Abstract
Altered stress reactivity is a predominant feature of post-traumatic stress disorder (PTSD) and may reflect disease vulnerability, increasing the probability that an individual will develop PTSD following trauma exposure. Environmental factors, particularly prior stress history, contribute to the developmental programming of the hypothalamic-pituitary-adrenal (HPA) stress axis. Critically, the consequences of stress experiences are transgenerational, with parental stress exposure impacting stress reactivity and PTSD risk in subsequent generations. Potential molecular mechanisms underlying this transmission have been explored in rodent models specifically examining the paternal lineage, identifying epigenetic signatures in male germ cells as possible substrates of transgenerational programming. Here, we review the role of these germ cell epigenetic marks, including post-translational histone modifications, DNA methylation, and populations of small non-coding RNAs, in the development of offspring stress axis sensitivity and disease risk.
Keywords: epigenetics, stress, transgenerational, PTSD, HPA axis, animal models
Introduction
PTSD, an anxiety disorder triggered by exposure to a severe traumatic event, presents in only a subset of individuals who experience such a stressor (1). Individual differences in stress sensitivity, primarily regulated by the HPA neuroendocrine stress axis, may be an underlying component of this variability in disease risk (2). In fact, disruption of stress neurocircuitry has been characterized as a pre-trauma vulnerability that increases the probability of developing PTSD, rather than a symptom that develops in response to a stressful experience (3). In particular, PTSD has been associated with a unique stress response profile, including low levels of plasma cortisol and increased noradrenergic and adrenergic activity, although these findings are not universal (4). The development of stress reactivity, and its potential consequences on PTSD predisposition, occurs through a dynamic interplay of genetic and environmental factors (G × E) (5). The role of the environment has been increasingly emphasized, with prior stress exposure highlighted as one of the strongest contributors to later stress sensitivity and PTSD presentation (6).
The behavioral and neuroendocrine consequences of stress exposure, as well as an increase in PTSD risk, have been observed not only in individuals directly exposed to stress, but also in their children (7). Potential mechanisms of this experience-dependent transgenerational transmission, or the reprogramming of offspring behavior and physiology in response to the parental environment, have been investigated in rodent models (8). Studies investigating maternal or paternal experience occurring prior to offspring conception, so-called lifetime exposures, suggest that transmission occurs through an epigenetic reprogramming of germ cells (9–13). In this review, we focus on these parental lifetime stress experiences and their consequences on subsequent generations’ stress reactivity in both humans and animals, and we consider potential molecular mechanisms of transmission, highlighting the role of germ cell epigenetic marks and how these signatures may alter offspring development to confer disease risk or resilience. While maternal stress during pregnancy also impacts offspring stress responses such experience likely does not rely on germ cell reprogramming and has been expertly reviewed elsewhere (see (14; 15)), and thus will not be included in our discussion.
Parental stress and PTSD risk
Epidemiological studies have offered strong evidence supporting altered offspring stress reactivity and neuropsychiatric disease risk following maternal or paternal lifetime stress exposure. For instance, adult children of Holocaust survivors were more likely to be diagnosed with a psychiatric disease such as depression, anxiety, and PTSD (16–18), and offspring disease risk was found to increase similarly following parental exposure to abuse or war-related trauma (19–22). In these studies of parental lifetime stress experience, offspring disease risk may have increased as a consequence of offspring HPA stress axis reprogramming (23–25). In fact, maternal PTSD among Holocaust survivors has been associated with increased offspring sensitivity to glucocorticoid stress hormones as well as with decreased methylation of their glucocorticoid receptor NR3C1 promoter region, both of which correlated with their PTSD risk (7; 26). Notably, the association between parental stress and offspring PTSD may be driven more by the presentation of parental pathology than by the initial parental trauma event (7), suggesting that the stress of chronic disease may also be required to induce offspring neurodevelopmental reprogramming.
Retrospective human studies have historically emphasized parental behavior as the primary mechanism by which lifetime stress experience can alter offspring stress reactivity and mental health (27–29). They have proposed that disease or prior stress exposure alters parentchild relationships so as to increase the level of stress experienced by offspring and thus impact disease presentation, drawing on the well-known relationship of early life stress with later stress axis dysregulation and PTSD risk (30–34). Interestingly, the impact of early childhood experiences has also been characterized as bidirectional, where healthy parental bonding, defined as the perception of low parental control and high affection, has been associated with lower PTSD risk (35). However, the contribution of alternative mechanisms of transgenerational transmission should be considered alongside the role of experience-dependent changes to parental behavior. Though not yet investigated following parental lifetime stress in humans, exposure to smoking and other environmental toxins has been associated with epigenetic changes in mature sperm, suggesting that molecular signatures in germ cells in addition to parental behavior may be poised to pass on information about the parental environment to their offspring (36; 37). The initiation of large-scale longitudinal studies, such as the Avon Longitudinal Study of Parents and Children that has followed approximately 14,500 English children from before birth into adulthood, offers the exciting opportunity to estimate the relative contributions of genetic, behavioral, and epigenetic factors in human transgenerational transmission (38–40).
Animal models of lifetime stress exposures
The first evidence of the transgenerational effects of parental lifetime stress experience in an animal model was reported nearly half a century ago, where exposure of adult female rats to stress before mating altered offspring behavioral stress responses, increasing exploratory behavior in a novel environment through 2 subsequent generations (41). In the years since, significant progress has been made in understanding mechanisms by which parental experience reprograms offspring stress-related behaviors and physiology, afforded by extensive evidence of offspring reprogramming in response to parental lifetime stress. For example, in our model of early prenatal stress, exposure to chronic variable stress in utero increased male HPA stress axis reactivity and altered male stress coping behaviors, including increased immobility in the tail suspension test, and these phenotypes transmitted to the next generation through the male lineage (9; 42). Postnatal stress has also been shown to induce stress dysregulation in subsequent generations, including observations of behavioral deficits on the forced swim task and decreased blood glucose in response to acute restraint in first and second generation offspring of male mice exposed postnatally to unpredictable maternal separation with maternal stress (10; 13; 43; 44). Notably, the transgenerational impact of parental lifetime stress is not restricted to the perinatal window, and changes in offspring stress-related behavior and physiology have been reported following parental exposure stress through adolescence or in adulthood (12; 45; 46). For example, in our lab, male exposure to chronic variable stress either over the pubertal window or only in adulthood programmed a blunted HPA stress axis response in male and female offspring, a stress phenotype reflecting that observed in PTSD (11). While sex-specific effects reported in some rodent models offer the intriguing possibility that parental experience contributes to sex differences in stress responsivity and, in humans, disease risk, the absence of these effects in other models contrasts this hypothesis. Further study of behavioral and physiological phenotypes in both male and female offspring will clarify potential sex-specific vulnerabilities as well as mechanisms by which they may be programmed.
Potential modes of transgenerational transmission have been investigated in rodent models specifically examining the paternal lineage, where the relative exclusion of behavioral and environmental factors affords the mechanistic evaluation of epigenetic marks in sperm, a readily accessible tissue (47). By contrast, transmission through the maternal lineage relies on the complex maternal-fetal/neonatal interaction, where changes in the intrauterine environment, parturition, lactation, and early maternal care may impact stress sensitivity in future generations (48). Few studies have investigated animal models of maternal stress exposure prior to offspring conception (12; 49), likely due to the confounding effects of the maternal milieu and behavior. Additionally, evaluation of potential epigenetic marks in these studies would require superovulation, a hormone-dependent process which may itself change marks in oocytes (50).
In paternal stress studies, epigenetic signatures in sperm have been highlighted as a likely substrate of offspring reprogramming (11; 13; 51), supported by evidence of altered patterns of retained histone modifications, DNA methylation, and/or populations of small noncoding RNAs in germ cells following diverse paternal insults (52–58). Though behaviorally-mediated mechanisms of transmission have been proposed in paternal studies, such as potential shifts in maternal investment in response to a perception of mate quality or the role of paternal behavior (59; 60), laboratory rodents typically are not bi-parental; males do not participate in rearing offspring, and male-female interactions can be limited to defined breeding windows to control for confounding effects of the male’s impact on the dam (47). Further, artificial reproductive techniques including in vitro fertilization and zygote microinjection have been used to directly assess epigenetic transmission through the male germ line, demonstrating the role of sperm epigenetic marks in transgenerational reprogramming (13; 45; 55). Recent development of enzymes capable of site-specific epigenetic modification may offer additional opportunities to investigate the role of specific epigenetic signatures in the sperm in the transgenerational transmission of paternal stress experience (61; 62).
Epigenetic signatures of stress experience
Three modes of epigenetic inheritance in male germ cells—post-translational histone modifications, DNA methylation, and RNA populations—have been proposed as likely substrates of transgenerational transmission, noting their potential ability to both respond to paternal stress experience and reprogram offspring stress reactivity (63). Evidence suggests that these germ cell marks are continuously vulnerable to environmental perturbations, as altered offspring phenotypes following an initial paternal stress experience prenatally, postnatally, in adolescence, or in adulthood have been reported (9; 11; 13; 45; 51). However, the effects of stress experience on the germline likely depend on the timing and duration of exposure. Gestational or early life exposures likely reprogram primordial germ cells in the embryonic gonadal ridge or immature postnatal testis, and insults later in life may instead impact germ cells further along in spermatogenesis (64). Once sperm are fully mature, they are stored in the epididymis where epigenetic marks are thought to be largely impervious to change or to the environment (65), although limited evidence of intercellular communication between mature sperm and the epididymal epithelial cells suggests that small noncoding RNA populations in mature sperm may still be altered (66; 67). In the following sections, we explore evidence of transgenerational transmission through the three classes of epigenetic marks in sperm cells, examining their regulation through spermatogenesis and highlighting potential consequences on offspring reprogramming.
Post-translational histone modifications
The basic unit of chromatin is the nucleosome, which consists of DNA tightly bound to a core of eight histone proteins. Covalent modifications, such as acetylation, methylation, or ubiquitination, of the N-terminus tails of these histone proteins determine the accessibility of chromatin and broadly regulates transcription (68). Until recently, the code of post-translational modifications on paternal histone proteins was considered to be lost during the process of spermatogenesis, when chromatin compaction is achieved through the incorporation of the unique nucleosome proteins known as protamines, suggesting therefore that these epigenetic marks could not directly mediate transgenerational transmission (69; 70). However, studies in C. elegans and Drosophila found that a subset of histone modifications persist through spermatogenesis and fertilization, and may act as a substrate of epigenetic inheritance (71; 72). Notably, a small percentage of histone proteins and their modifications have also been observed in condensed mammalian sperm, with approximately 1% of histone proteins retained in rodents and 10% in humans (73). Histone retention was found to occur preferentially at genomic regions critical to embryogenesis, including developmental gene promoters, microRNA clusters, and imprinted loci, supporting the potential of post-translational histone marks to afford paternal control over early offspring development (73–75). Future studies will need to examine the mechanisms by which paternal stress experience may influence post-translational modifications of retained histone proteins and their ability to alter their offspring’s phenotype.
DNA methylation
Methylation of cytosine residues at cytosine-phosphate-guanine dinucleotides (CpGs), as well as non-CpG sites, in sperm DNA has been characterized as a potential substrate of transgenerational transmission, with reports associating changes in specific or global methylation patterns in sperm with offspring phenotypes following early life stress (10; 44) or adult glucocorticoid administration (51). Paternal exposure to cocaine self-administration or olfactory fear conditioning has been similarly associated with changes in sperm DNA methylation patterns (53; 55). These environmental cues may reprogram DNA methylation in sperm through the direct interaction with germ cell membrane receptors, as has been proposed for cocaine (76; 77), or through activation of complex signaling cascades, perhaps involving the metabolic regulation of spermatogenesis by supportive Sertoli cells (78).
In order to impart offspring programming effects, experience-dependent changes in DNA methylation patterns must be maintained through two waves of global erasure and reprogramming, first immediately following fertilization and the second during primordial germ cell development (79). However, methylation patterns at notable loci, including genetically imprinted regions, can escape erasure, supporting the importance of methylation as a potential mechanism of transgenerational epigenetic transmission (80). While the maintenance of DNA methylation patterns through development is not fully understood, imprinted genes that escape erasure function primarily in embryonic and neonatal growth, perhaps reflecting differential evolutionary pressures in the maternal and paternal lineages (81). Defects in gene imprinting result in Angelman, Prader-Willi, and Beckwith-Weidemann syndromes (82), suggesting both that the careful regulation of DNA methylation is essential for offspring development and that it may be resistant to dynamic regulation by environmental stress. Interestingly, DNA methylation patterns in the early embryo may differ between developing somatic and germ cells (81). Depending on the timing of stress exposure, this distinction may underlie differences in transmission to first and second generation offspring, such as instances when phenotypes appear to skip a generation (i.e., the pattern of methylation affected in somatic cells does not produce a phenotype in the first generation, but affected germ cells giving rise to the second generation seemingly produce a phenotype).
Sperm RNAs
The third class of epigenetic mark implicated in transgenerational transmission, sperm RNA populations, was first proposed in C. elegans, where induction of RNA interference induced gene silencing through four to five generations in the paternal lineage (83). More recently, mRNA and small non-coding RNA populations have been identified in mammalian sperm (84–86), where changes in RNA populations have been implicated in the programming of offspring stress-related phenotypes following paternal stress exposure, including HPA axis hyporeactivity (11; 13). In fact, total RNA isolated from the sperm of stressed sires and injected into zygotes was shown to recapitulate aspects of the offspring phenotype (13). Sperm mRNA populations may mediate transgenerational transmission to some extent (87), however, due to their key role in regulating embryogenesis, small non-coding RNA populations have emerged as a primary mediator of transgenerational reprogramming (88).
Populations of small ~22bp RNA molecules known as microRNA are essential to early embryogenesis, where they regulate the selective degradation of stores of maternal mRNA in the zygote through the activity of the RNA-induced silencing complex (RISC) (88; 89). Loss of Dicer, the protein which preprocesses microRNA and loads them into the RISC, or of Argonaut 2, the functional component of the RISC with endonuclease activity, in mouse oocytes results in early embryonic lethality (90; 91). As each microRNA directly targets hundreds of different mRNAs, disruption of specific microRNA may elicit profound programmatic and developmental effects (92; 93). Recently, the targeted silencing of miR-34, a microRNA present in sperm but not oocytes, was reported to significantly restrict early embryogenesis, supporting a critical role of paternal microRNA in offspring development (94). We have also proposed more subtle, long-term functions of sperm microRNA, associating a significant increase in 9 specific sperm microRNA in a model of paternal stress with an altered HPA stress axis in their adult offspring (11).
Emphasizing the interdependence of epigenetic machinery, the increase in specific microRNA in our model of paternal stress represented a larger transcriptional shift, such as one mediated by underlying histone modification or DNA methylation patterns (11). This crosstalk between microRNA and other epigenetic marks may be crucial for the transgenerational transmission of paternal stress. The genomic loci of sperm microRNA are associated with regions of retained histone proteins, hypomethylation, and the histone mark H3K4me3 (86), suggesting that the expression of sperm microRNA may be controlled by these upstream epigenetic marks. Additionally, post-fertilization, microRNA may program lasting effects by inducing transcriptional activation or repression within the nucleus through other epigenetic mechanisms (95). In fact, microRNA can regulate de novo methylation in embryonic stem cells (96).
Far less is known about the potential role of PIWI-associated RNAs (piRNAs) as a potential mediator of epigenetic inheritance. piRNAs are small ~30bp noncoding RNAs expressed mostly in spermatids at much higher levels than microRNA, and primarily function to silence transposable elements during spermatogenesis (97; 98). piRNAs have been associated with transcriptional silencing in C. elegans through multiple generations (99), but their role in mammalian epigenetic inheritance requires further study (100).
Influence of germ cell marks on offspring neurodevelopment
The mechanisms by which sperm epigenetic marks program lasting consequences on offspring stress reactivity and shape disease susceptibility or resilience remain unclear. In this section, we explore potential pathways by which these molecular signatures may facilitate brain reprogramming; specifically how germ cell marks may influence maturation of the HPA axis and behavioral stress responses. Activation of the HPA axis is critical for the body’s physiological response to homeostatic challenge and, as described earlier, stress axis dysregulation is a predominant feature of neuropsychiatric diseases, including PTSD (7). Importantly, transgenerational effects of parental lifetime stress experience on offspring stress reactivity may be adaptive or maladaptive depending on the environmental context (101).
Epigenetic marks persist from sperm to brain
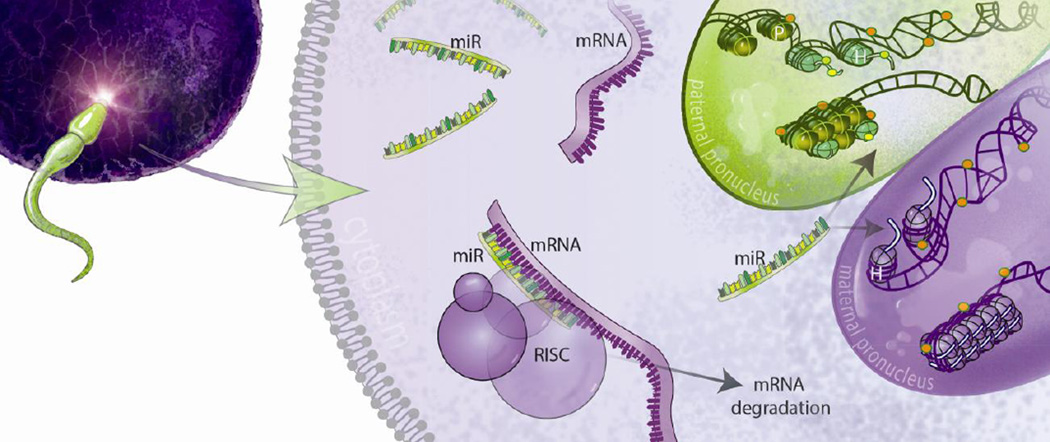
Transgenerational models of parental lifetime exposures first suggested that an initial epigenetic mark in germ cells may persist throughout developmental stages and into the adult brain, where it was directly or indirectly responsible for changes in behavior or physiology (Figure 1). For example, in a mouse model of early life stress, lasting changes in DNA methylation patterns were reported, with the methylation status of 2 specific genes, Crhr2 and Mecp2, altered in both the sperm of the stressed dads and in the brains of their female offspring (10). The persistence of microRNA changes has also been observed, where an increase in miR-339 initially identified in the sperm from stressed sires was similarly detected in their offspring’s hippocampus and associated with offspring behavioral changes (13).
Figure 1. Effects of sperm epigenetic marks following fertilization.
Histone modifications, DNA methylation patterns, and small non-coding RNA populations contained in the mature sperm may direct development in the zygote through transcriptional and post-transcriptional mechanisms, initiating a molecular cascade that may eventually impact the brain. Alternatively, these epigenetic marks may persist through development into the adult brain, exerting continual control over gene regulation. By altering these epigenetic signatures in sperm, environmental exposure to chronic stress can ultimately reprogram offspring behavior and/or physiology, influencing disease risk. Illustration by Jay LeVasseur / www.appliedartstudio.com.
Evidence from models of diverse paternal exposures corroborates the potential of epigenetic marks to persist from the mature sperm into the adult brain observed following early life stress (44; 53–55; 57), and the specificity and permanence of the proposed epigenetic signatures are remarkable. The precise activation of sperm epigenetic machinery at genetic loci involved in stress axis regulation suggests a very specific targeting mechanism that stands in contrast to the more commonly characterized role of epigenetic marks as a broad transcriptional control mechanism (69). However, such limited epigenetic changes reported may actually be representative of more global modifications that were not examined in these studies. Secondly, as gametes fuse to form the zygote, which is a pluripotent cell that gives rise to all differentiating cell lineages, an epigenetic modification that persists from sperm into neurons may be expected to propagate universally (65). Studies have reported correlations in epigenetic signatures between peripheral tissues such as white blood cells and postmortem brain tissues of psychiatric patients (102; 103), supporting the persistence of germ cell changes and offering a potential biomarker of brain epigenetic status or parental experience. However, others have described significant between-tissue variability (104; 105), perhaps because various cell types may not uniformly maintain inherited epigenetic marks across development (106; 107). Such differential maintenance of epigenetic marks may underlie the tissue- or brain region-specific outcomes observed in offspring following parental stress (13; 44).
Sperm epigenome alters the trajectory of offspring development
Alternatively, a germ cell epigenetic mark programmed by parental environmental exposure may initiate a cascade of molecular events in the early zygote, eventually altering offspring development and affecting long-term changes in offspring behavior or physiology (Figure 1). Evidence of dissimilar epigenetic signatures in sperm and offspring brain tissue following paternal perturbation support the notion of a cascading signal (9; 52; 58; 108). For example, our model of paternal stress elicited an increase in microRNA populations in sperm, but did not observe parallel changes in these miRs in key stress regulatory brain regions examined, the paraventricular nucleus and bed nucleus of stria terminalis (11). We instead found a broad reduction in gene expression in these regions, reflecting an as yet unknown upstream epigenetic mechanism in the adult brain that was likely responsible for the reported alterations in offspring HPA axis reactivity (11). Similarly, in a recent study examining the specific role of stress hormones in programming these epigenetic signatures in both sperm and offspring tissues, different methylation patterns were observed in the sire sperm and the offspring hippocampus or kidney following glucocorticoid administration (51).
Mechanistically, it is likely that epigenetic alterations in the zygote subtly shift developmental processes, where slight changes in cell proliferation, migration, or differentiation are the next step in the molecular cascade from the sperm epigenome to offspring brain function (65). The links between a germ cell epigenetic mark and eventual changes in offspring stress responsivity are no doubt complex and currently unknown, though recent evidence of brain microRNA regulating behavioral responses to chronic social defeat has emphasized the continual importance of epigenetic mechanisms in offspring stress susceptibility or resilience (109). A careful step-wise approach can forward our understanding of this cascade of molecular events and offer insight into transgenerational consequences of parental stress experience on offspring disease risk. Evidence of altered HPA axis regulation following maternal or paternal exposure to such diverse challenges as alcohol, lead, chronic stress, and social defeat suggests that the developing hypothalamus, the brain region ultimately responsible for stress axis reactivity, may be particularly susceptible to parental experience (42; 110–115).
Conclusion
It has been estimated that nearly 75% of the population experience a severe traumatic event in their lifetime, yet only a small percentage of these individuals will subsequently present with PTSD (116). Parental lifetime stress exposures, likely communicated to offspring through epigenetic marks in germ cells, contribute to the programming of subsequent generations stress axis development and reactivity, factors critical in individual disease vulnerability to PTSD, as highlighted in this special issue, but equally relevant to a wider array of neuropsychiatric diseases. In this light, the role of experience in conferring disease risk or resilience spans wider than the lifetime of an individual to encompass the lifetime of stress experienced by his or hers parents or grandparents, and perhaps generations before that. A growing appreciation for epigenetic inheritance as a source of individual variability will influence the future of clinical diagnosis and treatment of PTSD, affording the identification of at-risk individuals and allowing preventative or early intervention following trauma exposure.
Acknowledgements
Studies discussed in this review were funded in part by grants from the National Institutes of Health (NIH): MH073030, MH091258, MH087597, MH099910, and MH104184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
All authors report no biomedical financial interests or potential conflicts of interest.
References Cited
- 1.Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62:628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 4.Fink G. Stress controversies: post-traumatic stress disorder, hippocampal volume, gastroduodenal ulceration*. J Neuroendocrinol. 2011;23:107–117. doi: 10.1111/j.1365-2826.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- 5.Mehta D, Binder EB. Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology. 2012;62:654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- 7.Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, et al. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry. 2014;171:872–880. doi: 10.1176/appi.ajp.2014.13121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK. Nature, nurture and epigenetics. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan CP, Bale TL. Early Prenatal Stress Epigenetically Programs Dysmasculinization in Second-Generation Offspring via the Paternal Lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaidan H, Leshem M, Gaisler-Salomon I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol Psychiatry. 2013;74:680–687. doi: 10.1016/j.biopsych.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48C:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin Neurosci. 2014;16:297–305. doi: 10.31887/DCNS.2014.16.3/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, Duvdevani T. Vulnerability to posttraumatic stress disorder in adult offspring of Holocaust survivors. Am J Psychiatry. 1998;155:1163–1171. doi: 10.1176/ajp.155.9.1163. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda R, Halligan SL, Bierer LM. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. Journal of Psychiatric Research. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 18.Scharf M. Long-term effects of trauma: psychosocial functioning of the second and third generation of Holocaust survivors. Dev Psychopathol. 2007;19:603–622. doi: 10.1017/S0954579407070290. [DOI] [PubMed] [Google Scholar]
- 19.Dubowitz H, Black MM, Kerr MA, Hussey JM, Morrel TM, Everson MD, Starr RH. Type and timing of mothers' victimization: effects on mothers and children. Pediatrics. 2001;107:728–735. doi: 10.1542/peds.107.4.728. [DOI] [PubMed] [Google Scholar]
- 20.Miranda JK, la Osa de N, Granero R, Ezpeleta L. Maternal experiences of childhood abuse and intimate partner violence: Psychopathology and functional impairment in clinical children and adolescents. Child Abuse Negl. 2011;35:700–711. doi: 10.1016/j.chiabu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Rieder H, Elbert T. The relationship between organized violence, family violence and mental health: findings from a community-based survey in Muhanga, Southern Rwanda. Eur J Psychotraumatol. 2013;4 doi: 10.3402/ejpt.v4i0.21329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palosaari E, Punamäki R-L, Qouta S, Diab M. Intergenerational effects of war trauma among Palestinian families mediated via psychological maltreatment. Child Abuse Negl. 2013;37:955–968. doi: 10.1016/j.chiabu.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van IJzendoorn MH, Bakermans-Kranenburg MJ, Sagi-Schwartz A. Are children of Holocaust survivors less well-adapted? A meta-analytic investigation of secondary traumatization. J Trauma Stress. 2003;16:459–469. doi: 10.1023/A:1025706427300. [DOI] [PubMed] [Google Scholar]
- 25.van Zuiden M, Kavelaars A, Geuze E, Olff M, Heijnen CJ. Predicting PTSD: pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain Behav Immun. 2013;30:12–21. doi: 10.1016/j.bbi.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Lehrner A, Bierer LM, Passarelli V, Pratchett LC, Flory JD, Bader HN, et al. Maternal PTSD associates with greater glucocorticoid sensitivity in offspring of Holocaust survivors. Psychoneuroendocrinology. 2014;40:213–220. doi: 10.1016/j.psyneuen.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belsky J. Etiology of child maltreatment: a developmental-ecological analysis. Psychol Bull. 1993;114:413–434. doi: 10.1037/0033-2909.114.3.413. [DOI] [PubMed] [Google Scholar]
- 28.Oliver JE. Intergenerational transmission of child abuse: rates, research, and clinical implications. Am J Psychiatry. 1993;150:1315–1324. doi: 10.1176/ajp.150.9.1315. [DOI] [PubMed] [Google Scholar]
- 29.Schofield TJ, Lee RD, Merrick MT. Safe, stable, nurturing relationships as a moderator of intergenerational continuity of child maltreatment: a meta-analysis. J Adolesc Health. 2013;53:S32–S38. doi: 10.1016/j.jadohealth.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. Journal of Psychiatric Research. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Provencal N, Binder EB. The neurobiological effects of stress as contributors to psychiatric disorders: focus on epigenetics. Curr Opin Neurobiol. 2014;30C:31–37. doi: 10.1016/j.conb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci USA. 2013;110:8302–8307. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima AR, Mello MF, Andreoli SB, Fossaluza V, de Araújo CM, Jackowski AP, et al. The impact of healthy parenting as a protective factor for posttraumatic stress disorder in adulthood: a case-control study. PLoS ONE. 2014;9:e87117. doi: 10.1371/journal.pone.0087117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Li M, Liu Y, Song G, Liu N. A Microarray for MicroRNA Profiling in Spermatozoa from Adult Men Living in an Environmentally Polluted Site. Bull Environ Contam Toxicol. 2012;89:1111–1114. doi: 10.1007/s00128-012-0827-0. [DOI] [PubMed] [Google Scholar]
- 37.Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7:432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 38.Golding J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC) West Engl Med J. 1990;105:80–82. [PMC free article] [PubMed] [Google Scholar]
- 39.Golding J ALSPAC Study Team. The Avon Longitudinal Study of Parents and Children (ALSPAC)--study design and collaborative opportunities. Eur J Endocrinol. 2004;151(Suppl 3):U119–U123. doi: 10.1530/eje.0.151u119. [DOI] [PubMed] [Google Scholar]
- 40.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the “children of the 90s--”the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehmer F, Porter RH, Scales B. Pre-mating and pregnancy stress in rats affects behaviour of grandpups. Nature. 1970;227:622. doi: 10.1038/227622a0. [DOI] [PubMed] [Google Scholar]
- 42.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franklin TB, Linder N, Russig H, Thöny B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS ONE. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohacek J, Farinelli M, Mirante O, Steiner G, Gapp K, Coiret G, et al. Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.80. [DOI] [PubMed] [Google Scholar]
- 45.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. Paternal Transmission of Stress-Induced Pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saavedra-Rodríguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry. 2013;73:44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rando OJ. Daddy Issues: Paternal Effects on Phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bock J, Poeschel J, Schindler J, Börner F, Shachar-Dadon A, Ferdman N, et al. Transgenerational sex-specific impact of preconception stress on the development of dendritic spines and dendritic length in the medial prefrontal cortex. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0940-4. [DOI] [PubMed] [Google Scholar]
- 50.Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Periimplantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90:26. doi: 10.1095/biolreprod.113.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petropoulos S, Matthews SG, Szyf M. Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring. Biol Reprod. 2014;90:43. doi: 10.1095/biolreprod.113.115899. [DOI] [PubMed] [Google Scholar]
- 52.Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, et al. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 2014;345:1255903–1255903. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16:42–47. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Comms. 2013;4 doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2013;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerrero-Bosagna C, Weeks S, Skinner MK. Identification of Genomic Features in Environmentally Induced Epigenetic Transgenerational Inherited Sperm Epimutations. In: Ward WS, editor. PLoS ONE. Vol. 9. 2014. p. e100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milekic MH, Xin Y, O’Donnell A, Kumar KK, Bradley-Moore M, Malaspina D, et al. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.84. [DOI] [PubMed] [Google Scholar]
- 58.Fullston T, Ohlsson Teague EMC, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. The FASEB Journal. 2013;27:4226–4243. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 59.Mashoodh R, Franks B, Curley JP, Champagne FA. Paternal social enrichment effects on maternal behavior and offspring growth. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17232–17238. doi: 10.1073/pnas.1121083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braun K, Champagne FA. Paternal influences on offspring development: behavioural and epigenetic pathways. J Neuroendocrinol. 2014;26:697–706. doi: 10.1111/jne.12174. [DOI] [PubMed] [Google Scholar]
- 61.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31:1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Godmann M, Lambrot R, Kimmins S. The dynamic epigenetic program in male germ cells: Its role in spermatogenesis, testis cancer, and its response to the environment. Microsc Res Tech. 2009;72:603–619. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- 65.Gannon JR, Emery BR, Jenkins TG, Carrell DT. The sperm epigenome: implications for the embryo. Adv Exp Med Biol. 2014;791:53–66. doi: 10.1007/978-1-4614-7783-9_4. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 67.Belleannée C, Calvo É, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- 68.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 69.Jenkins TG, Carrell DT. The sperm epigenome and potential implications for the developing embryo. Reproduction. 2012;143:727–734. doi: 10.1530/REP-11-0450. [DOI] [PubMed] [Google Scholar]
- 70.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287– 301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 71.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seong K-H, Li D, Shimizu H, Nakamura R, Ishii S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell. 2011;145:1049–1061. doi: 10.1016/j.cell.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 73.Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 74.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatewood JM, Cook GR, Balhorn R, Bradbury EM, Schmid CW. Sequence-specific packaging of DNA in human sperm chromatin. Science. 1987;236:962–964. doi: 10.1126/science.3576213. [DOI] [PubMed] [Google Scholar]
- 76.Yazigi RA, Odem RR, Polakoski KL. Demonstration of specific binding of cocaine to human spermatozoa. JAMA. 1991;266:1956–1959. [PubMed] [Google Scholar]
- 77.Meizel S. The sperm, a neuron with a tail: “neuronal” receptors in mammalian sperm. Biol Rev Camb Philos Soc. 2004;79:713–732. doi: 10.1017/s1464793103006407. [DOI] [PubMed] [Google Scholar]
- 78.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–338. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- 79.Toth M. Mechanisms of Non-Genetic Inheritance and Psychiatric Disorders. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–452. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawson HA, Cheverud JM, Wolf JB. Genomic imprinting and parent-of-origin effects on complex traits. Nat Rev Genet. 2013;14:609–617. doi: 10.1038/nrg3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 84.Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond MP. A survey of small RNAs in human sperm. Hum Reprod. 2011;26:3401–3412. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawano M, Kawaji H, Grandjean V, Kiani J, Rassoulzadegan M. Novel small noncoding RNAs in mouse spermatozoa, zygotes and early embryos. PLoS ONE. 2012;7:e44542. doi: 10.1371/journal.pone.0044542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA. Stability, delivery and functions of human sperm RNAs at fertilization. Nucleic Acids Research. 2013;41:4104–4117. doi: 10.1093/nar/gkt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 88.Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12:136–149. doi: 10.1038/nrg2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 91.Lykke-Andersen K, Gilchrist MJ, Grabarek JB, Das P, Miska E, Zernicka-Goetz M. Maternal Argonaute 2 is essential for early mouse development at the maternal-zygotic transition. Mol Biol Cell. 2008;19:4383–4392. doi: 10.1091/mbc.E08-02-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 94.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts TC. The MicroRNA Biology of the Mammalian Nucleus. Mol Ther Nucleic Acids. 2014;3:e188. doi: 10.1038/mtna.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–267. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 97.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–1714. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- 99.Ashe A, Sapetschnig A, Weick E-M, Mitchell J, Bagijn MP, Cording AC, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu Q, Wang PJ. Mammalian piRNAs: Biogenesis, function, and mysteries. Spermatogenesis. 2014;4:e27889. doi: 10.4161/spmg.27889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 102.Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Human Molecular Genetics. 2011;20:4786–4796. doi: 10.1093/hmg/ddr416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13:R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shipony Z, Mukamel Z, Cohen NM, Landan G, Chomsky E, Zeliger SR, et al. Dynamic and static maintenance of epigenetic memory in pluripotent and somatic cells. Nature. 2014;513:115–119. doi: 10.1038/nature13458. [DOI] [PubMed] [Google Scholar]
- 107.Li R, Mav D, Grimm SA, Jothi R, Shah R, Wade PA. Fine-tuning of epigenetic regulation with respect to promoter CpG content in a cell type-specific manner. Epigenetics. 2014;9:747–759. doi: 10.4161/epi.28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 111.Hellemans KGC, Verma P, Yoon E, Yu W, Weinberg J. Prenatal alcohol exposure increases vulnerability to stress and anxiety-like disorders in adulthood. Ann N Y Acad Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 113.Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicology. 2006;27:11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. 2008;149:6406–6415. doi: 10.1210/en.2008-0347. [DOI] [PubMed] [Google Scholar]
- 115.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Breslau N, Kessler RC. The stressor criterion in DSM-IV posttraumatic stress disorder: an empirical investigation. Biol Psychiatry. 2001;50:699–704. doi: 10.1016/s0006-3223(01)01167-2. [DOI] [PubMed] [Google Scholar]