Abstract
Little is known about how patients undergoing stem cell transplantation (HCT) and their family caregivers (FC) perceive their prognosis. We examined prognostic understanding in patients undergoing HCT and their FC and its relationship with quality of life (QOL) and mood. We conducted a longitudinal study of patients (and FC) hospitalized for HCT. We used a questionnaire to measure participants’ prognostic understanding and asked the oncologists to estimate patients’ prognosis prior to HCT. We assessed QOL and mood weekly and evaluated the relationship between prognostic understanding and QOL and mood using multivariable linear mixed models. We enrolled 90 patients undergoing (autologous n=30); myeloablative (n=30) or reduced intensity allogeneic (n=30)) HCT. 88.9% of patients and 87.1% of FC reported it is ‘extremely’ or ‘very’ important to know about prognosis. However, 77.6% of patients and 71.7% of FC reported a discordance and more optimistic prognostic perception compared to the oncologist (P’s < 0.0001). Patients with a concordant prognostic understanding with their oncologists reported worse QOL (β = −9.4, P = 0.01) and greater depression at baseline (β = 1.7, P = 0.02) and over time ((β = 1.2, P < 0.0001). Therefore, Interventions are needed to improve prognostic understanding, while providing patients with adequate psychological support.
Introduction
Patients’ understanding of their prognosis and the likelihood of benefit with various therapies is an essential component of informed decision-making.1–3 This is especially true for patients with hematologic malignancies who are confronted with challenging decisions balancing the risks and benefits of high dose chemotherapy and hematopoietic stem cell transplantation (HCT). Although HCT carries considerable morbidity and risk of mortality, many patients face almost certain death without undergoing this treatment.4–7 The gravity of such decisions highlights the importance of patient access to accurate information about their prognosis.
Patients’ prognostic understanding plays a role in their medical decision-making.8 For example, patients’ understanding of the likelihood of achieving a cure is associated with their willingness to accept chemotherapy.9–11 Moreover, patients with advanced solid malignancies who overestimate their chances of survival are more likely to elect aggressive care at the end of life.12 Enhancing prognostic awareness may potentially empower patients to make informed decisions that are consistent with their preferences, facilitating a patient-centered approach to medical care.12, 13
Despite its importance, very little research has focused on prognostic understanding in patients with hematologic malignancies and those undergoing HCT.14, 15 In fact, the majority of data on prognostic awareness come from surveys of patients with incurable solid tumors, revealing marked misperceptions in prognostic understanding.16, 17 Moreover, studies are lacking in comparing the perceptions of patients, family caregivers (FC), and physicians regarding the likelihood of cure with HCT. As FC often play a pivotal role in helping patients make decisions regarding their care,18, 19 a better understanding of their prognostic awareness is warranted.
Data are also lacking examining the relationship between patients’ prognostic understanding and their QOL and mood.8 In a study of patients with advanced cancer, those who acknowledged the terminal nature of their illness reported lower QOL and worse anxiety compared to those with overly optimistic and inaccurate prognostic understanding.8 Patients undergoing HCT experience substantial distress during a prolonged and socially isolating hospitalization.5, 7, 20–23 Therefore, the relationship between prognostic awareness, QOL, and mood in these individuals and their families is particularly important, where the physical and psychological treatment burden is high.
We recently completed a prospective longitudinal study assessing patients’ QOL and mood during hospitalization for HCT.24 In this study, we also sought to evaluate both patient and FC preferences for receiving prognostic information, and compare their prognostic understanding to the treating oncologist’s perception of prognosis. Furthermore, we conducted an exploratory analysis to examine the association of patients’ and FCs’ prognostic understanding with their QOL and mood.
Methods
Participants
In this study, we included English-speaking patients (age ≥ 18) with hematologic malignancies admitted to Massachusetts General Hospital for HCT. We enrolled consecutively eligible participants within three transplant cohorts (1) autologous HCT (n = 30); (2) myeloablative allogeneic HCT (n=30); and (3) reduced intensity allogeneic HCT (n=30). We excluded patients with significant psychiatric or other comorbidities, which the treating oncologist believed impaired their ability to provide informed consent. We asked enrolled patients to identify a FC (a relative or a friend who either lived with the patient or had in-person contact with him/her at least twice per week) and invited this person to participate in the FC portion of this study. Patients without a willing or available FC were still eligible to participate.
Study Design and Procedures
This study was approved by the Dana Farber Harvard Cancer Center Institutional Review Board. We identified eligible patients with a planned HCT admission during the weekly bone marrow transplant team meeting. A trained research assistant obtained permission by email from the treating oncologist to approach eligible patients and their FC within 72 hours of admission. Willing participants (patients and FC) provided written informed consent and completed baseline questionnaires approximately six days prior to transplant (day -6). We then followed participants longitudinally and obtained self-reported measures weekly during hospitalization.
Study Measures
Information Preferences and Prognostic Understanding
We assessed information preferences and prognostic understanding using the 10-item Prognosis and Treatment Perception Questionnaire (PTPQ), which assesses patient beliefs regarding 1) likelihood of cure, 2) the importance of knowing about prognosis, and 3) preferences for information about treatment.8, 25 We previously performed cognitive interviews with patients to ensure the content validity, readability, and acceptability of the PTPQ and have utilized it in prior studies.8 We administered the PTPQ to patients and FC at the time of admission for HCT (6 days prior to transplant).
We used specific items from the PTPQ to evaluate patients’ and FC information preferences. For example, participants reported their preference for receiving information about diagnosis and treatment (“prefer not to hear a lot of details”, “want to hear details only in certain situations,” or “want to hear as many details as possible”). We also asked participants to rate the importance of knowing about the patients’ prognosis on a 5-point scale (ranging from “extremely important”, to “not at all important”). Additionally, participants reported the frequency of having a conversation with the oncologist about prognosis on a 5-point scale (ranging from “never”, to “very often”). We dichotomized participant’s responses into two categories: 1) very often or often; or 2) less often or never. Finally, we asked participants whether they had a discussion with the oncologist regarding end-of-life care (“yes” or “no”).
Finally, the patient, FC, and the treating oncologist, each reported their perceptions of the patients’ overall prognosis using a single item in which they rated the likelihood that he or she will be cured of their cancer on a 7-point scale (ranging from “very unlikely/ <10% ” to “extremely likely/ > 90% ”). We grouped responses into three categories: 1) extremely or very likely to be cured / 75%–100%; 2) moderately or somewhat likely to be cured/ 25%–74%); and 3) Unlikely to be cured / 0–24%. We then categorized patients and FC as either having a concordant prognostic understanding if their responses were concordant with the oncologist’s response or discordant prognostic understanding if their responses were inconsistent with the oncologist’s assessments.
Demographic and clinical factors
Each participant completed a demographic questionnaire that included age, gender, race, ethnicity, marital status, income, and educational level. We reviewed patients’ electronic medical records to obtain data on cancer diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, and comorbidities as measured by the HCT-Comorbidity Index.26
Quality of Life and Fatigue
We used the Functional Assessment of Cancer Therapy- BMT (FACT-BMT) questionnaire to assess patients’ QOL.27 The FACT-BMT contains 47 items that comprise 5 subscales assessing physical, functional, emotional, social well-being, and Bone Marrow Transplant (BMT) specific concerns during the past week. Higher total and subscale scores indicate better QOL. We also calculated the Trial Outcome Index (TOI), which is the sum of the scores on the physical well-being, functional well-being, and BMT subscales of the FACT-BMT. To measure patients’ fatigue, we used the FACT-Fatigue subscale, which consists of 13-items about fatigue symptoms during the past week.28 Lower scores on the FACT-Fatigue subscale indicate greater fatigue.
To evaluate QOL in FC, we utilized the Medical Outcomes Study 36-item Short Form Health Survey (SF-36), which measures physical functioning, role functioning-physical, bodily pain, general health, vitality, social functioning, role functioning-emotional, and mental health.29 We also calculated two summary scales derived from the SF-36: Physical Component Scale (PCS) and Mental Component Scale (MCS).29
Depression and Anxiety
We measured participants’ (patients and FC) anxiety and depression symptoms with the 14-item Hospital Anxiety and Depression Scale (HADS). The HADS consists of two subscales assessing anxiety and depression symptoms in the past week, with subscale scores ranging from 0 (no distress) to 21 (maximum distress).30 We also assessed mood using the Patient Health Questionnaire 9 (PHQ-9). The PHQ-9 is a nine-item measure that detects symptoms of major depressive disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).31
Attrition and missing data
Patients admitted to our institution for autologous and reduced intensity HCT are hospitalized on average for 1.5 weeks after their transplant, and those undergoing myeloablative HCT are hospitalized for 2.5–3 weeks after transplantation. Therefore, we analyzed data up to day +8 (8 days after HCT) for all participants in this analysis. Only 6.7% (6/90) of patients were missing day +1 evaluation, and 20% (18/90) were missing day +8 evaluation. Most missing evaluations (91%) were due deterioration in patients’ health status and being too symptomatic per self-report. We used several methods to compute missing QOL data and compared the results to those without imputations. Imputations involved (1) simple mean imputation and (2) last observation carried forward. We also utilized maximum likelihood estimation for missing data. As our results were similar with the different methods, we used the conservative approach of carrying last value forward to account for all missing patient and FC reported outcomes since QOL data were not missing at random.
Statistical Analysis
We first calculated descriptive statistics, including means or medians for continuous variables depending on the normality of the data, and proportions for categorical variables. For all analyses, we considered two-sided p-values < 0.05 to be statistically significant.
To examine prognostic and treatment understanding, we reported responses (frequencies) to PTPQ items, including perceptions of the likelihood of cure with HCT. We compared participant-provider responses using the chi square test. We measured agreement of participants and provider responses using the κ coefficient.
Finally, we explored unadjusted associations between prognostic understanding and participant’s demographic/clinical factors by conducting chi-square and Fisher exact tests for categorical variables as well as t-tests for continuous variables. Using linear mixed effect models adjusting for potential confounders identified in unadjusted analyses (age, type of HCT, and comorbidities), we examined the relationship between participants’ prognostic understanding and the following outcomes: patient’s QOL (FACT-BMT and TOI), patient’s fatigue (FACT-Fatigue), FC QOL (PCS and MCS), patients’ and FC psychological distress (HADS-depression, HADS-anxiety, and PHQ-9).
Results
Participants Characteristics
We enrolled 97% (90/93) of consecutively eligible patients admitted for autologous (n=30), myeloablative allogeneic (n=30), and reduced intensity allogeneic (n=30) HCT between 7/1/2012 and 3/10/2014. We also enrolled 47 FC and most (72.3%, 34/47) were a partner or spouse to the patient. Twenty one (23%) patients did not identify a FC that they were willing to have us approach for study participation. The remaining 24% (n=22) of FC either refused to participate or were not available during the allotted recruitment window to consent for study participation. Table 1 and Table 2 depict patients and FC characteristics, respectively. Patients were primarily white (91.1%, 82/90). The majority of the sample was male (58.9%, 53/90), and 50% (45/90) had a diagnosis of acute myeloid leukemia or myelodysplastic syndrome. The median age of patients and FC was 58.1 and 55.8, respectively.
Table 1.
Baseline Characteristics of Patients Participants
| Characteristic | All Patients (n=90) |
|---|---|
| Age mean (SD) | 58.1 (14.4) |
| Race: White | 82 (91.1%) |
| Male (%) | 53 (58.9%) |
| Type of Transplant | |
| Autologous HCT | 30 (33.3%) |
| Myeloablative Allogeneic HCT | 30 (33.3%) |
| Reduced Intensity Allogeneic HCT | 30 (33.3%) |
| Diagnosis (%) | |
| ALL | 7 (7.8%) |
| AML/MDS | 45 (50.0%) |
| MF/CML | 2 (2.2%) |
| Lymphoma | 23 (25.6%) |
| MM | 9 (10%) |
| Relationship status | |
| Married | 60 (66.7%) |
| Divorced | 7 (7.8%) |
| Single | 12 (13.3%) |
| Widowed | 11 (12.2%) |
| Education | |
| High school | 23 (25.6%) |
| College | 46 (51.1%) |
| Post graduate | 21 (23.3%) |
| Income | |
| <25,000 | 11 (12.2%) |
| 25,000–50,000 | 14 (15.6%) |
| 51,000–100,000 | 27 (30.0%) |
| 101,000–150,000 | 17 (18.9%) |
| >150,000 | 12 (13.3%) |
| Missing | 9 (10.0%) |
| HCT-Comorbidity Index (HCT-CI) median (range) | 1.0 (0–7) |
ALL = Acute lymphoblastic leukemia, AML = Acute myeloid leukemia, MDS = Myelodysplastic syndrome, MF = Myelofibrosis, CML = Chronic myeloid leukemia, MM = Multiple myeloma, HCT: Hematopoietic stem cell transplantation, SD = Standard deviation.
Table 2.
Baseline characteristics of Family Caregivers
| Variable | All Family Caregivers (n=47) |
|---|---|
| Age median (range) | 55.8(29–77) |
| Female (%) | 33 (70.2%) |
| Employment | |
| Full time | 22 (46.8%) |
| Part time | 6 (12.8%) |
| Retired | 14 (29.8%) |
| Paid leave | 2 (4.3%) |
| Unemployed | 3 (6.4%) |
| Race: White | 46 (97.9%) |
| Relationship | |
| Partners | 34 (72.3%) |
| Sibling | 3 (6.4%) |
| Child | 3 (6.4%) |
| Parent | 5 (10.6%) |
| Friend | 2 (4.3%) |
| Education | |
| High school | 13 (27.7%) |
| College | 23 (48.9%) |
| Post graduate | 11 (23.4%) |
SD = Standard deviation.
Information Preferences and Prognostic Understanding
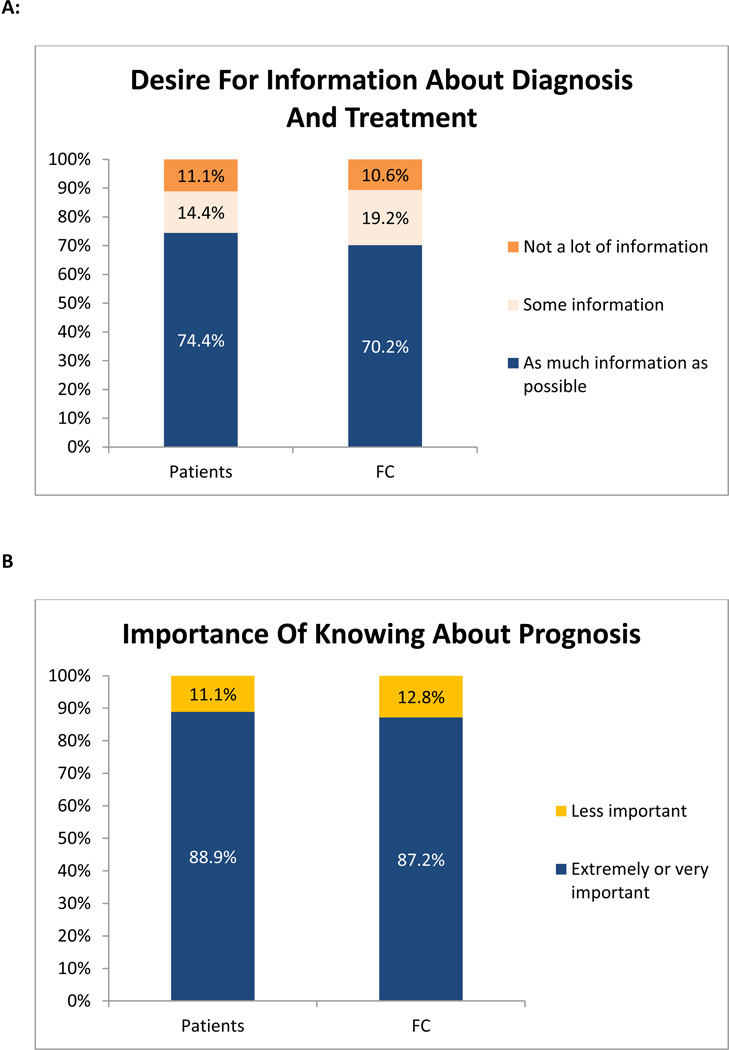
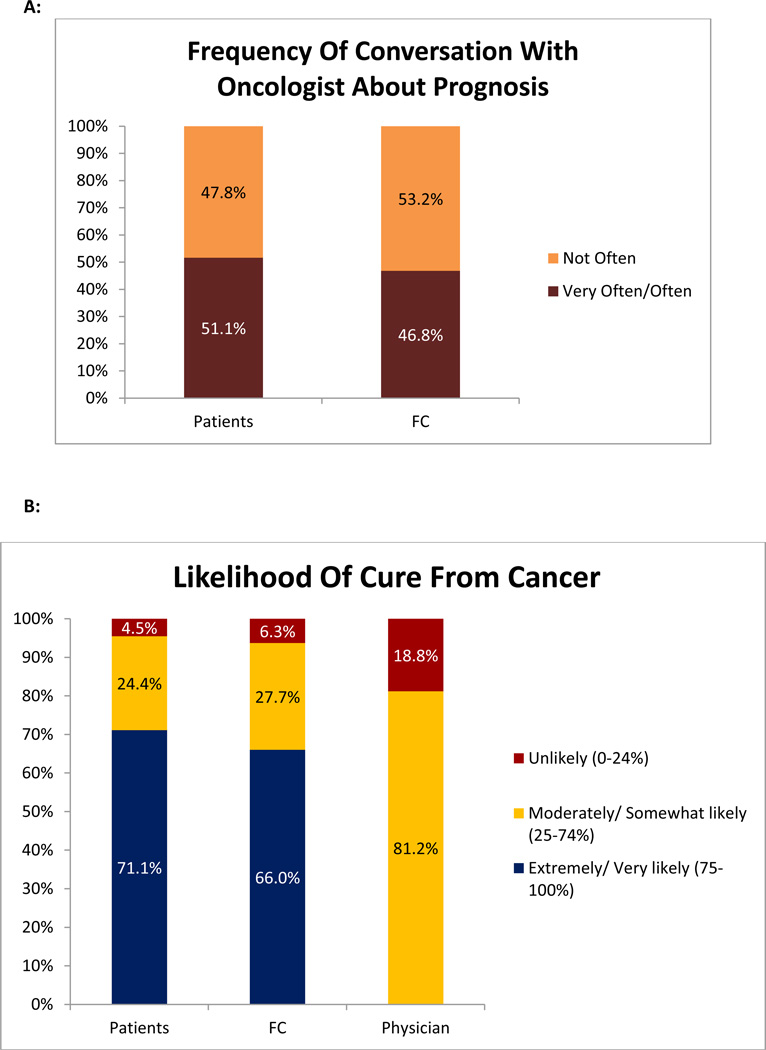
Most patients (74.4%, 67/90) and FC (70.2%, 33/47) desired as much information as possible about their diagnosis and treatment [Figure 1A]. Furthermore, 88.9% (80/90) of patients and 87.2% (41/47) of FC endorsed that it is ‘extremely’ or ‘very’ important to know about prognosis [Figure 1B]. However, only 51.1% (47/90) of patients and 46.8% (22/47) of FC reported that they discussed the patients’ prognosis with the treating oncologist frequently [Figure 2A]. The majority of patients (86.7%, 78/90) and FC (85.1%, 40/47) stated that they did not discuss end-of-life care with the treating oncologist.
Figure 1. Participants’ Preferences for Prognostic Information.
Figure 1A = Patients’ and Family Caregivers’ (FC) Desire for information about diagnosis and treatment; Figure 1B = Importance of knowing about prognosis for patients and Family Caregivers (FC).
Figure 2. Frequency of Prognostic Disclosure and Accuracy of Prognostic Perception.
Figure 2A: Patients’ and Family Caregivers’ (FC) report of frequency of discussing prognosis with the treating oncologist; Figure 2B: Perception of Prognosis among Patients, Family Caregivers (FC), and the treating oncologists.
Figure 2B depicts the perceptions of patients, their FC, and the treating oncologists regarding the likelihood of cure with HCT. The majority of patients (77.6%, 66/85) reported a more optimistic assessment of their prognosis, compared to their oncologist (P < 0.0001). The concordance rate between patients’ and their oncologists’ prognostic perception was low (k = 0.009, p = 0.74). Similarly, 71.7% (33/46) of FC reported a more optimistic assessment of their prognosis, compared to the oncologists (P < 0.0001). The concordance rate between FC and oncologists’ prognostic perception was similarly low (k = 0.04, p = 0.25). The majority of patients and FC (76.6%, 36/47) reported concordant views of the patients’ prognosis (k = 0.56, P < 0.0001). All patients and FC with discordant prognostic understanding compared to their oncologists reported a more optimistic perception of their prognosis compared to the oncologists.
Association of Prognostic Understanding with Demographic and Clinical Factors
Patients whose prognostic understanding was concordant with their oncologist did not differ by age, gender, education, income, comorbidities, ECOG performance status, cancer diagnosis, or relationship status, compared with those with discordant and more optimistic prognostic understanding. Similarly, there were no differences in age, gender, race, education, or relationship to the patient between FC with concordant (versus discordant) prognostic understanding.
Association of Prognostic Understanding with Quality of Life and Psychological Outcomes
After adjusting for potential confounders in multivariable analysis, patients whose prognostic understanding as concordant with their oncologist reported significantly lower QOL (FACT-BMT and FACT-TOI), and higher fatigue (FACT-Fatigue) throughout their hospitalization [Table 3]. Furthermore, patients with a concordant prognostic understanding reported significantly greater depressive symptoms (HADS-Depression and PHQ-9, Table 3) and a steeper increase in these symptoms during the transplant admission (β = 1.2, 95% CI 0.90–1.5, P < 0.0001).
Table 3.
Multivariable Analysis examining the association between patients’ prognostic understanding and their QOL and mood during hospitalization for HCT
| Variables of Interest | Estimate of Difference (β) |
95% CI | P value |
|---|---|---|---|
| QOL (FACT-BMT) | |||
| Accurate vs. inaccurate prognostic understanding | −9.4 | [−16.8,−2.1] | P = 0.01 |
| QOL (FACT-TOI) | |||
| Accurate vs. inaccurate prognostic understanding | −7.2 | [−12.8, −1.6] | P = 0.01 |
| Fatigue (FACT-Fatigue) | |||
| Accurate vs. inaccurate prognostic understanding | −7.0 | [−11.6, −2.3] | P = 0.003 |
| Depression (HADS-Depression) | |||
| Accurate vs. inaccurate prognostic understanding | 1.7 | [0.26, 3.1] | P = 0.02 |
| Anxiety (HADS-Anxiety) | |||
| Accurate vs. inaccurate prognostic understanding | −0.2 | [−1.7, 1.3] | P = 0.81 |
| Depressive Syndrome (PHQ9) | |||
| Accurate vs. inaccurate prognostic understanding | 1.3 | [1.0–1.6] | P < 0.0001 |
The estimate of difference: estimated difference in scores compared to the reference group (inaccurate prognostic understanding) based on the results of the multivariable linear mixed models adjusting for age, comorbidities (HCT-CI), type of HCT, and time.
Finally, across all domains of the SF-36, FC did not differ significantly between those with concordant versus discordant prognostic understanding in multivariable analysis [Table 4]. FC prognostic understanding was not associated with FC depression or anxiety [Table 4].
Table 4.
Multivariable Analysis examining the association between Family Caregivers (FC) prognostic understanding and their QOL and mood during patients’ hospitalization for HCT
| Variables of Interest | Estimate of Difference (β) |
95% CI | P value |
|---|---|---|---|
| QOL Physical Component Scale | |||
| Accurate vs. inaccurate prognostic understanding | −2.2 | [−7.4, 3.1] | P = 0.42 |
| QOL Mental Component Scale | |||
| Accurate vs. inaccurate prognostic understanding | −3.6 | [−11.0, 3.8] | P = 0.34 |
| Depression (HADS-Depression) | |||
| Accurate vs. inaccurate prognostic understanding | 0.79 | [−1.2, 2.8] | P = 0.45 |
| Anxiety (HADS-Anxiety) | |||
| Accurate vs. inaccurate prognostic understanding | 1.2 | [−1.1, 3.5] | P = 0.30 |
| Depressive Syndrome (PHQ9) | |||
| Accurate vs. inaccurate prognostic understanding | −0.83 | [−3.5, 1.9] | P = 0.54 |
The estimate of difference: estimated difference in scores compared to the reference group (inaccurate prognostic understanding) based on the results of multivariable linear mixed models adjusting for age, type of HCT, and time.
Discussion
Despite the high value patients and families place on the importance of knowing about prognosis, the majority reported an overly optimistic assessment of the likelihood of cure, compared with the oncologist. Our findings highlight major gaps in prognostic understanding among patients with hematologic malignancies undergoing HCT, similar to what has been reported in patients with solid tumors.17, 32, 33 Importantly, patients with concordant prognostic understanding with their oncologist reported lower QOL, and greater symptoms of fatigue and depression during their hospitalization for HCT.
The extent of the decrement in QOL and increase in both fatigue and depression during HCT hospitalization among patients with concordant prognostic perception with their oncologists are notable. While the medical community has focused on the value of prognostic disclosure,17 our findings stress the importance of studying better ways to support patients who do comprehend the likely outcome of their HCT. Patients who accept that they have a low chance of being cured with HCT may have a harder time enduring the symptoms during hospitalization, thereby leading to higher levels of distress. While our findings do not dispute the importance of prognostic disclosure, they do highlight the need to explore the complex relationship between prognostic understanding and patients’ QOL and mood.
While it is important for clinicians to provide patients and families with accurate prognostic information when they are making decisions regarding HCT, our results question whether clinicians should emphasize patients’ prognosis during the hospitalization period. Once a decision is made to proceed with HCT, there may be a benefit to focusing on enhancing coping strategies and building an optimistic framework while patients undergo intensive therapy during a prolonged hospitalization. On the other hand, prognostic awareness may be especially important for patients making decisions regarding alternative treatment options and weighing the potential risks and benefits of HCT. Future efforts should assess the optimal timing for prognostic disclosure in patients undergoing HCT and whether there are times in the illness trajectory when the emphasis should be placed on augmenting patients’ coping with their illness.
To our knowledge, this is the first study to examine FC’s prognostic understanding and its concordance with that of the patient and oncologist. FC and patients reported highly concordant perceptions of the patients’ prognosis, which were optimistic compared to the oncologists’ estimation of the likelihood of cure. Therefore, prognostic misperceptions affect both patients and their families alike, further impeding their ability to make informed decisions. It is possible that patients and FC are reinforcing each other’s misperceptions, further impeding their ability to accurately understand the patients’ prognosis. As family caregivers play a central role in the care of HCT patients,18, 19 addressing gaps in their prognostic understanding may have important implications on patients’ medical care and future decision-making.
Several of our findings are consistent with the results of previous research. In a study conducted in the 1990s, patients undergoing HCT reported an optimistic estimation of their disease-free-survival, compared to their physicians.15 Our findings in the modern era of HCT indicate that we have not made any progress in improving patients’ prognostic awareness. Another study in patients with advanced gastrointestinal cancers reported an association between patients’ acknowledgement of their terminal illness with worse QOL and higher anxiety.8 We report a similar association between less optimistic prognostic understanding and lower QOL and higher psychological distress in patients with hematologic malignancies. While these associations do not imply causation, detecting them in two different populations of cancer patients is intriguing and worthy of further exploration.
Our study has several important limitations. First, we included 90, mostly white patients, drawn from a single transplant center and thus our findings may not be generalizable to minority groups and patients in other geographic areas or transplant centers with difference practices. Second, although our results suggest an association between prognostic understanding and QOL and mood, the data do not allow us to determine the causality or direction of this association. Third, we used the oncologists’ assessment of the patients’ prognosis as the comparator to assess participants’ prognostic understanding. Studies have shown oncologists also hold overly optimistic perceptions of their patients’ prognosis,34 thus we may be underestimating the frequency of prognostic misperceptions among study participants. Fourth the FC enrolled in this study may or may not have been present during the initial HCT consultation when prognosis is usually discussed, and therefore may not have heard the oncologist’s perception of the patient’s prognosis. Fifth, the PTPQ has not been validated in the HCT population. Lastly, the use of imputation methods may introduce bias, as patients with missing patient-reported measures are more likely to be symptomatic. However, we utilized a conservative approach that biases the results towards the null hypothesis.
In this study, we demonstrated that the majority of patients undergoing HCT and their families report an overly optimistic perception of the patients’ prognosis, which is highly discordant with the treating oncologists’ estimation of the likelihood of cure. This discrepancy in prognostic understanding likely impedes the abilities of patients and families to make informed decisions, including weighing the risk and benefit of HCT. It is also noteworthy that patients with a less optimistic understanding of their prognosis reported lower QOL, and worse fatigue and depression. These patients might benefit from additional psychosocial support and symptom management during their hospitalization for HCT. Future research should focus on optimizing ways to enhance prognostic awareness in patients and families, especially as they make decisions regarding the pursuit of HCT, while providing the necessary psychological care to facilitate effective coping during the transplant process.
Supplementary Material
Acknowledgment
Research Support: This work was supported by funds from the MGH Cancer Center and K24 CA 181253 (Temel).
Footnotes
Conflict of Interest: None
References
- 1.Jonsen ARSM, Winslade W. Clinical ethics, 5th edition. 5th edition edn. New York: McGraw-Hill; 2002. [Google Scholar]
- 2.Faden R, Beauchamp TL. A History and Theory of Informed Consent. New York: Oxford University Press; 1986. [Google Scholar]
- 3.Veatch RM. Abandoning informed consent. The Hastings Center report. 1995;25(2):5–12. e-pub ahead of print 1995/03/01. [PubMed] [Google Scholar]
- 4.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. The New England journal of medicine. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. e-pub ahead of print 1997/03/27; doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 5.Duell T, van Lint MT, Ljungman P, Tichelli A, Socie G, Apperley JF, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Annals of internal medicine. 1997;126(3):184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. e-pub ahead of print 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 6.Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A. EBMT activity survey 2004 and changes in disease indication over the past 15 years. Bone marrow transplantation. 2006;37(12):1069–1085. doi: 10.1038/sj.bmt.1705377. e-pub ahead of print 2006/06/08; doi: 1705377 [pii] 10.1038/sj.bmt.1705377. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Joffe S, Kim HT, Socie G, Gilman AL, Wingard JR, et al. Physicians' attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104(7):2194–2200. doi: 10.1182/blood-2003-07-2430. e-pub ahead of print 2004/06/17; doi: 10.1182/blood-2003-07-2430 2003-07-2430 [pii] [DOI] [PubMed] [Google Scholar]
- 8.El-Jawahri A, Traeger L, Park ER, et al. Association among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2013 doi: 10.1002/cncr.28369. in press. [DOI] [PubMed] [Google Scholar]
- 9.Brundage MD, Davidson JR, Mackillop WJ. Trading treatment toxicity for survival in locally advanced non-small cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(1):330–340. doi: 10.1200/JCO.1997.15.1.330. e-pub ahead of print 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 10.Hirose T, Yamaoka T, Ohnishi T, Sugiyama T, Kusumoto S, Shirai T, et al. Patient willingness to undergo chemotherapy and thoracic radiotherapy for locally advanced non-small cell lung cancer. Psycho-oncology. 2009;18(5):483–489. doi: 10.1002/pon.1450. e-pub ahead of print 2008/10/24; doi: 10.1002/pon.1450. [DOI] [PubMed] [Google Scholar]
- 11.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. Bmj. 1998;317(7161):771–775. doi: 10.1136/bmj.317.7161.771. e-pub ahead of print 1998/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks JC, Cook EF, O'Day SJ, Peterson LM, Wenger N, Reding D, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA : the journal of the American Medical Association. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. e-pub ahead of print 1998/06/12; doi: joc71098 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Social science & medicine. 2000;51(7):1087–1110. doi: 10.1016/s0277-9536(00)00098-8. e-pub ahead of print 2000/09/27. [DOI] [PubMed] [Google Scholar]
- 14.Stiff PJ, Miller LA, Mumby P, Kiley K, Batiste R, Porter N, et al. Patients' understanding of disease status and treatment plan at initial hematopoietic stem cell transplantation consultation. Bone marrow transplantation. 2006;37(5):479–484. doi: 10.1038/sj.bmt.1705264. e-pub ahead of print 2006/01/26; doi: 1705264 [pii] 10.1038/sj.bmt.1705264. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Fairclough D, Antin JH, Weeks JC. Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA : the journal of the American Medical Association. 2001;285(8):1034–1038. doi: 10.1001/jama.285.8.1034. e-pub ahead of print 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 16.Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(17):2319–2326. doi: 10.1200/JCO.2010.32.4459. e-pub ahead of print 2011/05/11; doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 17.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients' expectations about effects of chemotherapy for advanced cancer. The New England journal of medicine. 2012;367(17):1616–1625. doi: 10.1056/NEJMoa1204410. e-pub ahead of print 2012/10/26; doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin DW, Cho J, Roter DL, Kim SY, Sohn SK, Yoon MS, et al. Preferences for and experiences of family involvement in cancer treatment decision-making: patient-caregiver dyads study. Psycho-oncology. 2013;22(11):2624–2631. doi: 10.1002/pon.3339. e-pub ahead of print 2013/07/31; doi: 10.1002/pon.3339. [DOI] [PubMed] [Google Scholar]
- 19.Zhang AY, Siminoff LA. The role of the family in treatment decision making by patients with cancer. Oncology nursing forum. 2003;30(6):1022–1028. doi: 10.1188/03.ONF.1022-1028. e-pub ahead of print 2003/11/07; doi: 10.1188/03.ONF.1022-1028. [DOI] [PubMed] [Google Scholar]
- 20.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. e-pub ahead of print 2009/04/02; doi: blood-2008-10-182592 [pii] 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fife BL, Monahan PO, Abonour R, Wood LL, Stump TE. Adaptation of family caregivers during the acute phase of adult BMT. Bone marrow transplantation. 2009;43(12):959–966. doi: 10.1038/bmt.2008.405. e-pub ahead of print 2008/12/24; doi: 10.1038/bmt.2008.405. [DOI] [PubMed] [Google Scholar]
- 22.Siston AK, List MA, Daugherty CK, Banik DM, Menke C, Cornetta K, et al. Psychosocial adjustment of patients and caregivers prior to allogeneic bone marrow transplantation. Bone marrow transplantation. 2001;27(11):1181–1188. doi: 10.1038/sj.bmt.1703059. e-pub ahead of print 2001/09/12; doi: 10.1038/sj.bmt.1703059. [DOI] [PubMed] [Google Scholar]
- 23.Foxall MJ, Gaston-Johansson F. Burden and health outcomes of family caregivers of hospitalized bone marrow transplant patients. J Adv Nurs. 1996;24(5):915–923. doi: 10.1111/j.1365-2648.1996.tb02926.x. e-pub ahead of print 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 24.El-Jawahri A, Traeger L, Kuzmuk K, et al. Qualit yof life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer. 2014 doi: 10.1002/cncr.29149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mack JW, Wolfe J, Grier HE, Cleary PD, Weeks JC. Communication about prognosis between parents and physicians of children with cancer: parent preferences and the impact of prognostic information. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(33):5265–5270. doi: 10.1200/JCO.2006.06.5326. e-pub ahead of print 2006/11/23; doi: 24/33/5265 [pii] 10.1200/JCO.2006.06.5326. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. e-pub ahead of print 2005/07/05; doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone marrow transplantation. 1997;19(4):357–368. doi: 10.1038/sj.bmt.1700672. e-pub ahead of print 1997/02/02; doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 28.Santana MJ, Au HJ, Dharma-Wardene M, Hewitt JD, Dupere D, Hanson J, et al. Health-related quality of life measures in routine clinical care: can FACT-fatigue help to assess the management of fatigue in cancer patients? Int J Technol Assess Health Care. 2009;25(1):90–96. doi: 10.1017/S0266462309090126. e-pub ahead of print 2009/01/08; doi: S0266462309090126 [pii] 10.1017/S0266462309090126. [DOI] [PubMed] [Google Scholar]
- 29.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Bmj. 1992;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. e-pub ahead of print 1992/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. e-pub ahead of print 1983/06/01. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. e-pub ahead of print 2001/09/15; doi: jgi01114 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattellari M, Voigt KJ, Butow PN, Tattersall MH. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(2):503–513. doi: 10.1200/JCO.2002.20.2.503. e-pub ahead of print 2002/01/12. [DOI] [PubMed] [Google Scholar]
- 33.Hagerty RG, Butow PN, Ellis PA, Lobb EA, Pendlebury S, Leighl N, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(9):1721–1730. doi: 10.1200/JCO.2004.04.095. e-pub ahead of print 2004/05/01; doi: 10.1200/JCO.2004.04.095. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan M, Temel JS, Wright AA, Bernacki R, Selvaggi K, Balboni T. Predicting life expectancy in patients with advanced incurable cancer: a review. The journal of supportive oncology. 2013;11(2):68–74. doi: 10.12788/j.suponc.0004. e-pub ahead of print 2013/08/24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.