Abstract
For a long time, clinically sized and mechanically functional cartilage could be engineered from young animal chondrocytes, but not from adult human mesenchymal stem cells that are of primary clinical interest. The approaches developed for primary chondrocytes were not successful when used with human mesenchymal cells. The method discussed here was designed to employ a mechanism similar to pre-cartilaginous condensation and fusion of mesenchymal stem cells at a precisely defined time. The formation of cartilage was initiated by press-molding the mesenchymal bodies onto the surface of a bone substrate. By image-guided fabrication of the bone substrate and the molds, the osteochondral constructs were engineered in anatomically precise shapes and sizes. After 5 weeks of cultivation, the cartilage layer assumed physiologically stratified histomorphology, and contained lubricin at the surface, proteoglycans and type II collagen in the bulk phase, collagen type X at the interface with the bone substrate, and collagen type I within the bone phase. For the first time, the Young’s modulus and the friction coefficient of human cartilage engineered from mesenchymal stem cells reached physiological levels for adult human cartilage. We propose that this method can be effective for generating human osteochondral tissue constructs.
Keywords: tissue engineering, cartilage, bone, mesenchymal stem cells, biomechanics
1. Introduction
Growing human cartilage in vitro would have tremendous clinical impact to millions of people whose cartilage has been lost to injury or arthritis. Tissue engineering is of particular interest to cartilage regeneration, as cartilage exhibits only minimal capability for intrinsic healing due to its avascular nature and low concentration of chondrocytes (1). The hallmarks of cartilage disease include the loss of mechanical function, degradation of the collagen network, loss and reduced synthesis of proteoglycans, and decreased cellularity (2, 3). The long-standing goal of the field of tissue engineering is to recreate the native biochemical content, stratified structure and functional quality (compressive stiffness and frictionless motion) of human cartilage by using the patient’s own cells.
Early studies demonstrated the ability to form small pieces of cartilage tissue following implantation of primary chondrocytes seeded into polymer matrix and subcutaneous implantation into rodents (4), and provided evidence that cartilage can be generated in vivo from cells on scaffolds. Subsequently, tissue engineering of cartilage in vitro using primary chondrocytes has been widely investigated. As a result, cartilage with properties approaching those of native parent cartilage was successfully grown in vitro using primary chondrocytes harvested from young animals cultured in agarose hydrogels (5, 6). Nevertheless, the use of primary chondrocyte was associated with several disadvantages, including the need for invasive harvesting of cartilage from another “less needed” area of a joint, limited quantity of donor tissue, and donor site morbidity.
Stem cells such as bone marrow-derived mesenchymal stem cells (BMSC) offer a clinically relevant cell source for tissue engineering, as they can be obtained in a less invasive way, expanded to large numbers without compromising the cell’s ability for chondrogenic differentiation (7, 8), and - most importantly, are not immunogenic (9, 10). For a long time, the utility of this clinically attractive cell source for cartilage tissue engineering was compromised by our inability to direct the BMSC differentiation into high quality tissue, and difficulties in generating clinically sized and shaped tissue constructs. The need to generate cartilage-bone grafts consisting of two very different tissues with an interface, rather than cartilage alone, requires the additional levels of complexity.
Previous studies attempted to engineer cartilage tissue from BMSC by replicating methods that were successfully used to engineer cartilage from primary chondrocytes. To this end, BMSC were encapsulated into hydrogels and cultured in vitro, but with only limited success (11). Even in long-term cultures, the BMSC generated only small amounts of glycosaminoglycan and collagen type II, the two most abundant extracellular matrix proteins in cartilage, compared to the cultures of primary chondrocytes, and in particular to the native cartilage tissue. For reasons that were poorly understood, the methods that were successful with primary chondrocytes, when applied to animal or human BMSCs, resulted in cartilaginous tissues with largely subnormal compositions and functional properties.
Interestingly, BMSC were much more efficiently induced toward chondrogenesis by scaffold-free self-assembly (5, 12, 13), a process involving cell condensation that is present during native skeletal development. BMSC were shown to spontaneously form condensed cellular bodies and undergo chondrogenesis in the presence of regulatory factors such as TGF-β (14, 15). Several groups have engineered cartilage tissues by utilizing self-assembly of BMSC (Table 1). These studies resulted in tissue constructs with increased levels of glycosaminoglycan and increased compressive moduli. However, the tissue structure and mechanical function remained subnormal and failed to match native cartilage.
Table 1.
Methods for engineering cartilage by cell self-assembly
| Cell/tissue format |
Number of Cells |
Anatomic shape |
Size | Compressive modulus (kPa) |
Friction coefficient |
Reference |
|---|---|---|---|---|---|---|
| Pressed layer of centrifuged cells |
1.5×106 | No | Layer 5mm×5mm 0.1mm thick |
n/a | n/a | (29) |
| Pressed layer of centrifuged cells |
1.5×106 | No | Layer 5mm×5mm undefined thickness |
~570 | n/a | (30) |
| Cells centrifuged onto agarose- coated well |
5.5×106 | No | Disc 4mm dia. 1mm thick |
n/a | n/a | (5) |
| Cells centrifuged onto a transwell |
5×105 | No | Disc 6.5mm dia. 1mm thick |
n/a | n/a | (13) |
| Pressed-mold fused pellets |
6×106 | No | Disc 5mm dia 0.5 mm thick |
~800 | <0.05 | (16) |
| Pressed-mold fused pellets |
50×106 | Yes | Articular surface 1 mm thick |
n/a | n/a | (16) |
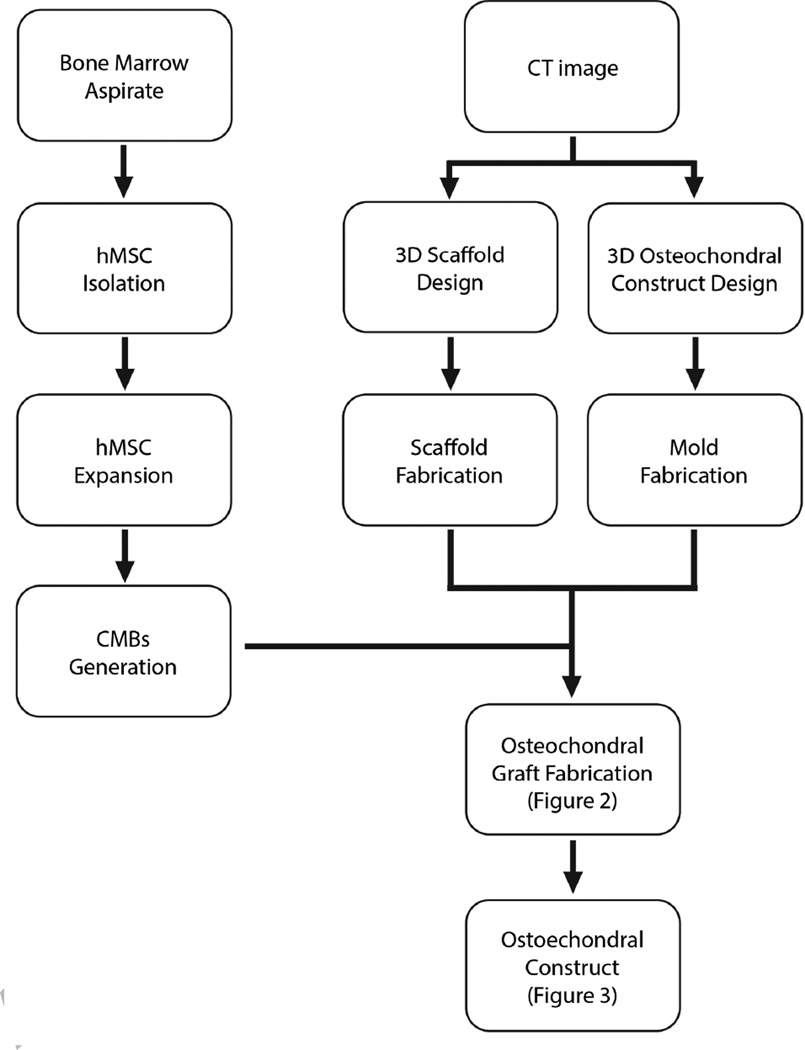
Here, we discuss an advanced method for generating human cartilage-bone grafts of a desired size and anatomical shape replicating an articulating surface that was recently developed in our laboratory (16). We utilized the self-assembly of BMSC that were directed to form condensed mesenchymal bodies (CMBs) and fuse together with the aid of press molding (16). Physiologically thick cartilaginous tissue integrated to the subchondral bone was engineered in vitro by inducing the fused CMBs while also establishing a cartilage-bone interface. After 5 weeks of cultivation, the cartilage tissue exhibited native-like, stratified structure and composition and reached – for the first time – physiological stiffness and friction coefficient. This method can generate large anatomically shaped pieces of cartilage overlaying bone, that are precisely defined by image-guided fabrication of the biomaterial scaffold and bioreactor chamber (Figure 1).
Figure 1. Key steps in engineering osteochondral constructs.
Flow chart shows the key steps of imaging-guided engineering of human osteochondral constructs, starting from the condensing mesenchymal bodies (CMBs) derived from human mesenchymal stem cells, interfaced with an anatomically shaped decellularized bone substrate (scaffold), and cultured in a matching bioreactor chamber.
2. Methods
2.1 Fabrication of the anatomical scaffold and bioreactor chamber
The grafts are designed based on the magnetic resonance or computer tomography images of the defect being repaired. The individual slices are converted into three-dimensional (3D) files using computer-aided software such as Mimics® Innovation Suite (Materialise, Belgium). After defining the exact anatomical fit for the graft being reconstructed, the 3D computer model of the graft is exported as a .STL file and used for the fabrication of the scaffold and the matching inner chamber of the bioreactor.
To fabricate the anatomical scaffold, the 3D model .STL file is imported into the SOLIDWORKS (Dassault Systemes SolidWorks, MA, USA) and a G-code for milling an anatomical scaffold from a block of material is generated using MasterCAM (CNC Software Inc., CT, USA) add-in in SolidWorks. The scaffold material used in the present study was the bovine trabecular bone harvested from calf knees from 1–4 month old animals, after removing the muscles, patella, fibrous connective tissues, ligaments, and meniscus and leaving only the femur head exposed. Using a table-top band saw, articular cartilage and cortical bone were removed to isolate spongy trabecular bone. Using a lathe (LittleMachineShop, CA, USA), the bone was shaped into cylindrical blocks with a diameter just larger than the width of the 3D graft model and the length approximately 3 cm longer than the 3D model so that there is enough structure to hold the blocks during machining. We used a four-flute 1/4” ball-end mill (McMaster-Carr, NJ, USA) to shape the anatomical bone scaffold from cylindrical bone block on the 4-axis CNC milling machine (LittleMachineShop, CA, USA). The anatomically shaped bone blocks were completely decellularized using hypotonic solution, detergent, multiple washes and DNAse/RNAse solution, by a method we previously established (17).
The same 3D image of each anatomical graft was then used to design the mold for fabricating the inner bioreactor chamber for cultivating the osteochondral construct. The desired cartilage thickness (1 mm or more, uniform or changing with the position like in native joints) was established by using the surface selection tool to set the shape of the articular surface in SolidWorks. The G-code was then created to mill a Teflon rod (McMaster-Carr, NJ, USA) to hold the construct. A negative mold was then made using the milled piece by pouring the SylGard® 184 Silicone Elastomer (Dow Corning, MI, USA) over the milled structure. Once cured, the silicone was cut into two pieces, one to hold the decellularized bone scaffold and the other to hold the condensed mesenchymal bodies (CMBs) that will be pressed inside the mold against the articulating surface. The strength of the elastomer was sufficient to maintain the shape of the mold under loading, and the geometry of the final construct corresponded closely to the original design defined by imaging. The two-piece mold was sterilized by autoclaving. The key steps of the process are schematically shown in Figure 2.
Figure 2. Fabrication of osteochondral grafts.
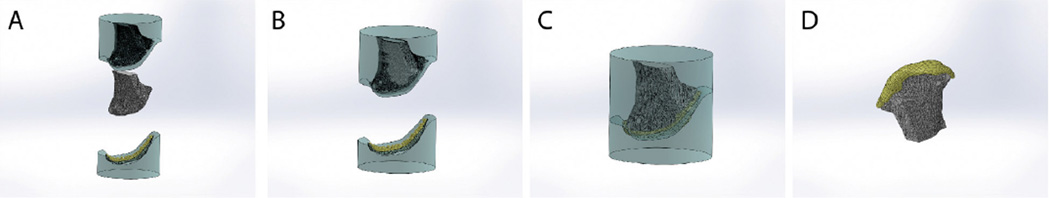
A Silicone mold was made in two pieces, and the CMBs were placed at the articular side. B An anatomically shaped scaffold machined by image-guided processing of native decellularized bone matrix is then placed into the other side of the mold. C The two-piece mold is fused together, to press the CMBs into a desired shape of an articular surface on the scaffold. D A compact layer of CMBs is formed at the surface of decellularized bone which can also be seeded by BMSCs. The resulting construct is then cultured in a matching, anatomically shaped bioreactor chamber. Image reproduced with permission from Bhumiratana et al., PNAS 111, 6940, 2014) (16).
2.2 Preparation of bone-marrow derived mesenchymal stem cells
Fresh human bone marrow aspirates were obtained either from orthopaedic surgeries (through a non-human IRB for fully de-identified surgical materials) or from a commercial supplier such as Cambrex (NJ, USA). Mesenchymal stem cells (BMSCs) were isolated by attachment to the cell culture plastics, and expanded in DMEM (Life Technologies) supplemented with 10% FBS (Life Technologies), 1% pen-strep (Life Technologies), and 0.1 ng/mL basic fibroblast growth factor (bFGF, Life Technologies). The cells were cultured up to the third passage and tested for the multi-lineage differentiation capability as in our previous studies (18).
2.3 Generation of condensed mesenchymal bodies
After trypsinization, BMSCs were suspended in chondrogenic medium consisting of high-glucose DMEM supplemented with 100 nM dexamethasone (Sigma), 50 µg/ml ascorbic acid-2-phosphate (Sigma), 100 µg/ml sodium pyruvate (Fisher), 40 µg/ml proline (Sigma), a mix of 1% insulin, transferrin, and sodium selenite (ITS+, Corning), 1% penicillin/streptomycin (Life Technologies), and 10 ng/ml Transforming Growth Factor-beta3 (TGF-β3, Peprotech), at the concentration of 5×105 cells/ml. 1 ml cell suspension was added to the wells of a deep round-bottom 96-well plate (NUNC®, Sigma-Aldrich, MO) and centrifuged at 250g for 5 min. The cells were incubated overnight at 37°C/5%CO2) to form spherical CMBs that were used to generate the cartilage layer of the osteochondral tissue construct.
2.4 Fabrication of the tissue constructs
After 3 days of culture, the spherical CMBs increased in size, to just above 1 mm in diameter. The number of CMBs used for each construct was calculated from the volume of the cartilage layer that needs to be engineered, using the equation:
For example, for a layer of articular cartilage that has a surface of 1 cm2 and 1 mm thick (including the 0.1 mm penetration into the bone substrate), one would need 190 CMBs that are transferred to the articular side of the mold using a sterile transfer pipette (without additional vacuum suction). The anatomical scaffold is then inserted into the other side of the mold and slowly pressed onto the articular side (Figure 2). By applying compressive force, the CMBs are made to fuse together at a pre-determined depth over the articular surface. It is important that a sufficient amount of the fused CMBs penetrates into the bone phase to drive the integration between the forming cartilage and bone. The osteochondral tissue constructs were then taken out of the mold and cultured for 5 weeks in chondrogenic media, using 100 µl medium per CMB, with a medium change twice a week.
3. Results
After press molding, the fused CMBs formed a dense cellular layer on the surface of the bone substrate inside the mold. The initial construct consisted of two clearly distinguished layers: (i) a dense cellular layer that will form a cartilage surface following cultivation, and (ii) a bone substrate. Penetration of fused CMBs into the bone substrate created an interface that gradually interlocked the two layers by forming a stratified structure.
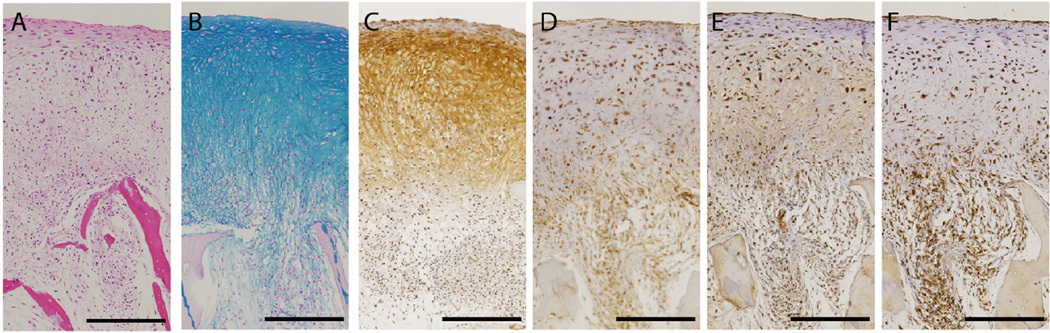
Following 5 weeks of cultivation in chondrogenic medium, an osteochondral tissue construct formed, with a dense layer of cartilaginous tissue, a bone tissue underneath and an interface in between (Figure 3). These constructs exhibited a well-developed stratification with a native-like gradient of structural compositional properties from the cartilage to the bone region. The cartilage region was rich in glycosaminoglycans and collagen type II, the main components of native articular cartilage, while collagen type I was mostly present in the bone region (Figure 3). Notably, lubricin - a protein essential for lubrication of the articulating cartilage surfaces (16, 19, 20), was present at the superficial surface of the cartilage layer.
Figure 3. Native-like histomorphology of human cartilage formed from MSCs at the surface of the bone.
After cultivation in chondrogenic medium for 5 weeks, the dense cellular layer developed into physiologically stratified cartilage. A H&E, B Alcian Blue, C Collagen type II, D Lubricin, E Collagen type I, and F Collage type X. [Bar: 500µm] Image reproduced with permission from Bhumiratana et al., PNAS 111, 6940, 2014) (16).
The interface consisted of cells residing in the scaffold pores that deposited only minimal amount of glycosaminoglycan and collagen type II, and instead were surrounded by collagen type I and collagen type X synthesized by the BMSCs in the scaffold pores, indicating the formation of calcified cartilage (16, 21).
This method resulted, for the first time, in cartilage engineered from human MSCs that achieved mechanical properties in physiological range. Both the Young modulus measured in compression and the friction coefficients measured in tribological studies of the tissue-engineered cartilage were comparable to those measured for native cartilage (Table 2).
Table 2.
Mechanical properties of tissue engineered cartilage (16) and native cartilage
4. Discussion
For a long time, cartilage has been a major focus of the field of tissue engineering, both due to the strong clinical need (cartilage is known to have only minimal ability to regenerate following injury or disease), the avascular nature of this tissue and low cell density (which helps maintain cell viability and function in vitro). Cartilage tissue engineering has progressed mostly by utilizing primary chondrocytes extracted from native cartilage of young animals (4, 6, 23). These highly active yet phenotypically stable and mature cells were shown to readily produce cartilaginous matrix produce within the right environment. In contrast to most other tissues, even simple culture systems enabled engineering of millimeters thick and viable cartilaginous tissue constructs (4). Experimental protocols that involved a timely application and withdrawal of regulatory factors (such as TGF-β3) and mechanical loading (6) and the cultivation of tissue constructs in bioreactors providing a dynamic flow enviroment (23) resulted in engineering of cartilaginous constructs with biochemical compositions and mechanical stiffness reaching the range of values measured for parent cartilage.
A particularly successful culture setting was the encapsulation of young bovine chondrocytes in agarose hydrogel, where the cells were grown in a lacunae-like environment similar to that present in native cartilage (6). The cells would form chondrons and surround themselves by a tissue matrix rich in proteoglycans and collagens. With time in culture, these tissue constructs would gradually develop a spatially homogeneous cartilaginous matrix.
However, the agarose-encapsulation of BMSCs did not provide a suitable environment for chondrogenic differentiation of BMSCs and the subsequent deposition of cartilage tissue matrix. Unlike primary chondrocytes, trapping BMSCs within a lacunae-like environment provided by agarose hydrogel would not enhance chondrogenesis (11). One could speculate that this is due to the differences between this culture environment, suitable for cartilage formation by primary chondrocytes and the “niche” within the bone marrow where BMSCs normally reside. In response to biological signals associated with e.g., bone fracture, the BMSCs migrate to the site of injury (24) and start forming a cartilage callus serving as a template for long bone repair (25). Also, BMSCs are known to facilitate the repair of non critical cartilage defects after microfracture procedures (26). Hence, it was apparent that BMSCs are capable of forming cartilage but only if provided with a suitable environment.
Interestingly, Bian et al. showed that – in contrast to agarose - hydrogels that promoted interactions with BMSCs leading to mesenchymal condensation via CD44 and CD168, have promoted chondrogenesis and improved cartilage formation in vitro (27). Nevertheless, the composition and mechanical properties of engineered cartilage were largely subnormal. Although tissue constructs consisted mostly of hydrogel and limited amounts of cartilage-like matrix, these studies suggested that hydrogels promoting aggregation of BMSCs are more favorable for inducing chondrogenesis than agarose.
Further advances in growing cartilaginous tissue in vitro from BMSCs were facilitated by the observations made by several research groups in parallel that the spontaneous aggregation of BMSCs, without any scaffolding material and in the presence of TGF-β, resulted in the formation of spherical pellets with high concentrations of glycosaminoglycan and collagen type II (8, 15, 28). This was an encouraging result that opened a new direction of research of BMSC-based cartilage constructs. However, the pellets were very small and not usable for cartilage repair. To increase the size of the cartilage constructs from aggregating BMSC, Noth et al. (29) induced chondrogenic differentiation by pressing pellets, containing 1.5×106 BMSCs each, onto a block made of polylactic acid. Under these conditions, the BMSCs deposited cartilaginous matrix inside the polymer scaffold (30). However, this method resulted in a composite construct without a defined cartilage layer.
Two methods were subsequently developed to generate cartilage by self-assembly (5, 13). In both cases, the BMSCs were centrifuged into low-attachment cell culture wells. In one study, non-adhesiveness was achieved using agarose-coated 96 well plates (5), and another study utilized polycarbonate transwell filters (13). Centrifugation forced the BMSCs to form a very thin and dense cellular sheet at the bottom of the well. As the cells proliferated and differentiated, the layer became thicker (up to 1 mm) and accumulated large amounts of cartilage matrix containing collagen type II, glycosaminoglycans, and aggrecans. The size of the engineered cartilage discs was defined by the size of the well. However, the mechanical properties of the discs were either not tested or were inferior to the native cartilage.
Despite many attempts to engineer cartilage from human mesenchymal stem cells (Figure 1), the resulting tissue constructs displayed only a limited structural organization and mechanical competence of the tissue matrix. All measured properties of these constructs were largely subnormal. Among all the techniques employed for cartilage tissue engineering from BMSC, the scaffold-free self-assembly method was the most promising as it allowed BMSCs to undergo chondrogenic differentiation and to deposit large amounts of extracellular matrix proteins and proteoglycans (5, 12, 13) (Table 1).
The method we describe in this article, recently reported by our lab (16), utilized the self-assembly of BMSCs with special regard to the mechanisms underlying the native process of mesenchymal condensation that precedes cartilage formation during limb development. One key element of successful application of this technique in cartilage tissue engineering is in the understanding of the conditions for setting of the outer boundary by the CMBs (16). Another key element was the interface with the bone substrate. Notably, the same CMBs were able to form a physiologically stiff layer of cartilage atop decellularized bone, and to heal fulldepth cartilage defects in an in vitro model of cartilage healing (16). Both factors – mesenchymal stem cell condensation and the presence of underlying bone substrate were critical contributors to the formation of physiologically strong and stratified human cartilage.
After forming a spherical cell body, the BMSCs begin to differentiate and deposit the extracellular matrix. One of key components of this matrix is tenascin, the protein deposited during the mesenchymal condensation that sets the tissue boundaries in vivo (14, 31, 32). During BMSCs condensation in vitro, tenascin starts to accumulate at the outer surfaces of mesenchymal bodies by 5 days post condensation, which was an indication for the setting of a boundary around each mesenchymal body. The setting of outer boundaries on mesenchymal bodies prevented their fusion even when they were pressed against each other (16). This in turn suggested that larger volumes of cartilage, alone or within osteochondral constructs, can only produced if fusion is induced within 5 days of condensation.
Indeed, we confirmed that the press-molding of the mesenchymal bodies formed by the condensation of BMSCs (Figure 2) resulted in a dense and spatially uniform layer of cells. This dense cellular layer resembled the layers that form in vivo during native mesenchymal condensation, and that serve as a platform for cartilage formation. In vitro, we found that these compact layers with very high cell density induced rapid deposition of extracellular matrix. As a result, the engineered tissue became mechanically strong, and both the Young’s modulus and the friction coefficient reached physiological levels (Table 2). At the same time, the compacted cell layer developed into physiologically stratified cartilage containing lubricin at the surface, proteoglycans and type II collagen in the bulk phase, collagen type X at the interface with the bone substrate, and collagen type I within the bone phase (Figure 3).
We thus propose that the fusion of mesenchymal bodies formed from BMSCs at the interface with a bone substrate can be an effective method for generating human osteochondral tissue constructs with physiological compositions, histomorphologies and mechanical properties. This approach enabled, for the first time, to engineer human cartilage from BMSCs with the physiological Young modulus and friction coefficient. Also, the method allows engineering of anatomically shaped osteochondral constructs using scaffolds made by imaging-guided fabrication. Constructs composed of cartilage and decellularized bone substrate can be cultured in simple well plate settings (16), due to the low mass transport requirements of cartilage tissue. In contrast, engineering of osteochondral constructs composed of living cartilage and bone require the use of bioreactors with medium perfusion. Such bioreactors have been developed in our lab both for simple geometries and for anatomically shaped constructs.
One current limitation of this technique is the need for large numbers of cells, if large grafts are to be grown. For example, the reconstruction of 1 cm2 of the cartilage surface, with the thickness of cartilage layer of 2 mm, requires almost 100 million BMSCs. To reconstruct a whole condyle, one would need hundreds of millions of cells. In light of these limitations, two directions of future research would be of interest: (i) modifications of the method that would enable the use of lower cell numbers, possibly by combining mesenchymal condensation with the use of scaffolds, and/or (ii) the utilization of easily accessible and abundant sources of mesenchymal cells, such as the adipose tissue. While the results of recapitulating the mesenchymal condensation are most promising, further optimization is needed, as well as animal testing to evaluate the actual clinical potential of the method.
Highlights.
Human mesenchymal stem cells from bone marrow aspirates were used to engineer a layer of mechanically functional, stratified cartilage
Cartilage was grown on anatomically shaped decellularized bone substrates to form clinically sized osteochondral constructs
Tissue engineering method recapitulating the developmental step of mesenchymal stem cell condensation was necessary to grow stratified human cartilage with physiological Young’s modulus and friction coefficient
Acknowledgments
We gratefully acknowledge funding support of this work by the NIH (grants DE016525, EB002520 and AR061988).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000 Jan 1;43:215. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001 Mar 1;19:242. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loadingduration dependent. J Orthop Res. 1999 Nov 1;17:870. doi: 10.1002/jor.1100170612. [DOI] [PubMed] [Google Scholar]
- 4.Cima LG, et al. Tissue engineering by cell transplantation using degradable polymer substrates. Journal of biomechanical engineering. 1991 May;113:143. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 5.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006 Apr;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 6.Lima EG, et al. The beneficial effect of delayed compressive loading on tissueengineered cartilage constructs cultured with TGF-beta3. Osteoarthritis and cartilage. 2007 Sep;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 10.Yoo KH, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cellular immunology. 2009;259:150. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis and cartilage. 2006 Feb;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mackay AM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue engineering. 1998 Winter;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 13.Murdoch AD, et al. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem cells. 2007 Nov;25:2786. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- 14.Mackie EJ, Murphy LI. The role of tenascin-C and related glycoproteins in early chondrogenesis. Microscopy research and technique. 1998 Oct 15;43:102. doi: 10.1002/(SICI)1097-0029(19981015)43:2<102::AID-JEMT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Tuli R, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. The Journal of biological chemistry. 2003 Oct 17;278:41227. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 16.Bhumiratana S, et al. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proceedings of the National Academy of Sciences of the United States of America. 2014 May 13;111:6940. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayson WL, et al. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue engineering. Part A. 2008 Nov;14:1809. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grayson WL, et al. Engineering anatomically shaped human bone grafts. Proceedings of the National Academy of Sciences of the United States of America. 2010 Feb 23;107:3299. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009 Jun;27:771. doi: 10.1002/jor.20798. [DOI] [PubMed] [Google Scholar]
- 20.Waller KA, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2013 Apr 9;110:5852. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyre D. Collagen of articular cartilage. Arthritis research. 2002;4:30. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter MJ, Basalo IM, Ateshian GA. The temporal response of the friction coefficient of articular cartilage depends on the contact area. Journal of biomechanics. 2007;40:3257. doi: 10.1016/j.jbiomech.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freed LE, Langer R, Martin I, Pellis NR, Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proceedings of the National Academy of Sciences of the United States of America. 1997 Dec 9;94:13885. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007 Nov;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 25.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. Journal of cellular biochemistry. 1994 Nov;56:283. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clinical orthopaedics and related research. 2001 Oct;:S362. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 27.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013 Jun 18;110:10117. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002 Apr 2;99:4397. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue engineering. 2002 Feb;8:131. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 30.Tuli R, et al. Human mesenchymal progenitor cell-based tissue engineering of a singleunit osteochondral construct. Tissue engineering. 2004 Jul-Aug;10:1169. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- 31.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays : news and reviews in molecular, cellular and developmental biology. 2000 Feb;22:138. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Koyama E, Leatherman JL, Shimazu A, Nah HD, Pacifici M. Syndecan-3, tenascin-C, and the development of cartilaginous skeletal elements and joints in chick limbs. Developmental dynamics : an official publication of the American Association of Anatomists. 1995 Jun;203:152. doi: 10.1002/aja.1002030204. [DOI] [PubMed] [Google Scholar]