Abstract
Better treatments are needed for patients with diffuse large B-cell lymphoma (DLBCL) at high risk of failing standard therapy. Avoiding apoptosis is a hallmark of cancer, and in DLBCL the redundantly functioning anti-apoptotic proteins BCL2 and MCL1 are frequently expressed. Here, we explore drugs that cause loss of MCL1, particularly the potent new cyclin-dependent kinase inhibitor dinaciclib, which knocks down MCL1 by inhibiting CDK9. Dinaciclib induces apoptosis in DLBCL cells but is completely overcome by increased activity of BCL2. We find clinical samples have frequent co-expression of MCL1 and BCL2, suggesting therapeutic strategies targeting only one will lead to treatment failures due to activity of the other. The BH3 mimetic ABT-199 potently and specifically targets BCL2. Single-agent ABT-199 had modest anti-tumor activity against most DLBCL lines and resulted in compensatory up-regulation of MCL1 expression. ABT-199 synergized strongly, however, when combined with dinaciclib and with other drugs affecting MCL1, including standard DLBCL chemotherapy drugs. We show potent anti-tumor activities of these combinations in xenografts and in a genetically accurate murine model of MYC-BCL2 double-hit lymphoma. In sum, we reveal a rational treatment paradigm to strip DLBCL of its protection from apoptosis and improve outcomes for high-risk patients.
INTRODUCTION
DLBCL is the most common aggressive non-Hodgkin lymphoma, making up ~30 percent of lymphoma diagnoses in western countries. Up-front chemoimmunotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) generates long-term disease-free survival in ~60% of patients.1–3 Relapsed or refractory patients, however, have poor prognosis, with only ~10% ultimately achieving cure, requiring aggressive salvage chemotherapy and transplant consolidation.4 Patients at high risk of failing R-CHOP can be identified before treatment with the International Prognostic Index (IPI) risk score, gene-expression profiling to determine cell of origin (COO), and immunohistochemical staining patterns, among other methods.5 Clinical efforts to improve outcome for these patients have largely involved intensification, modification, or replacement of the CHOP backbone.6 Though such alternatives may be offered by particular practitioners, none is recognized as a separate standard of care for high-risk disease, and prognosis for high-risk patients remains markedly compromised in the post-rituximab era.7
Though DLBCL has two major COO subtypes with disparate pathogenesis, recent clinicopathologic studies suggest mechanisms underlying high-risk disease are more unified. For example, co-expression of c-MYC and BCL2 detected by immunohistochemistry (IHC) is a negative prognostic finding independent of COO.8,9 A study of 893 cases highlighted increased frequency of MYC-BCL2 co-expression in the activated B-cell (ABC) subtype being one possible reason for its worse prognosis compared to the germinal center B-cell (GCB) subtype.10 Additionally, an elegant analysis by Monti et al. found cases carrying complex patterns of cytogenetic alterations had dramatically worse prognosis, and this again was independent of COO.11
Apoptotic defects are required for tumorigenesis,12 and in DLBCL the best annotated anti-apoptotic mechanism in clinical samples is over-expression of BCL2 or its functionally redundant family member MCL1. BCL2 and MCL1 are part of the BCL2 protein family, which regulates activation of the intrinsic apoptosis pathway, in which release of cytochrome C from mitochondria triggers a protease cascade ending in cell death.13 BCL2 and MCL1 both suppress apoptosis by sequestering the BH3-only protein BIM, which activates mitochondrial outer membrane permeabilization by the multi-domain pro-apoptotic proteins BAK and BAX. BCL2 is expressed in 40-80% of DLBCL, due to t(14;18)(q32;q21) found in 15-30% of cases, and through additional mechanisms that are not well defined.8–10,14 Frequent MCL1 expression in DLBCL, meanwhile, has been recognized for some time but was only recently quantified in a larger case series, showing IHC positivity in 50% of ABC and 30% of GCB tumors.15
In this study, we tested the potent and specific multi-CDK inhibitor dinaciclib16 and found broad ability to trigger apoptosis in DLBCL cell lines associated with lost MCL1 protein due to CDK9 inhibition. Correspondingly, BCL2 over-expression eliminated the activity of dinaciclib, and examination of BCL2 and MCL1 protein expression revealed DLBCL clinical samples can express either or both at high levels. We hypothesized combined targeting of MCL1 expression with dinaciclib and BCL2 activity with the third-generation BH3 mimetic ABT-199 would show greater anti-tumor activity than either alone. We found potent synergy in vitro and in vivo of this combination against both xenografted high-risk DLBCL cell lines and in an immunocompetent mouse model of MYC-BCL2 double-hit lymphoma. We extended our findings to combinations of ABT-199 with chemotherapy drugs that affect MCL1, revealing multiple potential therapeutic combinations that could be evaluated in patients.
MATERIALS AND METHODS
Cell Lines
Cell-culture conditions are described in Supplementary Data on the Leukemia website. All human DLBCL lines were subjected to short-tandem-repeat (STR) fingerprinting as described,17 with results compared to public databases. STR results are provided as Table S1.
Drugs
Dinaciclib, doxorubicin, etoposide, cytarabine, flavopiridol, SNS-032, and PHA-767491 were purchased from Selleck Chemicals (Houston, TX). ABT-199 was kindly provided by AbbVie Inc. (North Chicago, IL).
Overexpression of BCL2 and MCL1 and selection
BCL2 and MCL1 cDNAs were purchased from DNASU Plasmid Repository (Tempe, AZ) and cloned to pMIG vector. SU-DHL-4, TMD8, Riva, and U2932 cells were engineered to express the murine ecotropic receptor as previously described to permit efficient retroviral introduction (reagents a kind gift of the Staudt laboratory).18 Cells were treated with dinaciclib, ABT-199, or combination for 24 h and allowed to recover. GFP expression was assessed before drug treatment (initial infection) and after drug recovery through FACS analysis (Guava EasyCyte; Millipore). Data are reported as fold change in GFP in the main figures, with raw GFP results provided in the supplemental data.
Immunohistochemistry
Tumors from mice were prepared for H&E, Ki67, MCL1, and cleaved caspase-3 staining by the University of Arizona Cancer Center Tissue Acquisition Cellular/Molecular Analysis Shared Resource (TACMASR) using standard techniques. MCL1 IHC was performed as described.15 Staining intensity was scored by expert lymphoma hematopathologist (L.M.R.) as 0-3 per core, and the score for each case represents an average of 2 cores. We defined score categories as negative (<1), intermediate (1), and high (>1).
Viability and Apoptosis
Cells plated at 5,000 per well were exposed to serial drug dilutions and assessed for viability using CellTiter-Glo reagent (Promega, Madison, WI) by manufacturer's protocol. Apoptosis was assessed by flow cytometry on Guava EasyCyte using the Guava Nexin Reagent (Millipore, MA) by manufacturer's protocol.
Quantitative real-time PCR
RNA was extracted using RNeasy Mini Kit according to the manufacturer's specifications (Qiagen). cDNAs were synthesized by using TaqMan Reverse Transcription Reagents as directed by the protocol provided by the manufacturer (Life technologies, CA). Q-PCR was carried out using TaqMan Gene Expression Assays (Life technologies) and the Applied Biosystems 7500 Real-Time PCR System. Cycle threshold (Ct) values were calculated for each gene, and normalized to the reference gene GAPDH, and relative gene expression was determined using the ddCt method.
In Vivo Studies
All animal experiments were conducted in accordance with guidelines of the University of Arizona Institutional Animal Care and Use Committee (IACUC). Female severe combined immunodeficient (SCID) mice were flank injected with 2×106 cells. Tumors were generated using VavP-Bcl2 transgenic HSCs as previously described.19–21 Tumor-bearing mice for drug treatment were generated via tail-vein injection of tumor cells from primary mice to secondary recipients following 3G preparative irradiation. Drug administration protocols are described in Supplementary Data on the Leukemia website. Tumors dimensions were measured twice a week with digital calipers; volume = (minimum diameter)2 × (maximum diameter)/2. Mice were sacrificed if tumor volume reach 2000 mm3 or at the end of the experiment.
Statistical analyses
Statistical analyses were performed in the GraphPad Prism software version 6. Unless otherwise indicated, results indicate mean ± SEM of three independent experiments. p<0.05 was considered statistically significant, annotated throughout: *p<0.05; **p<0.01; ***p<0.001. Gene expression was normalized to GAPDH by using the ddCt method, and compared to IC50 (Log10) of dinaciclib by linear regression analysis. For drug-synergy assessment, combination index (CI) vs. fractional kill (Fa) curves were generated by CompuSyn version 1.0. CI results from CompuSyn at multiple Fa values for all combinations are in Table S2.
RESULTS
Dinaciclib Treatment of DLBCL Causes Rapid Loss of MCL1 and Apoptosis In Vitro and In Vivo
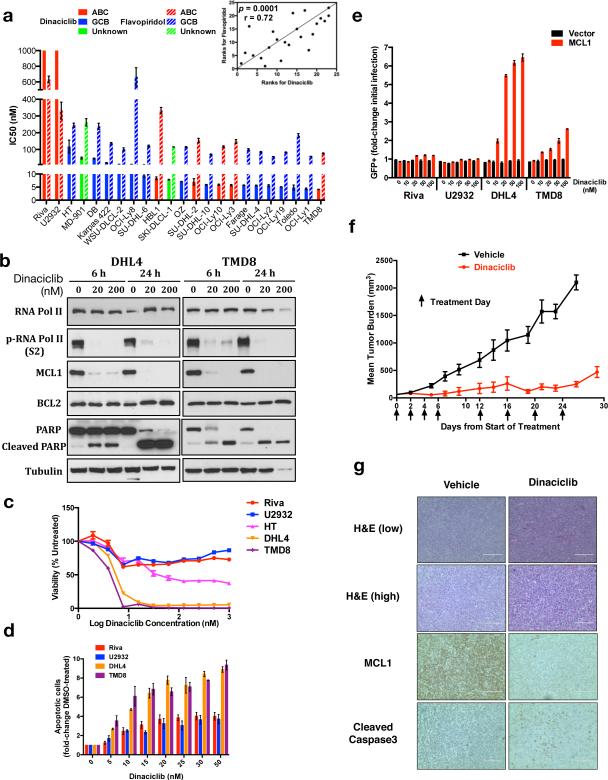
We tested 23 DLBCL cell lines for sensitivity to dinaciclib and compared its activity to that of flavopiridol.22 Because of dinaciclib's short half-life in vivo,16 we assessed viability at 24 hours. Overall, sensitivity to both drugs was independent of COO, and dinaciclib was significantly more potent (Figure 1a). Sensitivity patterns between the two drugs, however, are highly correlated (p=0.0001, Figure 1a inset). Multiple prior studies have shown flavopiridol and other CDK inhibitors including dinaciclib promote apoptosis of tumor cells due to decline of MCL1 protein resulting from inhibition of CDK9, which phosphorylates RNA polymerase II (RNAP II) to promote transcriptional elongation.23–26 Dinaciclib's in vivo antitumor activity in particular is known to be MCL1-dependent because of pharmacokinetic properties producing limited clinical duration of exposure.27 We investigated the time- and dose-dependent effects of dinaciclib and found exposure of either SU-DHL-4 (sensitive GCB cells) or TMD8 (sensitive ABC cells) to dinaciclib for 6 hours results in loss of phospho-RNAP II and MCL1 protein, while more stable BCL2 was unaffected even at 200 nM for 24 hours (Figure 1b). Significant PARP cleavage suggests induction of apoptosis. Sensitive lines showed complete loss of viability in response to dinaciclib, while the resistant lines U2932 and Riva and an intermediate-sensitive line, HT, displayed initial decreases that stabilized, consistent with a cytostatic effect (Figure 1c). Correspondingly, flow cytometry analysis showed dramatically increased apoptotic induction at 24 hours in sensitive compared to resistant cells (Figure 1d). Introduction of MCL1 with GFP co-expression to sensitive SU-DHL-4 and TMD8 cells and resistant U2932 and Riva cells followed by dinaciclib treatment results in potent GFP enrichment in sensitive lines only (Figure 1e, S1a-c). Dinaciclib therefore is potent in vitro against both GCB and ABC DLBCL associated with rapid loss of MCL1 and apoptosis, while forced MCL1 over-expression provides a selective advantage to sensitive cells during drug exposure.
Figure 1. Dinaciclib promotes loss of MCL1 and apoptotic death of DLBCL in vitro and in vivo.
(a) IC50s were calculated with non-linear curve-fit regression after exposure of 23 DLBCL cell lines to serially diluted dinaciclib or flavopiridol for 24 hours. Spearman correlation coefficient method compared results between the two drugs (inset). (b) SU-DHL-4 and TMD8 were exposed to 1:1000 DMSO or dinaciclib at the indicated times and concentrations and blotted as indicated. (c) Viability curves of selected lines after 24 hours’ dinaciclib exposure. (d) Apoptotic induction of selected cells. Cells were seeded at equal density and exposed as indicated to 1:1000 DMSO (0) or dinaciclib for 24 hours, then prepared with Guava Nexin Reagent, and analyzed by flow cytometry. (e) Fold change GFP+ cells in lines infected with MCL1 or empty vector after recovery from 1:1000 DMSO (0) or dinaciclib at the indicated concentrations for 24 hours. (f) Mean tumor volumes ± SEM of SU-DHL-4 flank tumors during treatment with vehicle or dinaciclib (n=9 per group). (g) Pathologic assessments of tumors harvested on day 16 four hours after treatment, imaged with EVOS XL Cell Imaging System (Life Technologies), scale bars 200 μm (H&E low), 100 μm (all others). (a, c) Mean of quadruplicates ± SEM. (d, e) Mean ± SEM of triplicates.
To further assess dinaciclib's therapeutic potential, we xenografted SU-DHL-4 cells to SCID mice, pair-matched animals by tumor size after engraftment, and treated them with dinaciclib vs. vehicle (n=9 per group). We dosed dinaciclib every other day (20+20 mg/kg split dosing)28 on the indicated days, resulting in dramatically reduced tumor burden compared to vehicle (Figure 1f). There were no major differences in mean weight (Figure S1d). We sacrificed two animals from each group on day 16, four hours after drug or vehicle dosing, for pathologic assessment. Dinaciclib-treated tumors appear more disorganized and necrotic on standard H&E (Figure 1g). IHC shows dramatic MCL1 decrease in drug-treated animals associated with increased cleaved caspase-3. Dinaciclib thus generates significant anti-tumor activity in vivo against DLBCL cells sensitive to the drug in vitro, associated with loss of MCL1 and induction of apoptosis.
BCL2 Activity Mediates Resistance to Dinaciclib in DLBCL
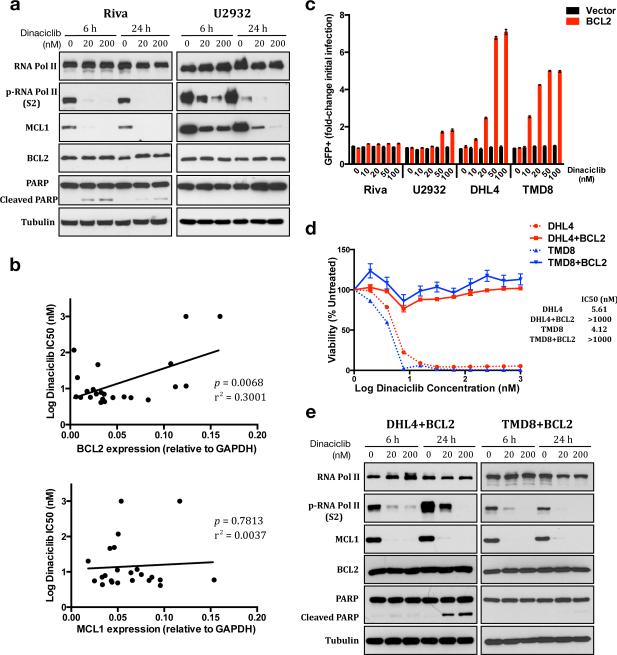
To understand the basis of dinaciclib resistance, we assessed its effects on resistant lines. Like sensitive lines, Riva and U2932 cells experience loss of phospho-RNAP II and MCL1 but greatly reduced or absent PARP cleavage (Figure 2a). As in the sensitive lines, BCL2 levels were not affected. Lymphoma cells dependent on multi-domain anti-apoptotic proteins, usually BCL2 or MCL1, have been described as “poised for death,” because the intrinsic apoptotic pathway is intact and ready to trigger without the protection they provide.29 Our data show MCL1 loss due to dinaciclib is sufficient to trigger apoptosis in some DLBCL cell lines but not others. We hypothesized BCL2's ability to compensate functionally for MCL1 is a major factor in determining sensitivity. We determined relative expression of MCL1 and BCL2 by qPCR in the 23 DLBCL cell lines and tested for association with dinaciclib sensitivity. Increased BCL2 but not MCL1 expression associated strongly with increased dinaciclib IC50 (p=0.0068, Figure 2b). Association between mRNA and protein levels was strong for BCL2, but less so for MCL1, whose protein levels are translationally regulated (Figure S2a).30,31 We retrovirally introduced BCL2-GFP to sensitive and resistant cells and treated them with dinaciclib for 24 hours. After recovery, sensitive cells showed strong enrichment of GFP-positive populations in contrast to resistant Riva or U2932 cells (Figure 2c, S2b-c). Consistently, we isolated 100% BCL2-GFP+ SU-DHL-4 and TMD8 cells and found them insensitive to dinaciclib, showing a viability response similar to Riva and U2932 (Figure 2d). BCL2 activity alone therefore can eliminate the anti-tumor effects of dinaciclib in sensitive cells. Like parent cells, the BCL2 over-expressing cells lost MCL1 protein in response to dinaciclib but had greatly reduced PARP cleavage (Figure 2e). Taken together, our data highlight key therapeutic implications of the ability of BCL2 and MCL1 to compensate for each other functionally in protecting cells from apoptosis.
Figure 2. Increased BCL2 eliminates dinaciclib's anti-DLBCL activity.
(a) Riva and U2932 cells were exposed to 1:1000 DMSO or dinaciclib at the indicated times and concentrations and blotted as indicated. (b) Expression of BCL2 and MCL1 in 23 DLBCL cell lines, normalized to GAPDH, and compared to IC50 (Log10) of dinaciclib using linear regression analysis. (c) Fold change GFP+ cells in lines infected with BCL2 or vector after recovery from 1:1000 DMSO (0) or dinaciclib at the indicated concentrations for 24 hours. (d) Comparison of cell viability after 24 hours dinaciclib exposure between parental and BCL2-over-expressing SU-DHL-4 and TMD8 cells. Mean of quadruplicates ± SEM. (e) BCL2-over-expressing SU-DHL-4 and TMD8 cells were exposed to 1:1000 DMSO or dinaciclib at the indicated times and concentrations and blotted as indicated. (b and c) Mean ± SEM of triplicates.
Co-Expression of MCL1 and BCL2 is Frequent in DLBCL Clinical Tumor Samples
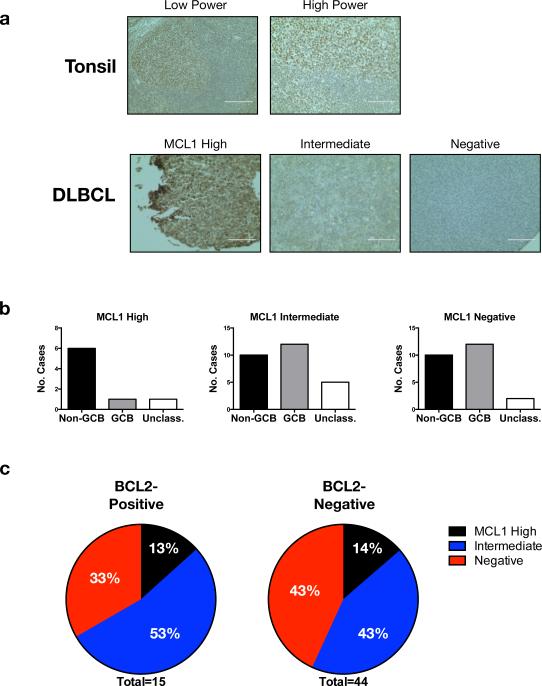
While MCL1 or BCL2 expression in DLBCL clinical samples has been described, the relationship between the two, to our knowledge, has not been analyzed. We performed MCL1 protein staining on tissue microarrays containing DLBCL cases for which BCL2 IHC results have been reported.32 We scored MCL1 as high, intermediate, or negative (Figure 3a). Consistent with a recent report,15 we found higher levels of MCL1 were more common in non-GCB cases (Figure 3b). Of 59 cases stained successfully for both BCL2 and MCL1, 15 were BCL2 IHC positive, defined as ≥ 50% of tumor cells positive using the standard monoclonal mouse 124 antibody.32 MCL1 staining was not different between BCL2-positive and -negative cases, with 10/15 (67%) of BCL2-positive and 25/44 (57%) of BCL2-negative cases being either MCL1 high or intermediate (p=0.5576, Figure 3c). Therefore, despite the redundant antiapoptotic functions of MCL1 and BCL2, there is no indication of mutually exclusive expression in DLBCL, with cases able to express either or both together at high levels.
Figure 3. Concurrent expression of MCL1 and BCL2 in DLBCL cases.
(a) MCL1 IHC of non-malignant tonsil and representative DLBCL TMA cores scored high, intermediate, and negative. Imaged with EVOS XL Cell Imaging System (Life Technologies), scale bars 200 μm (tonsil low power), 100 μm (others). (b) Distribution of MCL1 IHC results by COO. (c) Distribution of MCL1 IHC results in BCL2 IHC-positive (left) and -negative (right) cases.
Combined Targeting of MCL1 and BCL2 with Dinaciclib and ABT-199 is Highly Effective Against DLBCL in Vitro and in Vivo
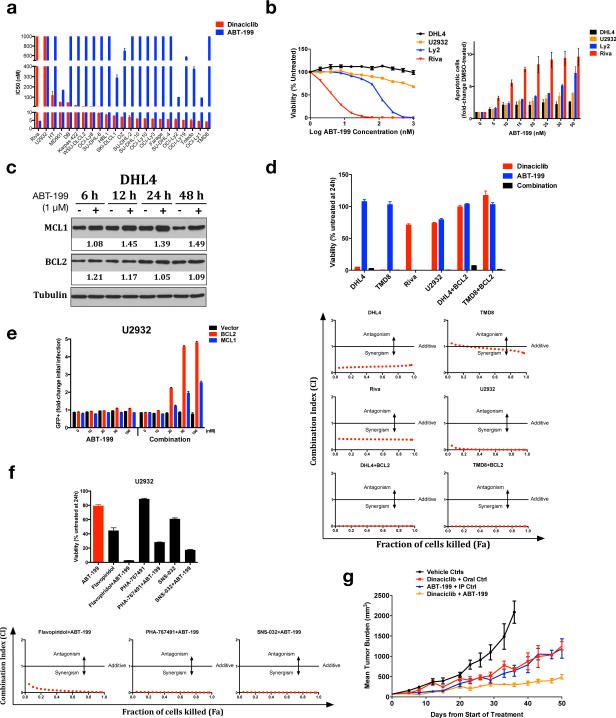
These data suggest targeting either MCL1 or BCL2 alone in DLBCL will select for cell populations able to express the other, leading to treatment failure, but combined targeting of both simultaneously may be more effective. ABT-199 is a BH3 mimetic in clinical trials that potently inhibits BCL2 while avoiding off-target activity against other BCL2 family members, particularly BCL-XL. Thrombocytopenia due to off-target activity against BCL-XL limited the clinical utility of ABT-199's predecessor ABT-263 (navitoclax).33 We evaluated ABT-199 against our DLBCL lines and found generally lower potency than dinaciclib (Figure 4a). Viability curves show rapid decline in sensitive cells as they reach IC50 but no effect on resistant cells, and sensitive cells are highly apoptotic after 24 hours' ABT-199 exposure, in contrast to resistant cells (Figure 4b). SU-DHL-4 contains t(14;18) and previously was reported to be BCL2-dependent based on BH3 profiling,29 but recently published data, like ours, showed insensitivity to BH3 mimetics.33 To further evaluate compensation between BCL2 and MCL1, we incubated SU-DHL-4 cells with and without ABT-199 and found drug-induced MCL1 protein up-regulation starting within six hours of exposure as well as BCL2 up-regulation to a lesser extent (Figure 4c).
Figure 4. Combined targeting of MCL1 and BCL2 is highly effective against DLBCL in vivo and in vitro.
(a) IC50s (mean of quadruplicates ± SEM) were calculated with non-linear curve-fit regression after exposure of 23 DLBCL cell lines to serially diluted ABT-199 for 24 hours. Dinaciclib results shown for comparison. (b) Viability curves and apoptotic induction of selected lines after 24 hours’ ABT-199 exposure. Cells were seeded at equal density and exposed to serially diluted ABT-199 for cell viability assay (mean of quadruplicates ± SEM), or exposed as indicated to 1:1000 DMSO (0) or ABT-199 for apoptosis assay (mean ± SEM of triplicates). (c) Western blot of SU-DHL-4 cells after incubation for the indicated times with 1:1000 DMSO (−) or 1 μM ABT-199. Drug-treated band intensities were quantified relative to the corresponding DMSO bands, following normalization to tubulin loading controls. (d) Viability was assessed in the indicated lines following 24 hours’ exposure to 250 nM dinaciclib, ABT-199, or the combination (mean of quadruplicates ± SEM); CompuSyn CI vs. Fa plots for dinaciclib + ABT-199 in the six lines. (e) Fold change in percentage of GFP+ cells in U2932 cells infected with BCL2, MCL1, or vector after recovery from 1:1000 DMSO (0), ABT-199, or dinaciclib+ABT-199 combination at the indicated concentrations for 24 hours. (f) Viability at 250 nM and CI vs. Fa for U2932 following 24 hours exposure to ABT-199, additional drugs with activity against CDK9, or the combinations. Mean of quadruplicates ± SEM. (g) U2932 cells were xenografted to SCID mice and treated with IP vehicle/PO vehicle (control group, n=5), IP dinaciclib/PO vehicle (dinaciclib group, n=5), IP vehicle/PO ABT-199 (ABT-199 group, n=5), or IP dinaciclib/PO ABT-199 (combination group, n=5).
These data further highlight a need for simultaneous targeting of MCL1 and BCL2 for therapeutic success in DLBCL. We therefore combined ABT-199 and dinaciclib against cells sensitive and resistant to both drugs as single agents (Figure 4d). In dinaciclib-sensitive cells (SU-DHL-4 and TMD8) or ABT-199-sensitive cells (Riva), addition of the other drug resulted in little further loss of viability and was non- or weakly synergistic. In U2932 cells, however, resistant to both drugs as single agents, the combination was highly potent and synergized strongly. The same was true for SU-DHL-4 and TMD8 cells engineered to over-express BCL2. Introduction of either MCL1 or BCL2 to U2932 cells led to enrichment in response to combined dinaciclib+ABT-199 but not either single agent, further highlighting the central importance of these apoptotic mediators to the anti-tumor effects of the combination (Figure 4e, S3a; dinaciclib results in 1e and 2c). To further evaluate the basis for synergy, we tested three additional drugs with activity against CDK9. Flavopiridol inhibits CDKs and several other kinases, although its anti-tumor effects in lymphoid cells are due to CDK9 and loss of MCL1.22,23,25 PHA-767491 is a dual inhibitor of CDC7 and CDK9,34 while SNS-032 has activity against CDK2, CDK7, and CDK9.35,36 All three synergized strongly with ABT-199 against U2932 cells (Figure 4f). Flavopiridol and SNS-032 also synergized strongly with ABT-199 against BCL2-over-expressing SU-DHL-4 and TMD8 cells, with PHA-767491 results less clear (Figure S3b). Because CDK9 is the only common target among all four drugs, these data strongly argue its inhibition and resulting loss of MCL1 is the basis for dinaciclib's synergy with ABT-199.
We chose U2932 as a model of high-risk disease for initial evaluation in vivo since both dinaciclib and ABT-199 showed poor single agent activity against these cells in vitro. We xenografted 20 SCID mice with U2932 cells and matched them by tumor size to four groups: control (IP vehicle/PO vehicle), dinaciclib (IP dinaciclib/PO vehicle), ABT-199 (IP vehicle/PO ABT-199), and combination (IP dinaciclib/PO ABT-199). Combination therapy produced markedly superior tumor control (Figures 4g, n=5 per group) and importantly no overt signs of toxicity, with animal weights similar between groups (Figure S3c). Combined targeting of MCL1 expression and BCL2 activity therefore is achievable in vivo and generates potent activity against tumors representing high-risk DLBCL.
High Efficacy of Targeting Both MCL1 and BCL2 in a Model of MYC-BCL2 Double-Expressing Lymphoma
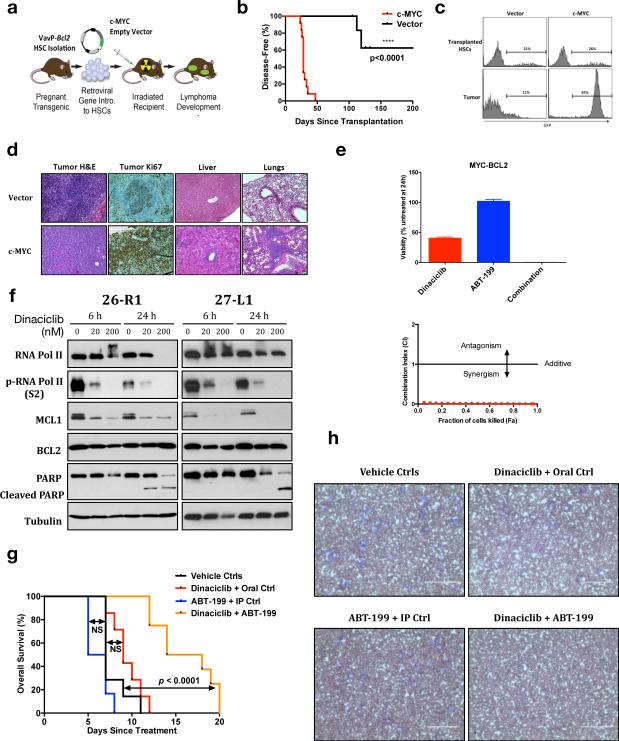
Double-Hit lymphoma is a subset of aggressive B-lymphoma overlapping with DLBCL carrying simultaneous presence of two chromosomal translocations involving the immunoglobulin heavy chain locus and c-MYC, BCL2, or BCL6. The combination of t(14;18)(q32;q21) involving BCL2 and t(8;14)(q24;q32) involving c-MYC is most common.37 Double-hit lymphoma responds poorly to standard chemoimmunotherapy with long-term survival in at best a quarter of patients, representing arguably the highest risk group of DLBCL and similar cancers.8,9,37 We retrovirally introduced c-MYC into hematopoietic stem cells (HSCs) derived from VavP-BCL2 mice38 as previously described19–21 and transplanted sub-lethally irradiated wild-type recipients (Figure 5a). MYC expression resulted in rapid lymphoma onset in recipient animals, with 100% penetrance by day 50 post-transplant (Figure 5b-c). Pathologic assessment showed a diffuse large B-cell tumor, highly proliferative and invasive to other organs, in contrast with non-invasive vector tumors that grow in a follicular pattern similar to baseline VavP-BCL2 animals (Figure 5d).38 This model therefore recapitulates the genetics, pathology, and aggressive behavior of MYC-BCL2 double-hit lymphoma in an immunocompetent animal system.
Figure 5. High efficacy of targeting both MCL1 and BCL2 in a model of MYC-BCL2 double-hit lymphoma.
(a) Schematic for generation of MYC-BCL2 genetically defined tumors (see text). (b) Tumor onset was detected with palpation and blood smear evaluation and compared using Kaplan-Meier analysis. (c) Representative FACS plots for GFP fluorescence of partially infected HSCs initially transplanted compared to resulting lymphoma cells at disease onset. (d) Representative pathology samples of c-MYC vs. vector tumors, imaged with EVOS XL Cell Imaging System (Life Technologies). (e) Lymphoma cells from a primary VavP-Bcl2/c-MYC recipient animal were cultured ex vivo and exposed to dinaciclib, ABT-199, or the combination for 24 hours. Viability at 250 nM and CI vs. Fa. (mean of quadruplicates ± SEM.) (f) Tumor cells from two different primary VavP-Bcl2/c-MYC recipients were exposed to dinaciclib and blotted as in Figure 1b. (g) Kaplan-Meier overall survival analysis of VavP-Bcl2/c-MYC tumor-bearing animals following initiation of treatment as indicated. The log-rank test was used to calculate p values. NS: p ≥ 0.05. (h) Representative blood smears of Vavp-Bcl2/c-MYC tumor-bearing mice during treatment. Blood smears were collected 7 days after treatment initiation, and stained with Hema-Quik II Stain Solutions. Imaged on EVOS XL Cell Imaging System (Life Technologies), scale bars 100 μm.
Oncogenic synergy between MYC and BCL2 was recognized nearly three decades ago due to BCL2's ability to prevent apoptosis resulting from high MYC activity.39 We hypothesized inhibition of BCL2 with ABT-199 would be effective against tumor cells derived from VavP-BCL2/c-MYC-transplanted animals. Strikingly, however, these tumor cells were mostly insensitive to single-agent ABT-199 (Figure 5e). Activity of single-agent dinaciclib, meanwhile, was consistent with a cytostatic effect. The combination, however, promoted strong synergy. A recent study in MYC-driven lymphoma showed particular dependence of these tumors on MCL1.40 Our data suggest that even with constitutive BCL2 expression promoting tumor formation, MCL1 maintains an important role in protecting cells from apoptosis. Consistently, we find dinaciclib-sensitive MCL1 expression in tumors derived from VavP-BCL2/c-MYC-transplanted animals, while dinaciclib-insensitive BCL2 expression prevents significant PARP cleavage (Figure 5f). We therefore tested dinaciclib + ABT-199 in vivo. Tumor-bearing animals treated with either dinaciclib/PO vehicle (n=7) or ABT-199/IP vehicle (n=6) showed no survival difference from those treated with PO vehicle/IP vehicle (n=7, Figure 5g). Those treated with ABT-199/dinaciclib (n=8), however, had greatly improved survival (p<0.0001) and dramatically increased clearing of tumor cells from the peripheral blood (Figure 5h). Inhibition of BCL2 alone therefore was inadequate therapy in an model of MYC-BCL2 double-hit DLBCL due to expression of MCL1 associated with high MYC activity, but combination with therapy promoting MCL1 loss was significantly more effective.
Chemotherapy Drugs Affecting MCL1 Levels Synergize Strongly with ABT-199
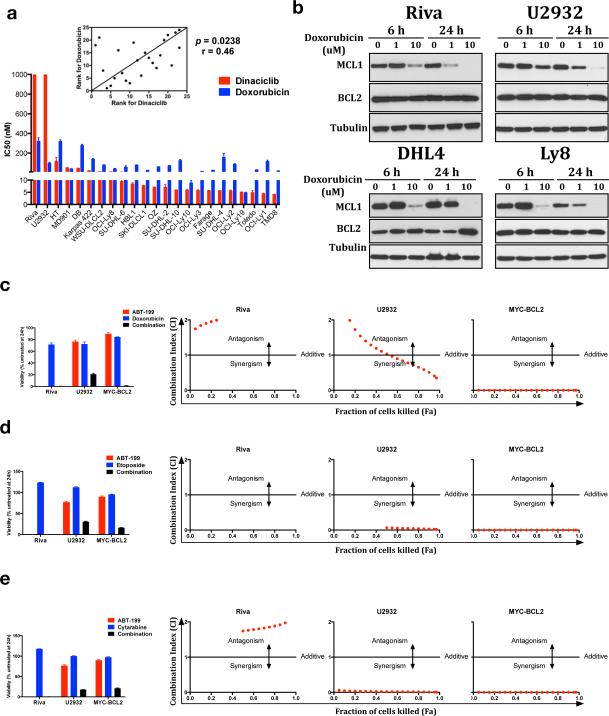
Dinaciclib and ABT-199 are unapproved drugs that cannot easily be combined in a clinical trial. Trials are underway or planned, however, combining ABT-199 with chemotherapy in B-NHL (NCT01594229, NCT01671904, NCT02187861, NCT02055820). We therefore investigated if our findings can help predict activity and inform trial design for such combinations. The anthracycline doxorubicin is considered the most active drug in R-CHOP and is known to have global effects on transcription that can result in reduced MCL1.41 Comparison of doxorubicin's activity to dinaciclib's in DLBCL cell lines shows correlation (p=0.0238), suggesting overlapping mechanism (Figure 6a). Similar to dinaciclib, doxorubicin caused loss of MCL1 at peak concentrations, and had no effect on BCL2 (Figure 6b). R-CHOP + navitoclax in vivo vs. xenografted mantle cell lymphoma cells previously was shown to be more potent than either alone.42 We found doxorubicin behaved like dinaciclib when combined with ABT-199, adding little to the ABT-199-sensitive Riva cells but synergizing against resistant U2932 and MYC-BCL2 murine tumor cells (Figure 6c). Etoposide and cytarabine are key components of DLBCL salvage regimens RICE43 and R-DHAP44 respectively and also can have global effects on transcription resulting in loss of MCL1.45 These drugs behaved nearly identically to doxorubicin and dinaciclib in combination studies with ABT-199 (Figure 6d-e). Notably, dinaciclib combined poorly with the same chemotherapy drugs, failing to promote a significant loss in viability in either Riva or U2932 cells (Figure S4). In sum, these data provide a basis to expect heightened therapeutic activity when ABT-199 is combined with chemotherapy drugs that affect MCL1. Until a more specific approach like dinaciclib to inhibit MCL1 transcription is available for combination with ABT-199, these findings can inform ABT-199+chemotherapy strategies for high-risk DLBCL.
Figure 6. ABT-199 synergizes strongly with lymphoma chemotherapy agents that affect MCL1 levels.
(a) Doxorubicin vs. dinaciclib activity comparison as in Figure 1a. (b) The indicated lines were treated with doxorubicin at the indicated times and concentrations and subjected to western blotting. (c-e) Viability and CI vs. Fa after 24 hours’ exposure to doxorubicin (c), etoposide (d), or cytarabine (e) alone or in combination with ABT-199 in Riva, U2932, and VavP-Bcl2/c-MYC murine tumor cells. Viability shown at 500 nM (500 ng/mL for doxorubicin; quadruplicates ± SEM.)
DISCUSSION
Improving outcome for high-risk DLBCL requires addressing underlying causes of poor response to standard therapy. Resistance to apoptosis is a hallmark of cancer12 and can promote escape from otherwise toxic therapies. Over-expression of multi-domain antiapoptotic proteins like BCL2 and MCL1 is a primary route to those ends. Despite their redundant function in apoptosis, however, we find both proteins can be expressed simultaneously in DLBCL tumors. A caveat to IHC is that it provides a snapshot in time of protein expression in particular tumors and not necessarily fixed properties. Instead, both our data and the work of others support show DLBCL cells have high propensity to express either or both these proteins to avoid programmed cell death, especially the poor-prognosis ABC subtype.10,15 Therefore, therapeutic targeting of either BCL2 or MCL1 alone seems destined to select for treatment failure driven by tumor populations able to express the other. Our study of the combined approach builds on work of others, who showed synergy using on older drugs (flavopiridol, roscovitine, navitoclax) whose off-target toxicities have prevented advancement to clinical approval.42,45,46 We now refine and improve the strategy using the newer more potent and specific inhibitors dinaciclib and ABT-199. By undertaking extensive in vivo analysis in independent, complementary model systems, we also provide a stronger basis for moving forward with clinical evaluation specifically in DLBCL.
Dinaciclib inhibits CDK1, 2, 5, and 9 with greater potency and specificity than flavopiridol or roscovitine and has a far superior therapeutic index in vivo.16 It has progressed through phase I clinically, where limited bone marrow suppression and electrolyte abnormalities were the main toxicities,26,47 and is now in phase 2 in combination with other therapies for multiple myeloma and solid tumors (NCT01783171, NCT01711528, NCT01434316). Though dinaciclib's four targets could produce diffuse effects on tumor-cell proliferation and survival, its effects on CDK9 leading to rapid loss of MCL1 predominate particularly in vivo where pharmacokinetics limit clinical duration of exposure.27 ABT-199, meanwhile, is a BH3 mimetic whose ability to spare platelets by avoiding anti-BCL-XL activity has enabled dramatic phase I activity against CLL/SLL. As such, the main dose limiting toxicity of ABT-199 in CLL is tumor lysis syndrome (TLS), which can be seen in particularly susceptible CLL patients after a single oral dose. TLS also has been reported for dinaciclib26 and flavopiridol48 in CLL, interestingly, so it is possible this toxicity is more disease-specific than drug-specific. Either way, the clinical experience with ABT-199 shows CLL's particular dependence on BCL2, while apoptosis protection in DLBCL and most other cancers is more complex. Results for ABT-199 in DLBCL have been reported for only nine relapsed or refractory patients, of whom three responded (3/8 treated with ≥ 600 mg daily, including one complete response), and TLS was not a significant issue.49 We found modest single-agent activity of ABT-199 against most DLBCL cell lines at 24 hours’ incubation, and our BCL2-MYC mouse model was more resistant in vivo than was reported recently for a genetically similar model.50 What's more important, however, is that ABT-199 in combination with MCL1 knockdown by dinaciclib resulted in strong synergy in all cases. Importantly, our in vivo experiments show significant anti-tumor activity of the combination is achievable without undue toxicity to host animals.
ABT-199 plus dinaciclib cannot easily be tested in patients in the near term until at least one of the drugs is approved, but more important is the principle of high anti-tumor activity when ABT-199 is combined with any drug resulting in a decrease of MCL1. By expanding our studies to ABT-199 plus chemotherapy agents with this activity, we show a short-term path to assess this strategy clinically until such time as assessment of the specific ABT-199-dinaciclib combination can be undertaken.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Abbvie Inc. for provision of ABT-199 for this study and Associate Scientific Director Joel D. Leverson for advice on its use in vivo. We thank Bethany Skovan, Gillian Paine-Murrieta, and Erica Sontz of the UACC Experimental Mouse Shared Service Resource for invaluable help with mouse studies. Funded by the NIH/NCI (P30CA023074-34 sub-award), the University of Arizona Bio5 Institute (JHS), the University of Arizona Cancer Center (JHS), and the Lymphoma Research Foundation (JHS).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supplementary information is available at Leukemia's website.
REFERENCES
- 1.Coiffier B, Lepage E, Briére J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Ann Oncol. 2014:mdu109. doi: 10.1093/annonc/mdu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George A, Tam CS, Seymour JF. High-risk diffuse large B-cell lymphoma: can we do better than rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone? Leuk Lymphoma. 2013;54:2575–2576. doi: 10.3109/10428194.2013.796059. [DOI] [PubMed] [Google Scholar]
- 7.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International Prognostic Index Remains a Valid Predictor of Outcome for Patients With Aggressive CD20+ B-Cell Lymphoma in the Rituximab Era. J Clin Oncol. 2010;28:2373–2380. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 8.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical Double-Hit Score Is a Strong Predictor of Outcome in Patients With Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent Expression of MYC and BCL2 in Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–4031. doi: 10.1182/blood-2012-10-460063. quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monti S, Chapuy B, Takeyama K, Rodig SJ, Hao Y, Yeda KT, et al. Integrative Analysis Reveals an Outcome-Associated and Targetable Pattern of p53 and Cell Cycle Deregulation in Diffuse Large B Cell Lymphoma. Cancer Cell. 2012;22:359–372. doi: 10.1016/j.ccr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Davids MS, Letai A. Targeting the B-Cell Lymphoma/Leukemia 2 Family in Cancer. J Clin Oncol. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn H, Ziepert M, Becher C, Barth TFE, Bernd H-W, Feller AC, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121:2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel S-S, Grau M, Mavis C, Hailfinger S, Wolf A, Madle H, et al. MCL1 is deregulated in subgroups of diffuse large B-cell lymphoma. Leukemia. 2013;27:1381–1390. doi: 10.1038/leu.2012.367. [DOI] [PubMed] [Google Scholar]
- 16.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol Cancer Ther. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 17.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci. 2001;98:8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 19.Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oricchio E, Ciriello G, Jiang M, Boice MH, Schatz JH, Heguy A, et al. Frequent disruption of the RB pathway in indolent follicular lymphoma suggests a new combination therapy. J Exp Med. 2014;211:1379–1391. doi: 10.1084/jem.20132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schatz JH, Oricchio E, Wolfe AL, Jiang M, Linkov I, Maragulia J, et al. Targeting cap-dependent translation blocks converging survival signals by AKT and PIM kinases in lymphoma. J Exp Med. 2011;208:1799–1807. doi: 10.1084/jem.20110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–2978. [PubMed] [Google Scholar]
- 23.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 24.MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–5407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 25.Gojo I, Zhang B, Fenton RG. The Cyclin-dependent Kinase Inhibitor Flavopiridol Induces Apoptosis in Multiple Myeloma Cells through Transcriptional Repression and Down-Regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 26.Flynn J, Jones J, Johnson AJ, Andritsos L, Maddocks K, Jaglowski S, et al. Dinaciclib is a novel cyclin dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic. Leukemia. 2015 doi: 10.1038/leu.2015.31. doi:10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booher RN, Hatch H, Dolinski BM, Nguyen T, Harmonay L, Al-Assaad A-S, et al. MCL1 and BCL-xL Levels in Solid Tumors Are Predictive of Dinaciclib-Induced Apoptosis. PLoS ONE. 2014;9:e108371. doi: 10.1371/journal.pone.0108371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuller A, Booher R, Cadzow L, Angagaw M, Harmonay L, Qu X, et al. Abstract 699: Optimized dosing strategies resulting in prolonged pathway inhibition enhance dinaciclib anti-tumor activity in preclinical xenograft models. Cancer Res. 2013;73:699–699. [Google Scholar]
- 29.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 Profiling Identifies Three Distinct Classes of Apoptotic Blocks to Predict Response to ABT-737 and Conventional Chemotherapeutic Agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Ertel F, Nguyen M, Roulston A, Shore GC. Programming cancer cells for high expression levels of Mcl1. EMBO Rep. 2013;14:328–336. doi: 10.1038/embor.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills JR, Hippo Y, Robert F, Chen SMH, Malina A, Lin C-J, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendrick SL, Redd L, Muranyi A, Henricksen LA, Stanislaw S, Smith LM, et al. BCL2 antibodies targeted at different epitopes detect varying levels of protein expression and correlate with frequent gene amplification in diffuse large B-cell lymphoma. Hum Pathol. 2014 doi: 10.1016/j.humpath.2014.06.005. doi:10.1016/j.humpath.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 34.Montagnoli A, Valsasina B, Croci V, Menichincheri M, Rainoldi S, Marchesi V, et al. A Cdc7 kinase inhibitor restricts initiation of DNA replication and has antitumor activity. Nat Chem Biol. 2008;4:357–365. doi: 10.1038/nchembio.90. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Wierda WG, Chubb S, Hawtin RE, Fox JA, Keating MJ, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009;113:4637–4645. doi: 10.1182/blood-2008-12-190256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conroy A, Stockett DE, Walker D, Arkin MR, Hoch U, Fox JA, et al. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009;64:723–732. doi: 10.1007/s00280-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 37.Oki Y, Noorani M, Lin P, Davis RE, Neelapu SS, Ma L, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 38.Egle A, Harris AW, Bath ML, O'Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004;103:2276–2283. doi: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- 39.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 40.Kelly GL, Grabow S, Glaser SP, Fitzsimmons L, Aubrey BJ, Okamoto T, et al. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28:58–70. doi: 10.1101/gad.232009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, et al. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell. 2012;21:547–562. doi: 10.1016/j.ccr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 43.Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 44.Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988;71:117–122. [PubMed] [Google Scholar]
- 45.Van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 47.Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou Y-M, et al. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med. 2013;11:259. doi: 10.1186/1479-5876-11-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blum KA, Ruppert AS, Woyach JA, Jones JA, Andritsos L, Flynn JM, et al. Risk factors for tumor lysis syndrome in patients with chronic lymphocytic leukemia treated with the cyclin-dependent kinase inhibitor, flavopiridol. Leukemia. 2011;25:1444–1451. doi: 10.1038/leu.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davids MS, Seymour JF, Gerecitano JF, Kahl BS, Pagel JM, Wierda WG, et al. Phase I study of ABT-199 (GDC-0199) in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL): Responses observed in diffuse large B-cell (DLBCL) and follicular lymphoma (FL) at higher cohort doses. [15 Oct 2014];J Clin Oncol. 2014 32:5s. http://meetinglibrary.asco.org/content/133739-144. [PubMed] [Google Scholar]
- 50.Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.