120-dB pulse-only trials. All five types of trials were presented eight times, each in pseudorandom order after the first four and before the last three pulse-only trials. The %PPI for each PPI was calculated using the following formula: %PPI =100× [(pulse alone) – (prepulse + pulse)]/pulse alone. Data were collected as Vmax amplitude. The entire test for a given animal lasted 28 min.
2.7. Drugs
Sevoflurane was manufactured by Fushimi-machi (Osaka, Japan). RU28318 was purchased from R&D Systems, Inc. (Minneapolis, MN). Bumetanide (Ben Venue Laboratories, Inc., Bedford, OH) was purchased from Bedford Laboratories™ (Bedford, OH). TTX, and QX314 were acquired from Sigma-Aldrich (St. Louis, MO). AP5 and DNQX were purchased from Tocris Cookson, Inc. (Ellisville, MO).
2.8. Statistical Analysis
Values are reported as mean ± SEM. Statistical analyses were carried out using JMP Pro software (SAS Institute, Cary, NC). Sevoflurane, maternal separation and facility rearing groups were compared using two-way ANOVA, with gender and treatment as the independent variables. Three-way ANOVA was used to analyze the PPI data, because the prepulse intensity added a third independent variable. A secondary analysis of all male treatment groups, including pretreatments with bumetanide and RU28318, was carried out with one-way ANOVA for corticosterone measurements and EPM testing and two-way ANOVA for PPI experiments. Multiple pairwise comparisons were done with the Student-Newman-Keuls method. Secondary analysis of the cellular electrophysiology data was done with Welch’s ANOVA, because of unequal variances among groups, followed by post hoc t-tests with correction for multiple comparisons. P < 0.05 was considered significant.
3. Results
3.1. Sevoflurane increased serum corticosterone levels in male and female P4–P6 pups. In adulthood, rats exposed to neonatal sevoflurane, responded to stress with heightened secretion of systemic corticosterone, with a greater increase in the levels of this glucocorticoid in male vs female rats. Bumetanide or RU28318 administered prior to the anesthetic exposure normalized the endocrine responses to stress in adulthood
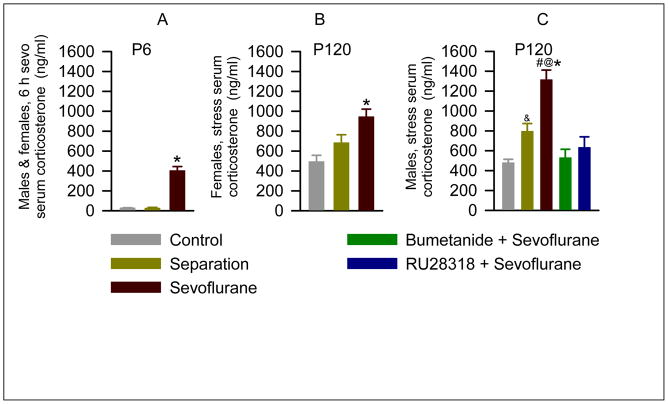
Six hours of anesthesia with 2.1% sevoflurane in P4–P6 rats caused a 14 fold increase in serum corticosterone levels (F(2,15) = 82.047, P < 0.001; Fig. 1A), compared to pups left with their dam (negative control) and pups that underwent maternal separation for 6 hours. Sevoflurane caused similar acute increases in serum corticosterone levels in male and female pups (t(4)=−1.674; P=0.169). A single 6-hour episode of maternal separation without exposure to sevoflurane did not change serum levels of corticosterone (P=0.958 vs negative control).
Figure 1.
Anesthesia of P4, P5 or P6 rats with sevoflurane for 6 h resulted in an acute increase in serum levels of corticosterone and in exacerbated endocrine responses to stress in adulthood. A: Histograms show serum levels of corticosterone immediately after 6 h of anesthesia with sevoflurane at P4, P5 or P6 in rats of both genders. Numbers of animals per treatment group: negative control (n = 6), maternal separation (n = 6), sevoflurane (n = 6). Each group consisted of 3 male and 3 female pups. *P < 0.001 vs. all other groups. B and C: Histograms showing serum levels of corticosterone in P120 female (B) and male (C) rats 5 min after physical restraint for 30 min. Each treatment group consisted of 6 rats. Two additional groups of male rats were pretreated with either bumetanide (1.82 mg/kg, intraperitoneally) or RU28318 (10 mg/kg, intraperitoneally) 15 min prior to initiation of anesthesia with sevoflurane for 6 h at P4, P5 or P6. *P < 0.001 vs. all other groups (B), and *P < 0.001 vs. all other groups (C).#P<0.001 vs. maternal separation, &P=0.006 vs. negative control (C). @P=0.002 vs. corresponding female treatment group (B and C).
In order to assess whether neonatal exposure to sevoflurane alters endocrine responses to stress in adulthood, serum corticosterone levels were measured in blood samples collected from P120 rats after physical restraint for 30 min. Overall, the levels of corticosterone in stressed male sevoflurane-exposed rats were significantly higher than those in stressed female sevoflurane-exposed rats (F(1,35)=6.51; P = 0.016, Fig. 1B,C). In multiple pairwise comparisons of corticosterone levels only the sevoflurane-treated animals differed by gender (P=0.002). In addition to the main effect of gender there was a statistically significant treatment by gender interaction (F(2,35)=3.40; P=0.047); i.e. the effect of the treatment differed across genders. The serum levels of corticosterone in male rats from the sevoflurane group stressed by restraint were markedly higher than those in male rats from the negative control and maternal separation only groups (P < 0.001; Fig. 1C). The maternal separation group had stress levels of corticosterone higher than the negative control group (P=0.006), but lower than in the sevoflurane group (P<0.001). P120 female rats exposed to sevoflurane at P4, P5 or P6, also had higher levels of corticosterone after restraint stress than their counterparts in the negative control (P < 0.001) and separation groups (P=0.02; Fig. 1B). In contrast to the stressed male rats, the stressed female rats in the maternal separation group did not have a significant increase in serum corticosterone (P=0.08 vs negative control).
In order to test whether sevoflurane-caused increases in corticosterone levels and GABAAR-mediated excitation at the time of anesthesia contribute to mechanisms initiating this altered stress response, separate groups of the P4, P5 or P6 male pups were pretreated prior to exposure to sevoflurane either with the NKCC1 inhibitor, bumetanide, or the corticosteroid receptor antagonist, RU28318. When compared to the sevoflurane only group, lower stress levels of corticosterone were detected in P120 male rats (F(4,25) = 18.36, P < 0.001; Fig. 1C) that were pretreated prior to exposure with sevoflurane either with bumetanide or RU 28318. Levels of corticosterone in rats pretreated with either bumetanide or RU28318 were not statistically different from those in facility reared or maternally separated animals.
3.2. In adulthood, rats that were exposed to neonatal sevoflurane had altered hippocampal synaptic activity. Neither bumetanide nor RU28318 significantly affected the long-term synaptic effects of neonatal sevoflurane
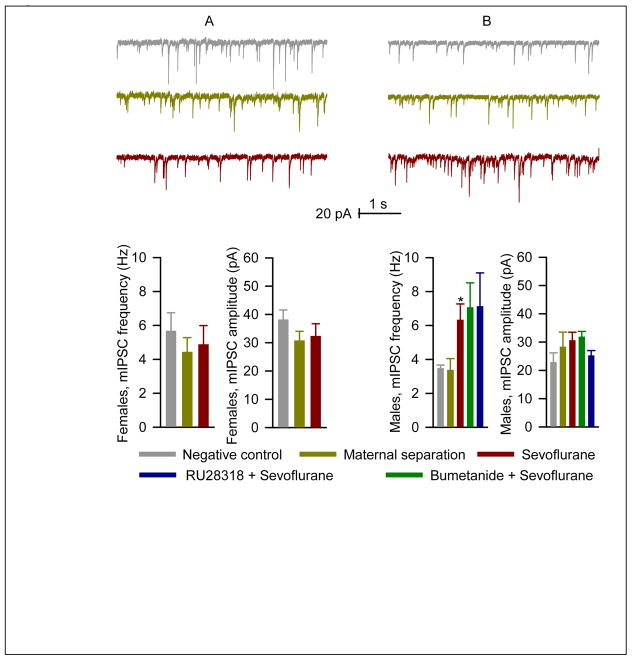
A comparison of the frequencies of mIPSCs in hippocampal pyramidal CA1 neurons between adult male and female rats exposed to either facility rearing, maternal separation or sevoflurane at a neonatal age revealed no overall statistically significant main effects of either gender (F(1,41)=0.62; P=0.44) or treatment group (F(2,41)=1.73; P=0.19, Fig. 2A,B). The amplitudes of mIPSCs tended to be smaller in males, but again did not differ significantly by treatment group (F(2,41)=0.13; P=0.88). Secondary analysis of the frequencies of mIPSCs in male rats with Welch’s ANOVA revealed a statistically significant difference between the 5 treatment groups (F(4,31)=3.76; P=0.03, Fig. 2B). Multiple pairwise comparisons showed that the sevoflurane treated animals tended to have higher mIPSC frequencies than animals exposed to facility rearing (P=0.01 uncorrected; P=0.07 corrected for multiple comparisons). Of note, pretreatment with either bumetanide of RU 28318 failed to decrease the mIPSC frequency towards control values. The rats pretreated with RU28318 tended to have lower mIPSC amplitude, but not sufficient to achieve statistical significance (P=0.086). Neonatal exposure to sevoflurane did not have any significant effect on miniature excitatory postsynaptic currents.
Figure 2.
The >P120 male, but not female, rats, anesthetized with sevoflurane for 6 h at P4, P5 or P6, had increased frequency of miniature inhibitory postsynaptic currents (mIPSCs) in the hippocampus. A: Examples of mIPSC recordings in hippocampal CA1 neurons of the >P120 female rats and respective histograms showing the frequency and amplitude of mIPSCs in rats from the negative control (n=7), maternal separation (n=9), and sevoflurane (n=8) groups. B: The mIPSC data for male rats (as described in A). Two additional groups of male rats were pretreated with either bumetanide ((1.82 mg/kg, intraperitoneally) or RU28318 (10 mg/kg, intraperitoneally) 15 min prior to initiation of anesthesia with sevoflurane for 6 h at P4, P5 or P6. Number of recorded cells for each treatment group: negative control (6), maternal separation (6), sevoflurane (6), bumetanide plus sevoflurane (n=7) and sevoflurane plus RU28318 (n=8). *P=0.01 (uncorrected) and *P=0.07 (corrected for multiple comparisons) vs. facility rearing.
3.3. Adult male, but not female, rats that were exposed to neonatal sevoflurane, spent shorter time in open arms of the elevated plus maze, an effect that was alleviated by pretreatment with either bumetanide or RU28318
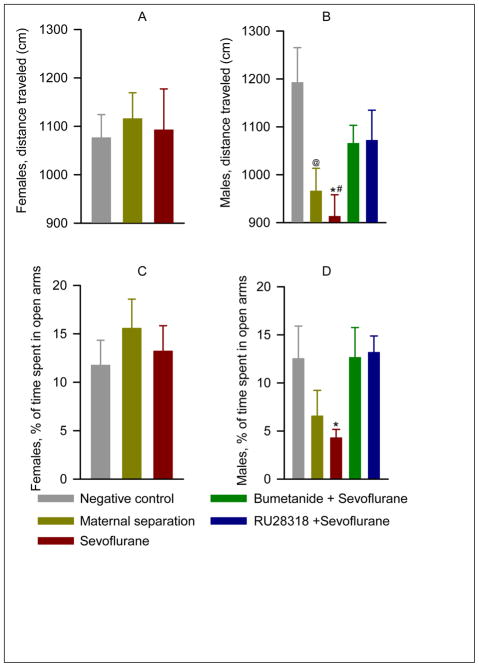
Analysis of locomotor activity in P60 rats indicated no statistically significant main effect of either gender (F(1,76)=2.0; P=0.16) or treatment (sevoflurane, maternal separation, or facility rearing; F(2,75)=2.39; P=0.099), but a significant interaction between gender and treatment ( F(2,75)=3.34; P=0.04, Fig. 3A, B). The male rats exposed to sevoflurane at P4-6 covered less distance than either unexposed males (P=0.004) or sevoflurane exposed females (P=0.03) (Fig. 3B). Maternally separated males also covered less distance than facility reared males (P=0.02). Pretreatment of male rats with either bumetanide or RU 28318 prior to sevoflurane exposure at P4-6 normalized locomotor activity (P= 0.25 and P= 0.14 compared to facility rearing for bumetanide and RU 28318, respectively). Time spent in the open arm of the maze was greater in females than in males (F(1,75)=7.90; P=0.006, Fig. 3C,D), but the overall differences in time spent in the open part of the maze between facility reared, maternally separated and sevoflurane treated animals did not achieve statistical significance (F(2,74)=1.03; P=0.36). Secondary analysis of the 5 male treatment groups reveled a significant effect of treatment on exploratory behavior (F(4,60)=3.42; P=0.014, Fig. 3B). P60 male rats from the sevoflurane group spent a significantly shorter time in the open arms of the EPM when compared to their counterparts from the negative control group (P = 0.03, Fig. 3D). Pretreatment of male rats with either bumetanide or RU 28318 prior to sevoflurane exposure at P4-6 normalized exploratory activity (P= 0.25 and P= 0.14 compared to facility rearing for bumetanide and RU 28318, respectively, Fig. 3D).
Figure 3.
Effects of neonatal exposure to sevoflurane on behavior in the EPM at P60. A and B: Histograms showing distance traveled (cm) by the female and male rats. C and D: Histograms showing % of time spent in open arms of the EPM by the female and male rats. The female rats: the negative control (n=11), maternal separation (n=12), and sevoflurane groups (n=14). The male rats: the negative control (n=11), maternal separation (n=12), and sevoflurane groups (n=14). Two additional groups of male rats were pretreated with either bumetanide (1.82 mg/kg, intraperitoneally, n=11) or RU28318 (10 mg/kg, intraperitoneally, n=10) 15 min prior to initiation of anesthesia with sevoflurane for 6 h at P4, P5 or P6. * P=0.004 vs. facility rearing, #P=0.03 vs. corresponding female treatment group, @P=0.02 vs facility rearing (B); *P=0.03 vs. facility rearing (D).
3.4. The ~P80 male, but not female, rats, exposed to sevoflurane at P4, P5 or P6, exhibited reduced PPI of startle responses. Bumetanide, but not RU28318, alleviated the effect of sevoflurane
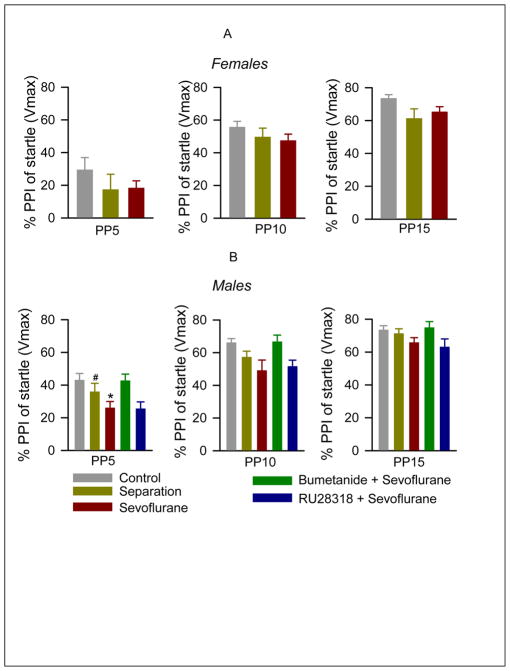
As expected from the experimental paradigm, louder prepulses caused a more profound inhibition of startle responses (F(2,213) = 99.77; P<0.001, Fig. 4A,B). In general female rats showed less inhibition of the startle response by prepulses than male rats (F(1,213) = 10.87; P<0.001). Facility reared rats differed significantly from maternally separated and sevoflurane exposed rats in the degree of PPI of startle responses (F(2,213) = 8.27; P<0.001). There were no significant interactions between the main effects gender, treatment and prepulse intensity in the 3-way ANOVA analysis. Multiple pairwise comparisons identified that both neonatal maternal separation and neonatal sevoflurane exposure significantly diminished PPI of startle responses (P<0.001 for sevoflurane and P=0.007 for maternal separation). Secondary analysis of the 5 male treatment groups with two way ANOVA revealed again a significant effect of prepulse intensity (F(2,165)=84.31; P<0.001) as well as significant differences between the treatment groups (F(4,165)=8.30; P<0.001, Fig. 4B). Multiple pairwise comparisons indicated that sevoflurane depressed PPI of startle responses (P<0.001). This depression of PPI of startle responses was reversed by pretreatment with bumetanide (P=0.88 versus facility rearing), but not by pretreatment with RU28318 (P<0.001 versus facility rearing). Startle stimuli by themselves caused similar responses in all five experimental groups of male rats. Startle response amplitudes were 1595.55 ± 218.26, 1775.85 ± 226.81, 1643.18 ± 270.84, 1477.5 ± 140.0 and 1384.95 ± 17.974 in the negative control, maternal separation, sevoflurane, bumetanide plus sevoflurane and RU28318 plus sevoflurane groups, respectively (F(4,56) = 0.386, P = 0.817; one-way ANOVA).
Figure 4.
The ~P80 male, but not female, rats, that were exposed to anesthesia with sevoflurane for 6 h at P4, P5 or P6, exhibited impaired PPI of the startle response. (A) Histogram showing PPI of startle in different treatment groups of female rats: negative control (n = 11), maternal separation (n = 13), and sevoflurane (n = 14). PP5–PP15: prepulse intensities in decibels above background. (B) Histogram showing PPI of startle in different treatment groups of male rats: negative control (n = 11), maternal separation (n = 12), and sevoflurane (n = 16). Two additional groups of male rats were pretreated with either bumetanide (1.82 mg/kg, intraperitoneally, n=11) or RU28318 (10 mg/kg, intraperitoneally, n=10) 15 min prior to initiation of anesthesia with sevoflurane for 6 h at P4, P5 or P6. *P <0.001 vs. facility rearing, #P=0.007 vs. facility rearing (B).
4. Discussion
Sevoflurane is the most frequently used general anesthetic in pediatric practice. The results of this study demonstrate that a single 6 h exposure of neonatal rats to sevoflurane anesthesia leads to both acute and long-term endocrine as well as neurobehavioral abnormalities in rats. Specifically, sevoflurane caused a multifold increase in systemic levels of corticosterone, a stress hormone, in rat pups of both genders at the time of exposure. Later in adulthood male rats that had been previously exposed to sevoflurane responded to stress with heightened secretion of corticosterone, had altered hippocampal synaptic activity, spent a shorter time in open arms of the EPM, and exhibited impaired prepulse inhibition of the acoustic startle response. Pretreatment of male pups prior to exposure to sevoflurane with the NKCC1 inhibitor bumetanide and the corticosteroid receptor antagonist RU28318 alleviated the effects of sevoflurane on endocrine activity and behavior in the EPM, while only bumetanide, but not RU28318, counteracted the developmental effects of sevoflurane on the PPI of acoustic startle responses. Neither bumetanide nor RU28318 significantly affected sevoflurane-induced alterations in hippocampal synaptic activity. These findings, together with previously published data, suggest a complex involvement of sevoflurane-induced increases in GABAAR-mediated depolarization and corticosteroid levels at the time anesthesia in the mechanisms causing the endocrine and neurobehavioral developmental effects of neonatal sevoflurane exposure.
Sevoflurane elicited increases in corticosteroid levels at the time of anesthesia may contribute to the development of long-term endocrine and some neurobehavioral abnormalities, since such abnormalities were alleviated by pretreatment with a mineralocorticoid receptor antagonist, administered prior to anesthesia. Our findings demonstrate that a single exposure to sevoflurane can provoke greater long-term neuroendocrine alterations than maternal separation of equal duration. This is consistent with the reported models of maternal separations, which employ up to two weeks of daily episodes of maternal separations to induce long-term developmental abnormalities (Macrí et al., 2004; Sousa et al., 2014). Such prolonged and repeated maternal separations lead to abnormalities in adulthood, which resemble those that we observed in neonatal rats exposed to sevoflurane, e.g., exacerbated corticosterone secretion in response to stress, anxiety-like behavior in the EPM test, and reduced PPI of startle (Brunson et al., 2005; Oomen et al., 2010; Li et al., 2013). It is unlikely that the accompanying maternal separation of neonatal pups during sevoflurane anesthesia contributes to the developmental neuroendocrine abnormalities, as these pups remain deeply sedated during the entire period of exposure.
Similar to the greater adverse behavioral effects of maternal separation in male rodents (Patchev et al., 1997; Mitev et al., 2003), we observed EPM and PPI of startle abnormalities in male, but not in female rats. Patchev et al (1997) suggested that the gender-dependent differences in behavioral effects of maternal separation could be due to counterbalancing effects of gonadal hormones in adult female rats. The nature of the gender-dependent behavioral effects of neonatal sevoflurane exposure remain to be elucidated, but it is plausible that these differences are linked to the anesthetic-induced long-lasting neuronal and endocrine processes that continue to develop after termination of anesthesia. Thus, sevoflurane caused similar acute increases in systemic corticosterone levels (this study) and in electroencephalographic excitation (Edwards et al., 2010; Cao et al., 2012; Seubert et al., 2013) in male and female P4–P6 rat pups. The developmental switch in GABAAR-mediated signaling from depolarizing/stimulatory to inhibitory occurs at younger age in female rat pups than in male rat pups (Galanopoulou, 2008; Akman et al., 2014). Given that exposures of P4–P6 rats to sevoflurane (this study) and propofol (Tan et al., 2014), resulted in long-lasting up-regulation of the GABA-ergic activity as seen in hippocampal slices, male rat pups may experience such abnormal GABAAR-mediated activation and GABAAR-mediated stimulation of the LHPA axis activity over a longer period.
Data in the literature link increased developmental depolarizing GABAergic signaling to neurodevelopmental disorders. As a specific example, Tyzio et al. (2014) demonstrated that up-regulated GABAAR-mediated signaling in naïve mice during the perinatal period led to development of symptoms seen in mouse models of autism, while bumetanide alleviated the symptoms in animal models of autism.
Our findings suggest that the different long-term developmental effects of sevoflurane are not initiated by identical mechanisms. Thus, both bumetanide and RU28318, administered to neonatal rats prior to exposure to sevoflurane, prevented sevoflurane-induced alterations in the endocrine responses to stress and EPM behavior, but only pretreatment with bumetanide was effective against sevoflurane-induced impairment of PPI of startle response. Finally, neither bumetanide nor the mineralocorticoid receptor antagonist, RU28318, were effective against sevoflurane-induced changes in hippocampal synaptic activity. Previously we have shown that exogenous corticosterone, administered to P4–P6 rats, induced an increase in GABAergic activity, similar to one observed in the sevoflurane exposed rats (Tan et al., 2014), suggesting that corticosterone may act through mechanisms that do not involve activation of the mineralocorticoid receptors to induce the observed alterations in GABAergic activity. Although, bumetanide and RU28318 at doses that were investigated in this study are widely used as selective inhibitors of the respective mechanisms in rats (Dzhala et al., 2005; Tyzio et al., 2014; Yuen et al., 2009; Willis et al., 2015), the fact that we tested the effects of these agents only at a single dose limits interpretation of the obtained results.
The possibility that neonatal sevoflurane may induce impairment in the PPI of the startle response by enhancing GABAAR-mediated depolarization is indirectly supported by findings from other laboratories which demonstrated that the neurosteroid agonist of GABAARs, allopregnanolone, administered to neonatal rats, induced development of a reduction in PPI of startle (Darbra et al., 2013), similar to that observed in this study in rats exposed to neonatal sevoflurane. This neurosteroid-mediated mechanism of impairment of PPI of startle may be applicable to the sevoflurane-induced reduction in PPI. If sevoflurane increases adrenal corticosterone secretion by activating the entire LHPA axis, then this is likely to be accompanied by increased production of allopregnanolone and other neurosteroids. Our unpublished observations indicate that exogenous allopregnanolone potentiated sevoflurane-induced hyperexcitation in neonatal rats (supplemental Figure 1). The potentiating effect of exogenous allopregnanolone was reduced by pretreatment with bumetanide, pointing to allopregnanolone-enhanced GABAAR-mediated excitation. Also, the neurosteroid synthesis inhibitor, finasteride, tended to decrease the hyperexcitatory effects of sevoflurane in neonatal rats. One plausible explanation of the alleviating effects of bumetanide on long-term developmental neuroendocrine abnormalities induced by sevoflurane is that bumetanide, by reversing GABA-initiated signaling from depolarizing/stimulatory to inhibitory, promotes adaptation of the neonatal brain to the stress-like effect of sevoflurane, at least in part, by increasing negative (inhibitory) feedback effects of sevoflurane and neurosteroids on the LHPA axis activity.
Sevoflurane is a volatile general anesthetic with polyvalent mechanism of actions that include enhancement of GABAAR activity. Recently, we reported that propofol, an intravenous general anesthetic, which is considered a relatively selective GABAAR enhancer, initiated similar acute and long-term developmental abnormalities when administered to neonatal rats (Tan et al., 2014; Willis et al., 2015) suggesting that similar mechanisms may be involved in initiation of the developmental effects of all general anesthetics that positively modulate GABA-ergic signaling.
In summary, we found that a single exposure of neonatal rats to sevoflurane results in acute and long-term developmental abnormalities reminiscent of those induced by stress, such as caused by prolonged and repeated maternal separations. The sevoflurane-induced abnormalities involve not only the brain, but also the endocrine system, e.g. heightened corticosterone levels at the time of anesthesia, and in adulthood exacerbated endocrine responses to stress, altered hippocampal synaptic activity, anxiety-like behavior in the EPM, and impaired sensorimotor gating function. Our results support the possibility that sevoflurane-enhanced neuronal activity and increase in corticosterone levels at the time of anesthesia may contribute to the mechanisms causing the long-term developmental abnormalities induced by the anesthetic. These findings form a basis for considering the role of the entire LHPA axis in mediating the developmental effects of neonatal anesthesia.
Supplementary Material
Highlights (for review).
Neonatal exposure to sevoflurane increased corticosterone acutely and exacerbated endocrine response to stress in adulthood
Sevoflurane induced long-term gender-dependent neurobehavioral abnormalities
Sevoflurane induced alterations in hippocampal synaptic activity that could be detected in adult male rats
Sevoflurane induced alterations in the elevated plus maze behavior and impairment in prepulse inhibition of startle in male rats
Antagonists of Cl− importer and corticosteroids, administered prior to neonatal anesthesia, alleviated some of the long-term effects of sevoflurane
1.5 million children under 12 months of age are exposed to anesthesia annually
Postnatal day 4, 5, or 6 rats were exposed to sevoflurane
Sevoflurane increased corticosterone acutely and exacerbated endocrine response to stress in adulthood
Sevoflurane induced neurobehavioral abnormalities predominantly in male rats
Antagonists of Cl− transporter and corticosteroids alleviated the effects of neonatal sevoflurane
Acknowledgments
Role of the funding sources
The present work was supported by grant No. R01 GM93036-01A1 from the National Institute of Health/ National Institute of General Medical Sciences, Bethesda, Maryland (to AEM), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to NG). The funding sources had no role in designing the study, collecting and analyzing the data.
The present work was supported by grant No. R01 GM93036-01A1 from the National Institute of Health/ National Institute of General Medical Sciences, Bethesda, Maryland (to AEM), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to NG). We would like to acknowledge technical contribution of Dyanet L. Puentes, Diana Infante, and Elizabeth Valdes (Students, University of Florida, Gainesville, Florida).
Footnotes
Contributors
A.E.M. conceptualized and designed the study. C.X., S.T., and J.Z. acquired data and performed data analysis. Analyses and interpretation of the data and writing of the article were performed by A.E.M., C.N.S., N.G., C.S., and T.V. All authors approved the final version of the article.
- John J McAuliffe, MD, MBA.FAAp, DABNM, Division of Neurobiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, John.McAuliffe@cchmc.org]
- Wise-Faberowski L, Lucile Packard Children’s Hospital, Stanford University School of Medicine, Palo Alto, CA 94305, USA. lwf1212@stanford.edu.
- Vladimir Patchev, Department of Neuroendocrinology, Max Planck Institute of Psychiatry, Clinical Institute, Munich, Germany. Vladimir.Patchev@Schering.de
- Sonia Darbra, Group of Neurosteroids and Behaviour, Institut de Neurociències, Departament de Psicobiologia i Metodologia de les Ciències de la Salut, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona, Spain. sonia.darbra@uab.cat.
- Galanopoulou AS., Department of Neurology, Department of Neuroscience, Laboratory of Developmental Epilepsy, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA. aristea.galanopoulou@einstein.yu.edu.
Conflicts of interest
The authors have no conflicts of interest or financial disclosures to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akman O, Moshé SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis. 2014;72:153–166. doi: 10.1016/j.nbd.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl) 2014;231:3619–3634. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Darbra S, Modol L, Vallée M, Pallarès M. Neonatal neurosteroid levels are determinant in shaping adult prepulse inhibition response to hippocampal allopregnanolone in rats. Psychoneuroendocrinology. 2013;38:1397–1406. doi: 10.1016/j.psyneuen.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J Neurophysiol. 2001;85:425–434. doi: 10.1152/jn.2001.85.1.425. [DOI] [PubMed] [Google Scholar]
- Glykys J, Dzhala VI, Kuchibhotla KV, Feng G, Kuner T, Augustine G, Bacskai BJ, Staley KJ. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–672. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Adler GK. Aldosterone: A forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehavioral Rev. 2010;34:80–86. doi: 10.1016/j.neubiorev.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Y, van den Pol AN. Reversed synaptic effects of hypocretin and NPY mediated by excitatory GABA-dependent synaptic activity in developing MCH neurons. J Neurophysiol. 2013;109:1571–1578. doi: 10.1152/jn.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013;1518:82–90. doi: 10.1016/j.brainres.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OF, Patchev VK. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Macrí S, Mason GJ, Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2012;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–739. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joëls M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OF. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth. 2013;110(Suppl 1):i53–72. doi: 10.1093/bja/aet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert CN, Zhu W, Pavlinec C, Gravenstein N, Martynyuk AE. Developmental effects of neonatal isoflurane and sevoflurane exposure in rats. Anesthesiology. 2013;119:358–364. doi: 10.1097/ALN.0b013e318291c04e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K. Biomedical Research. Researchers struggle to gauge risks of childhood anesthesia. Science. 2014;346:1161–1162. doi: 10.1126/science.346.6214.1161. [DOI] [PubMed] [Google Scholar]
- Sousa VC, Vital J, Costenla AR, Batalha VL, Sebastião AM, Ribeiro JA, Lopes LV. Maternal separation impairs long term-potentiation in CA1–CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol Aging. 2014;35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology. 2014;121:1010–1017. doi: 10.1097/ALN.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J, Zhu W, Tan S, Xu C, Perez-Downes J, Seubert CN, Gravenstein N, Martynyuk A. Propofol-induced electroencephalographic seizures in neonatal rats: The role of corticosteroids and GABA-A receptor mediated excitation. Anesth Analg. 2015;120:433–439. doi: 10.1213/ANE.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.