Abstract
Obesity is a risk factor for the development of hormone receptor–positive breast cancer in post-menopausal women. Obesity causes subclinical inflammation in white adipose tissue (WAT), characterized by macrophages surrounding dead or dying adipocytes forming crown-like structures (CLS). Estrogen synthesis is catalyzed by aromatase. Previously, we demonstrated CLS and elevated levels of pro-inflammatory mediators and aromatase in the mammary glands (MG) of obese mice and breast tissue of obese women. Here we tested the hypothesis that supplemental estrogen could prevent or reverse WAT inflammation (WATi) and related molecular changes in the MG. C57BL/6J mice were ovariectomized (OVX) to simulate the post-menopausal state. Supplementation with 17β-estradiol (E2) protected against high fat (HF) diet-induced weight gain and MG WATi. Expression of pro-inflammatory mediators (Cox-2, TNF-α, IL-1β) and aromatase were also reduced in the MG of mice that received supplemental E2. Next, to determine whether E2 supplementation can reverse WATi, obese OVX mice were treated with E2 or placebo and then continued on HF diet. E2 supplementation induced weight loss, reversed MG inflammation and down-regulated expression of pro-inflammatory mediators and aromatase. Finally, we determined whether the protective effects of E2 were mediated by estrogen receptor-α (ERα). Knocking out ERα in ovary intact mice fed a HF diet led to weight gain, WATi and elevated levels of pro-inflammatory mediators and aromatase mimicking the effects of OVX. Taken together, our findings indicate that estrogen via ERα protects against weight gain, WATi and associated increases in pro-inflammatory mediators and aromatase in the MG.
Keywords: estrogen, inflammation, adipose tissue, obesity, breast cancer
Introduction
Obesity is a risk factor for the development of hormone receptor (HR)-positive breast cancer in post-menopausal women (1, 2). In addition to increasing risk, obese patients with breast cancer have a worse prognosis (3-5). The development and growth of HR-positive breast cancers are commonly regulated by estrogens. Aromatase, a member of the cytochrome P450 superfamily of enzymes, catalyzes estrogen biosynthesis (6, 7). Inhibitors of aromatase which block the synthesis of estrogens reduce the risk of breast cancer and suppress the recurrence of HR-positive breast cancers (8). After menopause, peripheral aromatization of androgen precursors in adipose tissue is a key source of estrogen. A key unanswered question concerns the relative importance of breast adipose itself versus peripheral adipose tissue in the synthesis of estrogens that stimulate the formation and growth of HR-positive tumors. Although modestly increased levels of circulating estrogen are found in obese post-menopausal women (9), the levels are low and the importance of this source of estrogen in the development of breast cancer is unclear. In fact, The Women's Health Initiative Estrogen-alone Trial found that supplemental estrogen appeared to reduce the risk of breast cancer in post-menopausal women (10). The results of this clinical trial raise additional uncertainty about the link between mildly elevated circulating estrogen levels in obese post-menopausal women and increased breast cancer risk.
Clinically occult inflammation is commonly found in the visceral and subcutaneous white adipose tissue of obese women (11, 12). Macrophages infiltrate the white adipose tissue and form crown-like structures (CLS) around dead or dying adipocytes (11, 13). These macrophages produce a variety of pro-inflammatory mediators. Monocyte chemoattractant protein-1 (MCP-1) plays a significant role in the recruitment of these macrophages to the adipose tissue and may also stimulate macrophage proliferation within the CLS (14, 15). We demonstrated in both experimental models of obesity and in obese women that CLS occur in white adipose tissue of the mammary gland (MG) and breast, respectively (16-18). Ovariectomy (OVX) sensitized mice to both weight gain and MG inflammation (16). Furthermore, both the incidence and severity of breast white adipose inflammation are greater in post-menopausal than in pre-menopausal women suggesting an anti-inflammatory role for estrogen (19). Inflammation, as determined by the presence of CLS, was associated with elevated levels of pro-inflammatory mediators and aromatase suggesting that the obesity→inflammation→aromatase axis may contribute to the increased risk of HR-positive breast cancer in obese women (16, 17).
Menopause associated reductions in estrogen are linked to a significant increase in the incidence of obesity (20). In mouse models, exogenous estrogen attenuates OVX induced weight gain (21). In the current study, we investigated whether supplemental estrogen protected against or reversed histological inflammation and related molecular changes in the MG of mice. Our results suggest that obesity-related inflammation in the MG including elevated levels of pro-inflammatory mediators and aromatase can be attenuated by supplemental estrogen. These findings strengthen the rationale for evaluating whether supplemental estrogen can impact white adipose inflammation in post-menopausal women or women who have undergone surgical removal of the ovaries.
Materials and Methods
Materials
Lowry protein assay kits, glucose-6-phosphate, glycerol, pepstatin, leupeptin, glucose-6-phosphate dehydrogenase and rotenone were purchased from Sigma. 1ß-[3H]-androstenedione was purchased from Perkin-Elmer Life Science. RNeasy mini kits were purchased from Qiagen. MuLV reverse transcriptase, RNase inhibitor, oligo (dT)16, and Fast SYBR green PCR master mix were obtained from Applied Biosystems. Real-time PCR primers were synthesized by Sigma-Genosys. Mouse/rat estradiol Elisa kit was purchased from Calbiotech Inc. 17β-estradiol (E2) and placebo pellets were purchased from Innovative Research of America.
Animal models
Estrogen Prevention Study
At 5 wks of age, sham ovariectomized (sOVX) and OVX C57BL/6J female mice (Jackson Laboratories) were fed either low fat (LF) or high fat (HF) diets. The LF (12450Bi) and HF (D12492i) diets contain 10 kcal% fat and 60 kcal% fat, respectively (Research Diets). The sOVX mice were fed LF diet for 10 wks until sacrifice at 15 wks of age. The OVX mice were fed HF diet for 2 wks and then randomized (n=11/gp) to receive either a placebo pellet or an active 0.1mg E2 pellet (60-day continuous release). The pellets were implanted subcutaneously via 10-gauge trochar. The mice then continued on HF diets for an additional 8 wks until sacrifice at 15 wks of age. The weights of the mice and calories consumed were monitored throughout the experiment.
Estrogen Treatment Study
At 5 weeks of age, sOVX and OVX C57BL/6J female mice (Jackson Laboratories) were fed either LF or HF diets. The sOVX mice were fed LF diet and randomized to one of two groups (n=10/gp). One group was sacrificed at 15 wks of age after 10 wks on LF diet while the other group was sacrificed at 23 wks of age after 18 wks on LF diet. The OVX mice were fed HF diet for 10 wks to induce obesity and were then randomized to one of 3 groups (n=8-10/gp). One group was sacrificed at 15 wks of age after 10 weeks on HF diet. The second group received a placebo pellet and the third group received a 0.1 mg E2 pellet. Pellets (60-day continuous release) were implanted subcutaneously. The two groups that received placebo or E2 were continued on HF diet for an additional 8 wks until sacrifice at 23 wks of age. The weights of the mice and calories consumed were monitored throughout the experiment.
ERα KO Study
At 5 wks of age, sOVX and OVX C57BL/6J female mice (Jackson Laboratories) were fed either LF or HF diets. One group of sOVX mice was fed LF diet for 10 wks until sacrifice (n=10, same group described in the Treatment Study). The OVX mice were fed HF diet for 10 wks until sacrifice (n=10, same group described in the Treatment Study). Another group of sOVX mice was fed HF diet for 10 wks until sacrifice (n=10). The last group of sOVX mice was homozygous for deletion of ERα (ERα-/-, n=10, B6.129P2-Esr1tm1Ksk/J) and was fed HF diet for 10 wks until sacrifice. The ERα-/- mice had been backcrossed to C57BL/6 mice for ten generations. All mice were sacrificed at 15 wks of age. The weights of the mice and calories consumed were monitored throughout the experiment.
Following sacrifice, mammary glands were snap frozen in liquid nitrogen and stored at −80°C for molecular analysis or formalin fixed for histological analyses. The animal protocol was approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
Light microscopy
Four micron-thick sections were prepared from formalin-fixed, paraffin-embedded MG tissue and stained with hematoxylin and eosin. The total number of CLS per section was quantified by a pathologist (DG) and the amount of adipose tissue present on each slide was determined using NIH Image J. Inflammation was quantified as number of CLS per cm2 of adipose tissue.
Adipocyte diameter
Adipocyte diameter was quantified as previously described (17).
Quantitative real-time PCR
Total RNA was isolated using the RNeasy mini kit. For tissue analyses, poly A RNA was prepared with an Oligotex mRNA mini kit (Qiagen). Poly A RNA was reversed transcribed using murine leukemia virus reverse transcriptase and oligo (dT)16 primer. The resulting cDNA was then used for amplification. Primer sequences have been reported previously (16, 22). GAPDH was used as an endogenous normalization control. Real-time PCR was performed using 2x Fast SYBR green PCR master mix on a 7500 Fast Real-time PCR system (Applied Biosystems). Relative fold-induction was determined using the ddCT(relative quantification) analysis protocol.
Aromatase activity
To determine aromatase activity, microsomes were prepared from MG tissue by differential centrifugation. Aromatase activity was quantified by measurement of the tritiated water released from 1ß-[3H]-androstenedione (23). Aromatase activity was normalized to protein concentration.
PGE2 levels
PGE2 levels were quantified using an EIA kit from Cayman Chemical. Protein levels were determined by the method of Lowry (24). Levels of PGE2 were normalized to protein concentrations and expressed as pg/μg protein.
Serum estradiol concentration
Blood was collected from mice by cardiac puncture after sacrifice and allowed to clot at room temperature for 20 min. The blood was then centrifuged at 8,000 rpm at 4°C and serum was isolated and stored at −80°C until assayed. E2 concentration in serum was determined using an ELISA kit. Absorbance was measured at 450nm on an absorbance microplate spectrophotometer.
Statistical analysis
In the mouse experiments, endpoints of interest include mouse weight over time, mouse caloric consumption over time, and various biomarker measurements obtained from mouse MG tissues including number of CLS/cm2, average adipocyte diameter, relative mRNA expression level and aromatase activity and PGE2 levels. Mouse growth rates were calculated under linear growth assumption for defined period of time based on study design. For endpoints that conform to normality assumption (including mouse weight at a specific time point, mouse growth rate and caloric consumption during a specific periods of time, and average adipocyte diameter), ANOVA was used to examine the differences across experimental groups. Pair-wise comparisons were carried out using Tukey's method which adjusts the p-values for multiple comparisons by controlling experiment-wide error rate. For biomarker data including number of CLS/cm2, relative mRNA expression level, aromatase activity and PGE2 levels, the non-parametric Kruskal-Wallis test was used to examine the difference in biomarker levels across experimental groups. The non-parametric Wilcoxon rank-sum test was used to examine the difference in biomarker levels between pairs of groups of interest. P-values were adjusted for multiple comparisons by controlling the false discovery rate.
Results
Estrogen protects against diet-induced obesity and associated histological and molecular changes in the mammary gland
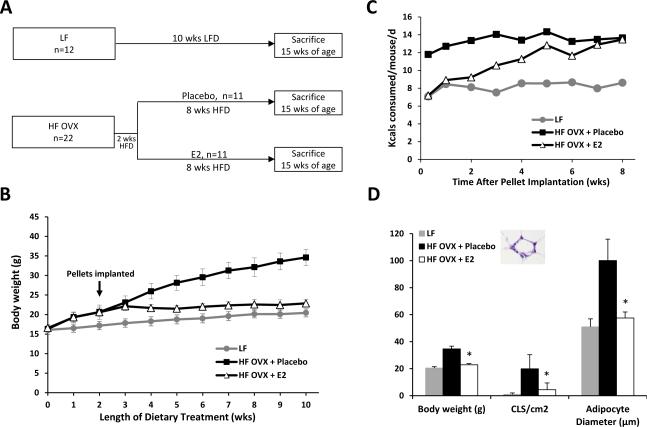
To determine whether estrogen supplementation prevented OVX mice from becoming obese with HFD feeding, HF OVX mice were implanted subcutaneously with E2 or placebo pellets. Following implantation, the mice continued on HFD for 8 wks (Fig. 1A). Ovary intact mice fed a LFD for 10 wks served as lean controls. At sacrifice, serum estradiol levels were similar in ovary intact mice and OVX mice that received supplemental E2 (data not shown). As shown in Fig. 1B, a marked increase in weight occurred in the placebo group over time while the group supplemented with E2 failed to gain weight with HFD feeding. Consistent with the suppression of weight gain, caloric consumption was also initially reduced in the E2 treated group compared to the placebo group (Fig. 1C). Histological analysis of MG tissue was conducted to determine if the inhibition of weight gain in the E2 group was associated with decreased white adipose tissue inflammation. Treatment with the HFD led to a significant increase in MG inflammation, as defined by the number of CLS/cm2, an effect that was attenuated by supplemental E2 (Fig. 1D). Adipocyte size correlates with the number of CLS in both mice and women (17, 22). Accordingly, we next measured adipocyte diameters in all treatment groups. In accord with the inflammation findings, supplemental E2 blocked the increase in adipocyte diameter found in the MG of OVX mice fed the HFD (Fig. 1D).
Figure 1. Supplemental estrogen protects against high fat diet-induced weight gain and mammary gland inflammation.
A, Study schema. A group of ovary intact mice (n=12) was fed a low fat (LF) diet for 10 wks until sacrifice at 15 wks of age. Mice that were subjected to ovariectomy (OVX) were fed high fat (HF) diet for 2 wks and then randomized (n=11/gp) to receive either a placebo pellet or 0.1 mg 17β-estradiol (E2) pellet (subcutaneous implantation, 60-day continuous release). The mice then continued on HF diets for an additional 8 wks until sacrifice at 15 wks of age. B, Body weights of mice in different treatment groups. E2 supplementation prevented HFD induced weight gain. C, Caloric consumption was monitored weekly in mice in each of the three groups. D, In pair-wise comparisons, at sacrifice, mice given E2 supplementation had significantly lower body weights (*, P<0.001), mammary gland inflammation as defined by presence of crown-like structures (CLS/cm2; *, P<0.001), and smaller adipocyte diameter (*, P<0.001) compared to HF mice treated with placebo. Inset, example of a CLS in the MG as shown by hematoxylin and eosin stain.
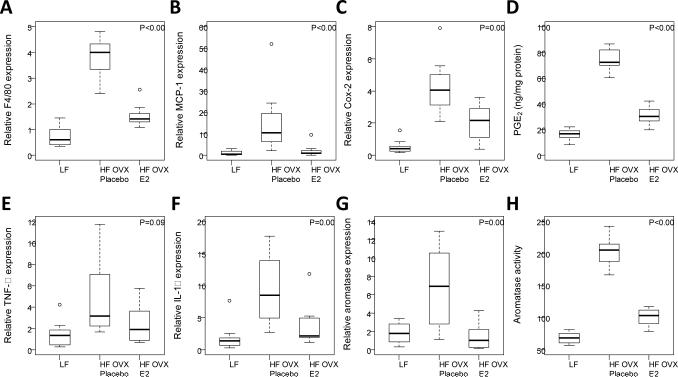
Because supplemental E2 suppressed HFD-mediated increases in the number of CLS/cm2 in the MG, we next conducted real-time PCR to assess expression of the macrophage marker F4/80 in the MG (Fig. 2A). Consistent with the CLS findings, treatment with E2 attenuated the increase in F4/80 mRNA levels mediated by the HFD (Fig. 2A). MCP-1 plays a role in the recruitment of blood monocytes which become macrophages in adipose tissue. Given the ability of E2 to suppress the increase in macrophages in the MG of mice fed the HFD, levels of MCP-1 were quantified. As shown in Fig. 2B, supplemental E2 also blocked the increase in MCP-1 levels in mice fed the HFD. Next we assessed levels of pro-inflammatory mediators. Here too supplemental E2 suppressed the increase in levels of pro-inflammatory mediators (Cox-2, PGE2, TNF-α, IL-1β) found in the MG of mice fed the HFD (Fig. 2, C-F). Because these pro-inflammatory mediators can modulate aromatase expression, we also assessed both aromatase mRNA levels and aromatase activity. The increase in aromatase in the MG of mice fed the HFD was attenuated by supplemental E2 (Fig. 2, G and H).
Figure 2. Estrogen supplementation suppresses the increased levels of pro-inflammatory mediators and aromatase found in the mammary glands of obese mice.
Real-time PCR was carried out on RNA isolated from the mammary gland (MG) of mice in each of the three groups (n=11-12/gp). EIA was used to quantify PGE2. Box-plots of F4/80, MCP-1, Cox-2, PGE2, TNF-α and IL-1β are shown (A-F). In pair-wise comparisons, HF OVX +E2 mice compared to HF OVX +placebo mice had significantly lower MG levels of all biomarkers (P<0.05) except TNF-α (P=0.09). G and H, Mammary gland aromatase expression and activity were also significantly decreased in HF OVX mice supplemented with E2 compared to placebo (P=0.003 and P<0.001, respectively).
Obesity-related mammary gland inflammation and elevated aromatase levels are reversed by estrogen treatment
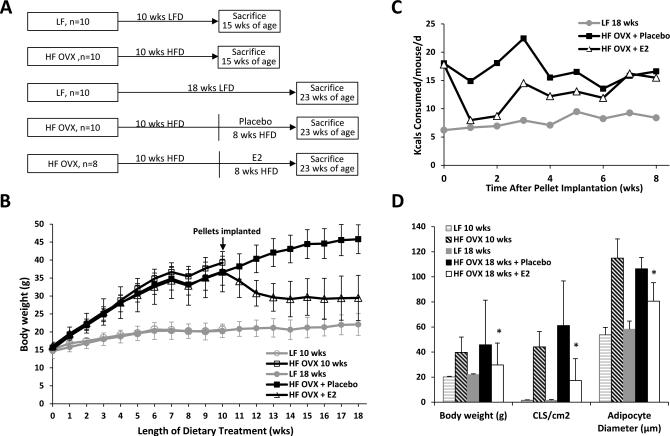
Next, we conducted a treatment study to determine if estrogen supplementation could reverse obesity-related MG inflammation. OVX mice were made obese with HFD feeding for 10 weeks (Fig. 3A). Mice were then either sacrificed or implanted with a placebo or E2 pellet and then continued on HFD for an additional 8 wks. Ovary intact mice fed a LFD for 10 or 18 weeks served as lean controls. At sacrifice, serum estradiol levels were similar in ovary intact mice and OVX mice that received supplemental E2 (data not shown). Immediately after implantation with E2, there was a significant decrease in body weight while the placebo group continued to gain weight normally (Fig. 3B). Consistent with the decreased weight, E2 supplementation also caused a significant decrease in calorie consumption (Fig. 3C). Histological analysis of MG tissue also showed decreased CLS/cm2 and smaller adipocyte diameters in the E2 treated group compared to the placebo group (Fig. 3D). In fact, both the number of CLS/cm2 and adipocyte diameters were also reduced compared to the HFD group that was sacrificed prior to pellet implantation. Therefore, E2 treatment led to weight loss which was associated with both reduced adipocyte size and decreased MG inflammation.
Figure 3. Supplemental estrogen reverses high fat diet-induced weight gain and mammary gland inflammation.
A, Study schema. Ovary intact mice were fed LF diet for 10 or 18 weeks until sacrifice (n=10/gp). The mice that had been subjected to ovariectomy (OVX) were fed HF diet for 10 wks and were then randomized to one of three groups (n=8-10/gp). One group was sacrificed immediately after 10 weeks on HF diet. The second group received placebo and the third group received 0.1 mg 17β-estradiol (E2) pellet (subcutaneous implantation, 60-day continuous release). Both groups continued on HFD for an additional 8 wks. B, Body weight was monitored weekly in the different treatment groups. E2 supplementation reversed HFD-induced weight gain. C, Caloric consumption was monitored weekly in the HFD fed mice following implantation of E2 or placebo and compared to the group that received the LFD. D, In pair-wise comparisons, at sacrifice, mice treated with E2 had significantly lower body weight (*, P<0.001), MG inflammation (CLS/cm2, *, P=0.02), and average adipocyte diameter (*, P<0.001) compared to HFD fed mice given placebo.
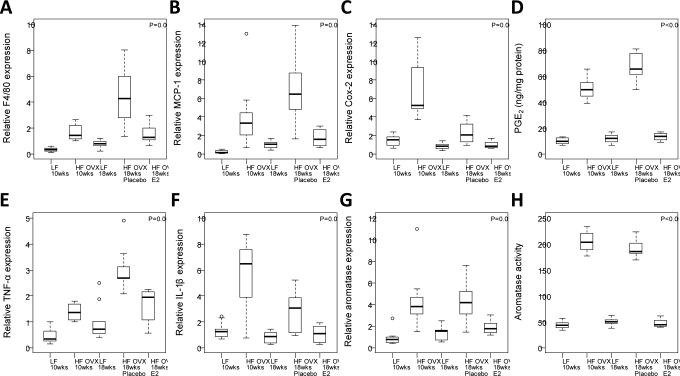
Levels of F4/80, MCP-1 and pro-inflammatory mediators (Cox-2, PGE2 and TNF-α) were all significantly decreased in HFD-fed mice treated with E2 compared with placebo (Fig. 4, A-E). There was also a trend toward decreased expression of IL-1β following E2 treatment (Fig. 4F). As in in the E2 prevention study, aromatase expression and activity paralleled the changes in levels of the pro-inflammatory mediators (Fig. 4, G and H).
Figure 4. Supplemental estrogen reverses the increased levels of pro-inflammatory mediators and aromatase found in the mammary gland of obese mice.
Real-time PCR was carried out on RNA isolated from the mammary gland (MG) of mice in each of five groups (n=8-10/gp). EIA was used to quantify PGE2. Box-plots of F4/80, MCP-1, Cox-2, PGE2, TNF-α, and IL-1β are shown (A-F). In pair-wise comparisons, HF OVX +E2 mice compared to HF OVX + placebo mice had significantly lower MG levels of all biomarkers (P<0.05) except IL-1β (P=0.07). G and H, Mammary gland aromatase expression and activity were also significantly decreased in HF mice supplemented with E2 compared to placebo (P=0.01 and P<0.001, respectively).
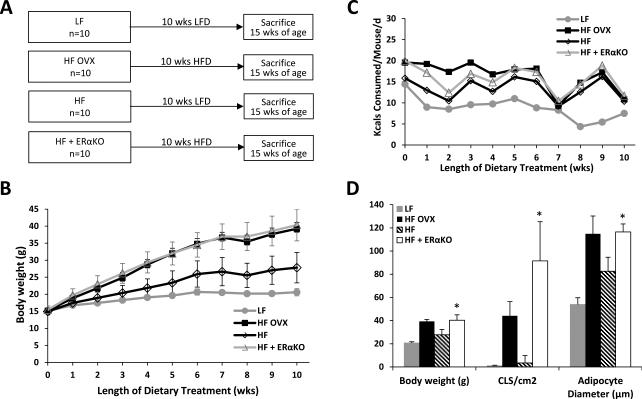
Estrogen acts via ERα to inhibit weight gain and inflammation in the MG
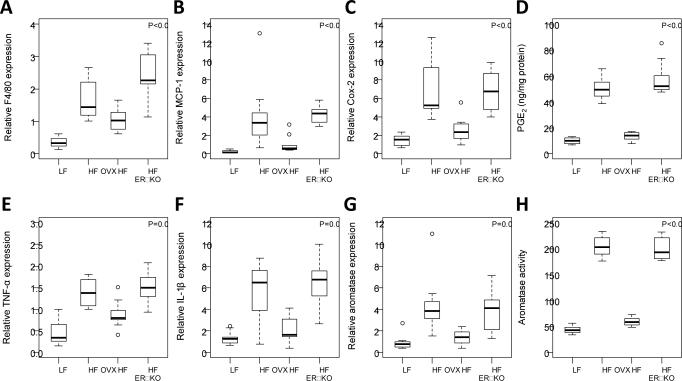
ERα plays a key role in both adipose tissue biology and metabolic processes. Therefore, an experiment was carried out to determine whether knocking out ERα mimicked the effects of OVX. As indicated in Fig. 5A, we compared the effects of HFD in OVX mice, ovary intact mice and ovary intact ERα knockout mice. Ovary intact mice fed a LFD served as lean controls. Weight gain and calorie consumption were increased in ovary intact ERα knockout mice fed a HFD compared with ovary intact wild-type mice fed a HFD (Fig. 5, B and C). In fact, the weight increase observed in ERα knockout mice mirrored the increase found in OVX mice suggesting that E2 acts via ERα to suppress weight gain. Consistent with the weight gain that was observed in ERα knockout vs. wild-type mice, both adipocyte size and the number of CLS/cm2 were increased in the MG of ERα knockout mice (Fig. 5D). Additionally, levels of F4/80, MCP-1, Cox-2, PGE2, TNFα, IL-1β and aromatase were all significantly increased in the ERα knockout vs. wild-type control mice (Fig 6A-H). The fact that both the histological changes and related molecular endpoints found in ERα knockout mice mirrored the changes induced by OVX suggests that the protective effects of estrogen are mediated via ERα.
Figure 5. Estrogen receptor-α mediates the protective effects of estrogen on weight gain and mammary gland inflammation.
A, Study schema. At 5 wks of age, ovary intact mice and mice that had been subjected to ovariectomy (OVX) were fed either low fat (LF) or high fat (HF) diets. The ovary intact wild type mice were fed LF or HF diets for 10 wks until sacrifice (n=10/gp). The OVX mice were fed HF diet for 10 wks (n=10). The last group of ovary intact mice was homozygous for deletion of ERα (ERα knockout) and was fed HF diet for 10 wks. B, Body weight was monitored weekly in the different treatment groups. Weight gain in ERα KO mice mimicked the weight gain observed in OVX mice. C, Calorie consumption was monitored weekly. D, In pair-wise comparisons, at sacrifice, HF OVX and HF + ERα KO mice had greater body weights (*, P<0.001), MG inflammation (CLS/cm2; *, P<0.001), and MG adipocyte diameter (*, P<0.001) than HF mice.
Figure 6. Levels of pro-inflammatory mediators and aromatase are increased in ERα knockout mice compared to wild-type mice.
Real-time PCR was carried out on RNA isolated from the mammary gland of mice in each of the four groups (n=10/gp). EIA was used to quantify PGE2. Box-plots of F4/80, MCP-1, Cox-2, PGE2, TNF-α and IL-1β are shown (A-F). In pair-wise comparisons, HF OVX and HF + ERα KO mice had significantly higher levels of all biomarkers compared to HF mice (P<0.05). Levels of all biomarkers were similar in ERα KO mice compared to levels in the OVX mice (P>0.1). G and H, mammary gland aromatase expression and activity were also significantly increased in OVX and ERα KO mice compared to ovary intact mice (P<0.01).
Discussion
Chronic inflammation of several organs increases the risk of epithelial malignancies (25). Previously, we demonstrated that subclinical inflammation of the breast manifested as CLS-B occurs in most obese and overweight women. Importantly, menopause is an independent determinant of CLS-B (19). Histological inflammation was associated with adipocyte hypertrophy and elevated levels of pro-inflammatory mediators and aromatase (17, 18). We concluded that the obesity→inflammation→aromatase axis was likely to contribute to the development of HR-positive breast cancer in post-menopausal women. These findings in women were predicted by earlier work in a diet-induced mouse model of obesity (18). In the mouse model, OVX sensitized the mice to HFD-induced obesity, adipose inflammation and related molecular changes in the MG. The fact that ovary intact mice were resistant to these effects led us to focus on the role of estrogen in the current study.
Initially, we determined whether supplemental estrogen protected against diet-induced obesity and associated histological and molecular changes in the MG of OVX mice. Supplemental E2 suppressed caloric consumption and weight gain. Moreover, HFD induced adipocyte hypertrophy, white adipose inflammation, and increased levels of pro-inflammatory mediators and aromatase were suppressed by supplemental E2 in OVX mice. To complement this prevention study, we treated obese OVX mice with E2. Here, treatment with E2 led to weight loss with associated decreases in adipocyte size, white adipose inflammation and levels of pro-inflammatory mediators and aromatase. Although these effects of exogenous estrogen on the MG were previously unknown, the findings are consistent with the known biological effects of estrogen. More specifically, supplemental estrogen has been reported to suppress weight gain, protect against adipocyte hypertrophy and attenuate adipose inflammation in other fat depots in mice (21, 26, 27). Our results are also consistent with findings in women where loss of ovarian function due to natural aging or surgical removal of the ovaries is associated with weight gain and increases in adipose tissue (20, 28). The observed anti-inflammatory effects of E2 in the MG could be a consequence of its ability to inhibit weight gain or stimulate weight loss. However, estrogen is also known to possess anti-inflammatory activity. For example, estrogen can block lipopolysaccharide-mediated induction of pro-inflammatory pathways in macrophages (29, 30). NF-κB activation can be blocked by estrogen (30, 31). Hence, it is unclear if treatment with E2 led to improved white adipose inflammation exclusively due to reductions in weight or if there were additional anti-inflammatory effects. The fact that the findings in ERα knockout mice mirrored the effects of OVX is a clear indication that the effects of E2 were mediated by ERα. ERα can mediate its effects via the nucleus, the plasma membrane or both (32). Future studies are warranted to determine whether one or both of these functions is important for regulating energy balance and inflammation. Our results also raise the possibility of a feedback loop whereby loss of ovarian function leads to up regulation of aromatase. This increase in aromatase was blunted by treatment with exogenous E2. Given the significance of estrogen in mediating a multitude of important biological effects, such a feedback loop could well exist. Estrogen affects energy balance via effects on both appetite and metabolism (33, 34). We showed that E2 suppressed calorie consumption which helps to explain the observed changes in weight. This finding fits with prior reports that higher levels of estradiol during the menstrual cycle and pregnancy lead to decreased food intake and fat accumulation (35). By contrast, OVX and menopause lead to increased food intake, an effect that is reversed by treatment with estradiol. Estradiol has anorexigenic functions in the brain (35). More specifically, E2 has direct actions in the hypothalamus that affect feeding behavior. In fact, silencing of ERα in the ventromedial nucleus of the hypothalamus of mice leads to obesity (36). Overall, our results suggest that E2 via ERα suppressed caloric intake leading to weight changes. Because estradiol can also affect energy expenditure leading to weight changes (33, 34), future studies are warranted to determine if E2-mediated changes in metabolism contribute to the observed suppressive effects on adipose inflammation and related molecular changes in the MG.
It is important to consider the possible clinical implications of this study. As mentioned above, we recently showed that menopause is associated with both an increased incidence and severity of breast white adipose inflammation (19). The current preclinical results raise the possibility that supplemental estrogen will either protect against or reverse white adipose inflammation and related molecular changes in the breast and other adipose depots. Because estrogen stimulates endometrial hyperplasia, this type of question will be best addressed in women who have undergone a hysterectomy. Our results also support the goal of developing agents that target estrogen delivery to the brain as an approach to improving the therapeutic index. Such an agent might prove useful in modulating weight and secondarily breast adipose inflammation. In support of this possibility, a glucagon-like peptide-1-estrogen conjugate was recently reported to improve energy balance, glucose and lipid metabolism in the absence of the hallmark side effects of estrogen such as reproductive endocrine toxicity (37). A limitation of the current study is that we did not study the effects of supplemental estrogen in lean mice. This was purposeful because the white adipose tissue is uninflamed in lean mice. Hence, our preclinical findings are potentially relevant to the subset of women with occult adipose inflammation. Taken together, our data raise the intriguing possibility that supplemental estrogen will be beneficial in modulating white adipose inflammation, a process linked to insulin resistance, cardiovascular disease and possibly breast cancer (12, 38).
Acknowledgments
Grant Support:
This work was supported by grants NIH/NCI R01CA154481 (to A.J. Dannenberg), R01 CA185293 (to A.J. Dannenberg and C.A. Hudis), UL1TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College (to X. K. Zhou), the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick; to A.J. Dannenberg), and the Breast Cancer Research Foundation (to A.J. Dannenberg and C.A. Hudis).
Footnotes
Disclosures: None
References
- 1.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 5.Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9:1091–101. doi: 10.1586/era.09.71. [DOI] [PubMed] [Google Scholar]
- 6.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 7.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 10.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Iyengar NM, Morris PG, Zhou XK, A. G, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila) doi: 10.1158/1940-6207.CAPR-14-0243. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 21.Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51:861–70. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6:282–9. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Subbaramaiah K, Hudis C, Chang SH, Hla T, Dannenberg AJ. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J Biol Chem. 2008;283:3433–44. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 25.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 26.Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, et al. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab. 2012;303:E445–56. doi: 10.1152/ajpendo.00638.2011. [DOI] [PubMed] [Google Scholar]
- 27.Stubbins RE, Najjar K, Holcomb VB, Hong J, Nunez NP. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab. 2012;14:58–66. doi: 10.1111/j.1463-1326.2011.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–58. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vegeto E, Ghisletti S, Meda C, Etteri S, Belcredito S, Maggi A. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J Steroid Biochem Mol Biol. 2004;91:59–66. doi: 10.1016/j.jsbmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 31.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–68. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin ER. Extranuclear estrogen receptor's roles in physiology: lessons from mouse models. Am J Physiol Endocrinol Metab. 2014;307:E133–40. doi: 10.1152/ajpendo.00626.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–99. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–6. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–56. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]