Abstract
Background
Functional endoscopic sinus surgery (FESS) was historically predicated on targeted widening of narrow anatomic structures that caused post-obstructive persistent sinus inflammation. It is now clear that chronic rhinosinusitis (CRS) is a multi-factorial disease with subsets of patients which may require a more extensive surgical approach. This study compares quality-of-life (QOL) and disease severity outcomes after FESS based on the extent of surgical intervention.
Methods
Participants with CRS were prospectively enrolled into an on-going, multi-institutional, observational, cohort study. Surgical extent was determined by physician discretion. Participants undergoing bilateral frontal sinusotomy, ethmoidectomy, maxillary antrostomy, and sphenoidotomy were considered to have undergone ‘complete’ surgery, while all other participants were categorized as receiving ‘targeted’ surgery. Improvement was evaluated between surgical subgroups with at least 6-month follow-up using the 22-item Sinonasal Outcome Test (SNOT-22) and the Brief Smell Inventory Test (BSIT).
Results
311 participants met inclusion criteria with 147 subjects undergoing complete surgery and 164 targeted surgery. A higher prevalence of asthma, ASA sensitivity, nasal polyposis, and a history of prior sinus surgery (p≤0.002) was present in participants undergoing complete surgery. Mean improvement in SNOT-22 (28.1[21.9] vs. 21.9[20.6];p=0.011) and BSIT (0.8[3.1] vs 0.2[2.4];p=0.005) was greater in subjects undergoing complete surgery. Regression models demonstrated a 5.9[2.5] greater relative mean improvement on SNOT-22 total scores with complete surgery over targeted approaches (p=0.016).
Conclusions
Complete surgery was an independent predictor of greater postoperative SNOT-22 score improvement, yet did not achieve clinical significance. Further study is needed to determine the optimal surgical extent.
Keywords: Sinusitis, diagnosis, quality of life, endoscopy, therapy
Introduction
Since the introduction of functional endoscopic sinus surgery (FESS), surgical intervention has been targeted at objective signs of inflammation seen on either radiographic or endoscopic exam.1 The rationale behind this targeted approach is in part based on the techniques of Messerklinger who placed a large emphasis on anatomic configurations that predispose a given sinus to chronic rhinosinusitis (CRS), while minimizing surgical intervention and risk to the patient.1 Over the past 30 years our understanding of the underlying mechanisms of CRS has dramatically evolved and how we manage this disease both surgically and medically.
Chronic rhinosinusitis may evolve for a variety of reasons beyond anatomic and ostial obstruction that warrants a multifactorial treatment approach. Intrinsic mucosal inflammation, dysbiosis, and mucociliary dysfunction can all play independent roles in the pathophysiology of CRS,2 that may benefit from a more complete surgical approach. CRS is classically associated with an inflammatory infiltrate that is successfully treated with anti-inflammatory topical therapies.3,4 It has been proposed that complete marsupialization of the sinuses improves delivery of topical therapies,5 which a targeted approach cannot achieve. In targeted surgery the natural ostia of the sinuses may be predisposed to cicatricial scarring, secondary to instrumentation of the sinonasal mucosa as the sinus is not widely opened. This stems from the concept that the risk of scarring is theoretically highest when opposing mucosal cut edges are in close proximity, which may occur at the junction between the dissected and undissected anatomy. For example, a frontal Draf I sinusotomy allows for a remnant agger nasi and ethmoid bulla an opportunity to adhere in contrast to a Draf IIa frontal sinusotomy that can allow for a larger frontal postoperative opening if achieved with mucosal sparing technique. Finally, given that little is known about the natural history of CRS, it is highly plausible that surgical interventions may be undertaken too early in the disease process. This may result in a subset of patients that fail initial surgical management that go on to require either targeted revision or completion surgery as the disease progresses and involves more sinuses.
The goal of the present study was to compare both postoperative endoscopic examination findings and mean improvements in QOL outcomes in study subjects that have undergone “complete” FESS to subjects with “targeted” FESS. We hypothesized that subjects that undergo more complete surgery have both greater QOL and better postoperative endoscopic findings than subjects that underwent more minimal or targeted sinus surgery.
Materials & Methods
Patient Population and Inclusion Criteria
Adult (≥18 years) study participants were enrolled and followed across four academic, tertiary care rhinology practices including the Oregon Health & Science University (OHSU, Portland, OR, USA), the Medical University of South Carolina (Charleston, SC, USA), Stanford University (Palo Alto, CA, USA), and the University of Calgary (Calgary, Alberta, Canada). All subjects had a diagnosis of CRS based on the Rhinosinusitis Task force criteria6 and were enrolled dependent on the subjects electing to pursue FESS after failing either broad-spectrum and/or culture-directed antibiotics and a trial of oral and topical steroid therapy. The Institutional Review Board at each enrollment location provided oversight and annual review of the informed consent process and all investigational protocols, while central review and coordination services were conducted at OSHU (eIRB #7198). This data is part of an on-going, multi-institutional, prospective, observational cohort study that has been previously reported.7-15
Patient Study Data Collection
Study participants were required to speak and understand English and complete all necessary baseline evaluations in addition to informed consent. Consented participants were asked to provide demographic, social and medical history cofactors including, but not limited to: age, gender, race, ethnicity, asthma, nasal polyposis, known allergies (reported by patient history or confirmed skin prick or radioallergosorbent testing), acetylsalicylic acid (ASA) sensitivity, depression, current tobacco use, history of prior sinus surgery, and ciliary dyskinesia/cystic fibrosis.
Exclusion Criteria
Study participants diagnosed with either a current exacerbation of recurrent acute sinusitis or ciliary dyskinesia was excluded from the final study cohort due to the heterogeneity of those comorbid disease processes. Participants were also excluded from final analyses if they failed to complete all required baseline study evaluations or had not yet either entered into the minimum follow-up appointment time window or completed follow-up evaluations within 18 months after enrollment.
Outcome Measurements
All study participants were asked to complete 22-item Sinonasal Outcome Test (SNOT-22) at baseline and at follow-up.13,16 The SNOT-22 is a validated, 22-item treatment outcome measure applicable to chronic sinonasal conditions (©2006, Washington University, St. Louis, MO, USA). Higher scores on the SNOT-22 survey items suggest worse patient functioning or symptom severity (total score range: 0-110). Participants were asked to complete the SNOT-22 survey at both baseline appointments and at least 6-months after surgical intervention. The last available follow-up of at least six months was used for determining interval change in SNOT-22 scores.17 Patients were lost to follow-up if they did not complete any survey evaluations within 18 months after enrollment. Physicians at each site were blinded to all patient-based survey responses for the study duration.
Olfactory function was evaluated at both the initial enrollment period and during follow-up visits and operationalized using The Brief Smell Identification Test (B-SIT; Sensonics, Inc., Haddon Heights, NJ, USA). The B-SIT is a validated 12-item, standardized, noninvasive test of olfactory function that employs 12 microencapsulated odorant strips in a “scratch-‘n-sniff” format (score range: 0-12), with higher scores indicating a better sense of smell. Complete B-SIT scores ≥9 are defined as “normal” for healthy males and females of all ages.18 Change in both SNOT-22 and olfactory scores were defined as mean interval change between baseline scores and postoperative score at the last available follow-up.
Clinical Disease Severity Measures
During routine initial clinical / enrollment visits, all study subjects completed a medical history, head and neck clinical examinations, sinonasal endoscopy and computed tomography (CT) imaging of the coronal plane using 1.0-3.0mm axial slices. Endoscopic examinations were scored using the Lund-Kennedy endoscopy scoring system where higher scores represent worse bilateral disease severity (score range: 0-20).19 Computed tomography images were evaluated and staged in accordance with the Lund-Mackay bilateral scoring system where higher scores represent higher bilateral severity of disease (score range: 0-24).20 Both CT imaging and endoscopy scores were assessed by the enrolling physician at each enrollment site.
Surgical Extent: “Complete” and “Targeted” Surgical Definitions
Surgical extent was dictated by individual disease processes and the intraoperative clinical judgment of the enrolling surgeons. Participants were either primary or revision surgery cases. Surgical procedures included various combinations of the following: unilateral or bilateral maxillary anstrostomy, partial or total ethmoidectomy, sphenoidotomy, middle or inferior turbinate reduction, frontal sinus procedures (Draf I, IIa/b, or III), and septoplasty. For the purposes of this investigation the surgical interventions were operationalized into either a “complete” surgical intervention, which was defined by bilateral maxillary antrostomies, bilateral total ethmoidectomies, bilateral sphenoidotomies, bilateral frontal sinusotomies (Draf IIa, IIb, or III). Any surgical intervention short of this definition was operationalized as a “targeted” surgery. The extent of surgery was examined relative to the extent of disease by each sinus. Radiographic extent of disease was defined as non-zero scores on the Lund-Mackay grading scale. Extent of the surgical interventions was extrapolated from current procedural terminology (CPT) codes recorded at the time of surgery. Surgery involved the frontal sinusotomy if CPT #31276 was coded, anterior and posterior ethmoid sinuses for CPT #31255 (total ethmoidectomy), anterior ethmoid sinus for CPT #31254 (partial ethmoidectomy), maxillary sinus for CPT #31256 and CPT #31267, and sphenoid sinus for CPT #31287 and CPT #31288. Draf III frontal sinusotomy was signified as a bilateral frontal sinus intervention.
Data Management and Statistical Analysis
Study data was collected at each enrollment site using standardized clinical research forms and transferred to a central database collection system (Microsoft Office Access 2007; Microsoft Inc., Redmond, WA, USA). The final study dataset was analyzed using SPSS statistical software (SPSS v22.0, IBM Corporation, Armonk, NY, USA). Descriptive statistics (means [standard deviations], frequencies) and graphical analysis was complete for all final study cohort factors to evaluate assumptions of normality. Two-sided sample t-tests, Mann-Whitney U, and analysis of variance (ANOVA) omnibus tests with Bonferroni adjustments for multiple comparisons were used to identify significant differences between all continuously measured variables between surgical subgroups where appropriate. Pearson's chi-square (χ2) testing was used to compare differences in the prevalence of demographic and comorbid factors between surgical subgroups. Match paired t-tests or Wilcoxon signed-rank tests were used to evaluate significant improvement in each outcome measure over time. Furthermore, to best reflect the magnitude of postoperative change relative to initial symptom severity, individual percent changes [((Post – Pre)/Pre)*100] were calculated for each subject not reporting summarized total or domain preoperative scores of zero.21 Linear regression modeling was performed to adjust for potential independent cofactors associated with postoperative improvement in SNOT-22 scores. Eight preliminary models were built and included all independent covariates with univariate significance (p<0.250) while manually controlling for potential enrollment site / surgeon experience and follow-up time (months). The main exposure variable of interest was surgical extent (complete vs. targeted) while the main outcomes of interest was change in SNOT-22 total scores, five SNOT-22 domain scores, endoscopy scores, and B-SIT scores. Additional covariates were introduced into preliminary models to assess potential confounding of the effect estimate for the main exposure variable. Final models were selected using forward selection (p=0.100) and a manual stepwise backwards elimination (p=0.050) process. Final model results were reported using unadjusted and adjusted effect estimates (β), standard error means (SE), 95% confidence intervals, and corresponding p-values. Coefficient of multiple determination values (R2) were used to assess model fit. Variance inflation factors (VIFs) were used to quantify any multi-collinearity in final model factors.
Results
Study Population and baseline characteristics
A total of 535 participants undergoing FESS were enrolled between February, 2011 and April, 2014. Of these subjects, 342(63.9%) had available follow-up, and after application of inclusion and exclusion criteria a total of 311 subjects were included in the final analysis (Figure 1). Average follow-up for the entire cohort (n=311) was 13.0[5.5] months. Categorization of the subjects into complete and targeted surgical subgroups yielded 147(47.3%) and 164(52.7%) in each subgroup, respectively. Loss to follow-up was similar between subjects undergoing targeted and complete surgery. After removal of subjects with RARS and ciliary dyskinesia, 169 of 193 subjects without follow-up were found to have similar prevalence of complete and targeted surgery (n=77 (45.6%) and n=92 (54.4%); p=0.721, respectively) compared to the 311 subjects with follow-up. Compare to study subjects lost to follow-up, subjects with follow-up had a significantly higher mean age (47.7[14.5] vs. 52.5[15.0] years; p<0.001), lower prevalence of allergies (49.7% vs. 38.9%; p=0.022) and inferior turbinate reduction (33.1% vs. 15.8%; p<0.001), as well as significantly worse baseline mean CT scores (11.1[6.2] vs. 12.5[6.0]; p=0.021) and endoscopy scores (5.5[3.8] vs. 6.3[3.8]; p=0.039).
Figure 1. Derivation of final study cohort.
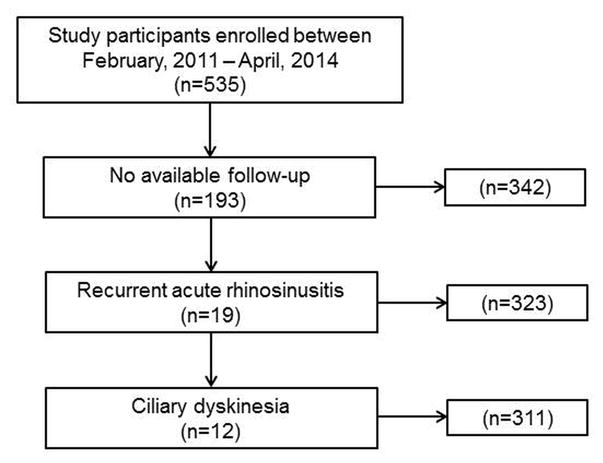
Compared to subjects undergoing targeted surgical interventions, subjects undergoing complete sinus surgery were more likely to have asthma (p<0.001), ASA sensitivity (p<0.001), a history prior sinus surgery (p=0.002), and nasal polyposis (p<0.001; Table 1). Average preoperative CT scores, endoscopy scores, and B-SIT olfactory scores reflected a significantly worse burden of disease in subjects undergoing complete surgery across all measures (p<0.001). Disease burden and symptom severity was also higher in subjects undergoing complete surgery as measured by total SNOT-22 (p<0.001) scores and across all subdomains with the exception of ‘Ear/Facial symptoms’ (p=0.923). Subjects undergoing targeted surgery were more likely to undergo inferior turbinate reduction (p<0.001) while subjects undergoing complete surgery were more likely to undergo image-guided surgery (p<0.001) and middle turbinate resection (p=0.015). The prevalence of radiographic disease and corresponding surgical intervention are displayed for each sinonasal region (Table 2).
Table 1. Comparisons of baseline characteristics of subjects undergoing targeted and complete endoscopic sinus surgery.
| Complete Surgery (n=147) | Targeted Surgery (n=164) | ||||
|---|---|---|---|---|---|
| Demographics: | Mean [SD] | N(%) | Mean [SD] | N (%) | p-value |
| Follow-up duration (months) | 12.6 [5.3] | 13.4 [5.7] | 0.188 | ||
| Age (years) | 52.4 [15.3] | 52.6 [14.7] | 0.910 | ||
| Males | 78 (53.1) | 71 (43.3) | ---- | ||
| Females | 69 (46.9) | 93 (56.7) | 0.085 | ||
| White / Caucasian | 121 (82.3) | 138 (84.1) | 0.665 | ||
| Hispanic / Latino | 7 (4.8) | 10 (6.1) | 0.605 | ||
| Clinical characteristics: | |||||
| Asthma | 74 (50.3) | 41 (25.0) | <0.001 | ||
| Allergies (skin prick / RAST confirmed) | 61 (41.5) | 60 (36.6) | 0.375 | ||
| ASA sensitivity | 22 (15.0) | 4 (2.4) | <0.001 | ||
| Depression | 21 (14.3) | 31 (18.9) | 0.276 | ||
| Tobacco use/current smoker | 7 (4.8) | 13 (7.9) | 0.256 | ||
| Alcohol consumption | 64 (43.5) | 77 (47.0) | 0.546 | ||
| COPD | 11 (7.5) | 6 (3.7) | 0.139 | ||
| Steroid dependency | 10 (6.8) | 11 (6.7) | 0.973 | ||
| Previous sinus surgery | 90 (61.2) | 72 (43.9) | 0.002 | ||
| Nasal polyposis | 90 (61.2) | 30 (18.3) | <0.001 | ||
| Septal deviation | 64 (43.5) | 68 (41.5) | 0.712 | ||
| Hypertrophy turbinate | 16 (10.9) | 31 (18.9) | 0.049 | ||
| Outcome measures: | |||||
| SNOT-22 total score | 57.4 [18.9] | 49.8 [18.9] | <0.001 | ||
| Rhinologic symptom score | 18.8 [5.6] | 14.6 [6.1] | <0.001 | ||
| Extra-nasal rhinologic score | 9.1 [3.3] | 7.9 [3.6] | 0.003 | ||
| Ear / Facial symptoms | 9.3 [5.0] | 9.3 [4.9] | 0.923 | ||
| Psychological dysfunction score | 17.0 [8.4] | 15.1 [8.3] | 0.050 | ||
| Sleep dysfunction score | 14.5 [6.9] | 12.9 [6.6] | 0.031 | ||
| Computed tomography score | 15.8 [4.8] | 9.6 [5.5] | <0.001 | ||
| Endoscopy score | 8.2 [3.6] | 4.6 [3.1] | <0.001 | ||
| BSIT Olfaction score | 7.8 [3.6] | 9.7 [2.5] | <0.001 | ||
| Adjuvant Surgical Procedures: | |||||
| Image guidance | 131 (89.1) | 77 (47.0) | <0.001 | ||
| Septoplasty | 67 (45.6) | 58 (35.4) | 0.067 | ||
| Inferior turbinate reduction* | 11 (7.5) | 38 (23.2) | <0.001 | ||
| Middle turbinate resection* | 31 (21.1) | 18 (11.0) | 0.015 | ||
SD, standard deviation; RAST, radioallergosorbent; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; SNOT-22, 22-item Sinonasal Outcome Test; BSIT, Brief Smell Identification Test;
either unilateral or bilateral
Table 2. Surgical extent data by targeted versus complete endoscopic sinus surgery.
| Sinonasal region: | Complete Surgery (n=147) |
Targeted Surgery (n=164) |
||||||
|---|---|---|---|---|---|---|---|---|
| RIGHT SIDE | LEFT SIDE | RIGHT SIDE | LEFT SIDE | |||||
| Operated (%) | Radiographic Evidence of Disease (%)* | Operated (%) | Radiographic Evidence of Disease (%)* | Operated (%)2 | Radiographic Evidence of Disease (%)* | Operated (%)2 | Radiographic Evidence of Disease (%)* | |
| Sphenoid | 100 | 91.9 | 100 | 87.8 | 41.5 | 48.8 | 41.5 | 43.9 |
| Maxillary | 100 | 96.6 | 100 | 96.6 | 79.3 | 80.5 | 82.3 | 83.5 |
| Anterior Ethmoid1 | 100 | 98.0 | 100 | 96.6 | 22.6 | 73.1 | 22.6 | 74.4 |
| Posterior Ethmoid1 | 100 | 98.0 | 100 | 98.0 | 56.1 | 60.4 | 58.5 | 64.0 |
| Frontal3 | 100 | 91.8 | 100 | 89.8 | 42.1 | 52.5 | 39.0 | 47.0 |
| Ostiomeatal complex | --- | 78.9 | --- | 75.5 | --- | 49.4 | --- | 53.0 |
Disease on CT is defined as a non-zero score on Lund-Mackay score
Operated sinus data was extracted from current procedural terminology codes recorded at the time of surgery. Surgery involved the frontal sinus if CPT 31276 was coded, anterior and posterior ethmoid sinuses for CPT 31255 (total ethmoidectomy), anterior ethmoid sinus for CPT 31254 (partial ethmoidectomy), maxillary sinus for CPT 31256 and CPT 31267, and sphenoid sinus for CPTs 31287 and 31288. Draf III were counted as a bilateral frontal sinus intervention
Either unilateral or bilateral surgery
Frontal sinus surgery included Draf IIa, Draf IIb and Draf III surgeries
Postoperative QOL and Objective Outcomes
Significant absolute mean improvement of SNOT-22 total and subdomain scores was found for the total cohort, as well as for the complete and targeted surgical subgroups (Table 3). Subjects undergoing complete sinus surgery were found to experience greater absolute improvement on SNOT-22 total scores, as well as rhinologic symptom domain scores, extra-nasal rhinologic symptom domain scores, endoscopy, and B-SIT olfaction scores (Table 4). Comparisons between absolute mean improvement in outcome scores were also evaluated across subjects undergoing complete surgery and various independent categories of targeted surgery, including surgery consisting of just maxillary antrostomy and partial ethmoidectomy (“Mini-FESS”), unilateral surgery, and other targeted procedures with and without frontal sinusotomy (Table 5). After adjustment for multiple comparisons, significantly greater mean improvement in SNOT-22 total scores were reported by subjects undergoing complete surgery compared to unilateral surgery (p=0.012). Similarly, complete surgery was also associated with significantly greater improvement on rhinologic symptom domain scores compared to both unilateral surgery (p=0.001) and targeted surgery without any type of frontal sinusotomy (p=0.002), as well as greater improvement in mean extra-nasal rhinological symptom domain scores compared to unilateral surgery (p=0.050) and mean endoscopy scores compared to unilateral (p=0.025) and targeted surgery without frontal sinusotomy (p=0.013). Interestingly, significantly greater mean improvement in SNOT-22 ear / facial symptom domain scores were reported by subjects undergoing mini-FESS compared to unilateral procedures (p=0.046).
Table 3. Mean improvement and relative differences in outcome measures after endoscopic sinus surgery.
| Outcomes measures: | Baseline | Follow-up | Absolute Improvement | |
|---|---|---|---|---|
| Total cohort (n=311) | Mean [SD] | Mean [SD] | Mean [SD] | p-value |
| SNOT-22 total score | 53.4 [19.3] | 28.5 [21.4] | 24.8 [21.4] | <0.001 |
| Rhinologic symptom score | 16.6 [6.2] | 8.4 [6.3] | 8.1 [7.3] | <0.001 |
| Extra-nasal rhinologic score | 8.5 [3.5] | 4.6 [3.6] | 3.8 [3.8] | <0.001 |
| Ear / Facial symptoms | 9.3 [5.0] | 4.5 [4.6] | 4.8 [4.9] | <0.001 |
| Psychological dysfunction score | 16.0 [8.4] | 8.9 [8.6] | 7.1 [8.4] | <0.001 |
| Sleep dysfunction score | 13.7 [6.8] | 7.9 [6.9] | 5.7 [6.8] | <0.001 |
| Endoscopic Score | 6.3 [3.8] | 3.5 [2.9] | 3.1 [4.2] | <0.001 |
| BSIT Olfaction score | 8.9 [3.2] | 9.0 [2.7] | 0.2 [2.8] | 0.317 |
| Complete surgery (n=147) | ||||
| SNOT-22 total score | 57.4 [18.9] | 29.3 [21.0] | 28.1 [21.9] | <0.001 |
| Rhinologic symptom score | 18.8 [5.6] | 8.8 [6.2] | 10.0 [7.2] | <0.001 |
| Extra-nasal rhinologic score | 9.1 [3.3] | 4.9 [3.8] | 4.2 [3.7] | <0.001 |
| Ear / Facial symptoms | 9.3 [5.0] | 4.3 [4.4] | 4.9 [5.0] | <0.001 |
| Psychological dysfunction score | 17.0 [8.4] | 9.2 [8.7] | 7.8 [8.9] | <0.001 |
| Sleep dysfunction score | 14.5 [6.9] | 8.1 [7.1] | 6.4 [6.9] | <0.001 |
| Endoscopic Score | 8.2 [3.6] | 4.2 [3.0] | 4.1 [4.5] | <0.001 |
| BSIT Olfaction score | 7.8 [3.6] | 8.6 [2.7] | 0.8 [3.1] | 0.020 |
| Targeted surgery (n=164) | ||||
| SNOT-22 total score | 49.8 [18.9] | 27.8 [21.7] | 21.9 [20.6] | <0.001 |
| Rhinologic symptom score | 14.6 [6.1] | 8.1 [6.3] | 6.4 [7.1] | <0.001 |
| Extra-nasal rhinologic score | 7.9 [3.6] | 4.5 [3.5] | 3.4 [3.9] | <0.001 |
| Ear / Facial symptoms | 9.3 [4.9] | 4.7 [4.8] | 4.6 [4.9] | <0.001 |
| Psychological dysfunction score | 15.1 [8.3] | 8.6 [8.4] | 6.5 [7.9] | <0.001 |
| Sleep dysfunction score | 12.9 [6.6] | 7.7 [6.6] | 5.1 [6.7] | <0.001 |
| Endoscopic Score | 4.6 [3.1] | 2.7 [2.6] | 2.1 [3.7] | <0.001 |
| BSIT Olfaction score | 9.7 [2.5] | 9.4 [2.6] | 0.2 [2.4] | 0.432 |
SD, standard deviation; SNOT-22, 22-item Sinonasal Outcome Test; BSIT, Brief Smell Identification Test;
Table 4. Comparison of absolute mean score improvements between treatment groups.
| Complete Surgery | Targeted Surgery | |||
|---|---|---|---|---|
| Outcomes: | Mean [SD] Improvement | Mean [SD] Improvement | t-test | p-value |
| SNOT-22 total score | 28.1 [21.9] | 21.9 [20.6] | 2.550 | 0.011 |
| Rhinologic symptom score | 10.0 [7.2] | 6.4 [7.1] | 4.414 | <0.001 |
| Extra-nasal rhinologic score | 4.2 [3.7] | 3.4 [3.9] | 1.893 | 0.037 |
| Ear / Facial symptoms | 4.9 [5.0] | 4.6 [4.9] | 0.543 | 0.587 |
| Psychological dysfunction score | 7.8 [8.9] | 6.5 [7.9] | 1.392 | 0.165 |
| Sleep dysfunction score | 6.4 [6.9] | 5.1 [6.7] | 1.613 | 0.108 |
| Endoscopic Score | 4.1 [4.5] | 2.1 [3.7] | 3.331 | 0.001 |
| BSIT Olfaction score | 0.8 [3.1] | 0.2 [2.4] | -2.913 | 0.005 |
SD, standard deviation; SNOT-22, 22-item Sinonasal Outcome Test; BSIT, Brief Smell Identification Test;
Table 5. Comparison of absolute mean score improvements between complete and various targeted subgroups.
| Complete Surgery (n=147) | Mini-FESS Surgery (n=18) | Unilateral Surgery (n=34) | Targeted Surgery w/o Frontal Sinusotomy (n=51) | Targeted Surgery w/ Frontal Sinusotomy (n=61) | |||
|---|---|---|---|---|---|---|---|
| Outcomes: | Mean [SD] Improvement | Mean [SD] Improvement | Mean [SD] Improvement | Mean [SD] Improvement | Mean [SD] Improvement | F-test | p-value |
| SNOT-22 total score | 28.1 [21.9] | 29.7 [16.9] | 14.8 [19.1] | 21.1 [20.8] | 24.1 [21.4] | 3.409 | 0.007 |
| Rhinologic symptom score | 10.0 [7.2] | 8.6 [4.7] | 4.5 [6.2] | 5.7 [7.9] | 7.4 [7.1] | 6.315 | <0.001 |
| Extra-nasal rhinologic score | 4.2 [3.7] | 4.5 [3.5] | 2.2 [3.9] | 3.1 [3.7] | 4.0 [3.9] | 2.662 | 0.038 |
| Ear / Facial symptoms | 4.9 [5.0] | 7.1 [4.6] | 3.0 [4.3] | 4.2 [5.1] | 5.1 [4.9] | 2.398 | 0.049 |
| Psychological dysfunction score | 7.8 [8.9] | 8.1 [7.6] | 4.6 [8.6] | 6.9 [7.0] | 6.7 [8.3] | 1.121 | 0.198 |
| Sleep dysfunction score | 6.4 [6.9] | 6.9 [6.1] | 3.5 [6.1] | 5.1 [7.1] | 5.5 [6.9] | 1.465 | 0.256 |
| Endoscopic Score | 4.1 [4.5] | 1.9 [2.2] | 0.9 [4.1] | 1.3 [2.8] | 3.4 [4.1] | 4.634 | 0.001 |
| BSIT Olfaction score | 0.8 [3.1] | 0.1 [1.5] | 0.3 [1.9] | 0.2 [2.3] | 0.2 [2.9] | 2.124 | 0.483 |
ESS, endoscopic sinus surgery; SD, standard deviation; SNOT-22, 22-item Sinonasal Outcome Test; BSIT, Brief Smell Identification Test; p-values represent omnibus test statistic results using ANOVA.
Mean relative improvement was comparable between completed and all targeted surgery subgroups, but the rhinologic domain had greater relative improvement in subjects undergoing complete surgery (p=0.040; Table 6).
Table 6.
Comparison of mean percentage improvements between surgical treatment groups
| Complete Surgery |
Targeted Surgery |
||
|---|---|---|---|
| Outcomes: | Mean % Improvement | Mean % Improvement | p-value |
| SNOT-22 total score | 47.7 | 41.7 | 0.229 |
| Rhinologic symptom score | 51.1 | 35.4 | 0.040 |
| Extra-nasal rhinologic score | 48.4 | 36.6 | 0.054 |
| Ear/Facial symptoms | 49.6 | 47.0 | 0.688 |
| Psychological dysfunction score | 27.5 | 38.9 | 0.572 |
| Sleep dysfunction score | 40.6 | 26.1 | 0.197 |
| Endoscopic Score | 29.4 | 33.9 | 0.723 |
| BSIT Olfaction score | 41.9 | 2.6 | <0.001 |
SNOT-22, 22-item Sinonasal Outcome Test; BSIT, Brief Smell Identification Test;
Objective findings paralleled QOL outcomes. Post-operative endoscopic scores were also greater in subjects undergoing complete surgery (p=0.001). No significant improvement was found in B-SIT scores for the total cohort, but significant mean postoperative improvement was reported by patients undergoing complete sinus surgery (p=0.020), and this improvement was greater than subjects undergoing targeted surgery (p=0.005; Table 4).
Linear Regression Modeling Outcomes
Final models for independent variables associated with sinonasal inflammation found that subjects undergoing complete surgery experienced a -6.1[2.4] greater interval change in the SNOT-22 (Table 7) compared to subjects undergoing targeted surgery. Controlling for the confounders of gender, ASA sensitivity, steroid dependency, previous sinus surgery, and septoplasty, the mean difference was durable and favored complete surgery (β = -5.9[2.5]; p=0.016). There was significantly greater improvement across all domain scores with the exception of the ear/facial symptoms domain. No significant difference were found between enrollment site / surgeon experience for any final model (p≥0.294).
Table 7. Linear regression effect estimates for complete endoscopic sinus surgery.
| Outcome Improvements: | Unadjusted β Mean (SE) | Adjusted β Mean (SE) | 95% CI | p-value | R2 |
|---|---|---|---|---|---|
| SNOT-22 total score | -6.1 (2.4) | -5.9 (2.5)1 | (-10.7, -1.1) | 0.016 | 0.140 |
| Rhinologic symptom score | -3.6 (0.8) | -1.9 (0.9)2 | (-3.6, -0.1) | 0.037 | 0.187 |
| Extra-nasal rhinologic score | -0.8 (0.4) | -0.8 (0.4)3 | (-1.7, 0.1) | 0.064 | 0.063 |
| Ear/Facial symptoms | -0.3 (0.6) | -0.3 (0.6)4 | (-1.4, 0.8) | 0.573 | 0.115 |
| Psychological dysfunction score | -1.3 (1.0) | -1.9 (1.0)5 | (-3.8, -0.1) | 0.044 | 0.073 |
| Sleep dysfunction score | -1.3 (0.8) | -1.7 (0.8)6 | (-3.3, -0.2) | 0.026 | 0.105 |
| Endoscopic Score | -1.9 (0.6) | -1.0 (0.6)7 | (-2.2, 0.3) | 0.121 | 0.109 |
| BSIT Olfaction score | 1.0 (0.3) | 0.5 (0.4)8 | (-0.3, 1.2) | 0.238 | 0.072 |
β, effect estimate; CI, confidence interval, R2, coefficient of multiple determination; SNOT-22, 22-item Sinonasal Outcome Test, BSIT, Brief Smell Identification Test. All models were manually controlled for site differences and follow-up time. Variance inflation factors (VIFs) were used to evaluate collinearity. VIFs for all models were < 2.00. Models were designed using targeted surgery as the referent group so all models should be described using the effect estimate associated with complete surgery. (eg. complete surgery is associated with a 6.1 unit better improvement on total SNOT-22 scores on average compared to the targeted surgery group without adjustment for other cofactors).
Adjusted for significant independent predictors (p<0.050) including: gender, ASA sensitivity, steroid dependency, previous sinus surgery, and septoplasty.
Adjusted for significant independent predictors (p<0.050) including: ASA sensitivity, alcohol consumption, steroid dependency, previous sinus surgery, nasal polyposis, and septoplasty.
Adjusted for significant independent predictors (p<0.050) including: ASA sensitivity, steroid dependency, previous sinus surgery.
Adjusted for significant independent predictors (p<0.050) including: age, ASA sensitivity, steroid dependency, previous sinus surgery.
Adjusted for significant independent predictors (p<0.050) including: gender, steroid dependency, previous sinus surgery.
Adjusted for significant independent predictors (p<0.050) including: gender, steroid dependency, previous sinus surgery, and septoplasty.
Adjusted for significant independent predictors (p<0.050) including: nasal polyposis.
Adjusted for significant independent predictors (p<0.050) including: nasal polyposis, ASA sensitivity.
Discussion
Subjects undergoing complete sinus surgery were more likely to have clinical characteristics associated with greater sinonasal inflammation and overall disease severity including: asthma, ASA sensitivity, and nasal polyposis. These subjects also reported greater preoperative subjective burden of disease as measured by the SNOT-22. Regardless, subjects undergoing complete FESS experienced a significantly greater mean improvement in SNOT-22 total scores of 5.9 units compared to subjects who underwent targeted surgery. Importantly, this significance was preserved after controlling for asthma, ASA sensitivity, and nasal polyposis using linear regression modeling. Subjects undergoing complete surgery also experienced greater gains in olfaction as measured on the B-SIT scores in both absolute and relative improvement (Tables 4 and 6). A subset analysis targeted surgery subgroups revealed that subjects undergoing unilateral surgery and surgery without a frontal sinusotomy experienced lesser improvement in some outcome measures relative to subjects undergoing complete surgery. These preliminary findings highlight the need for further study to help identify how surgical extent can differentially impact QOL gains.
The data in the present study cannot explain the underlying mechanisms by which subjects undergoing complete FESS experience significantly greater average improvements in QOL, but recent work on the underlying pathophysiology of CRS offers some insight into evolving endoscopic surgical philosophy. Since the introduction of FESS there have been great strides in understanding the underlying mechanisms of CRS. In the initial understanding of CRS obstruction and inflammation at anatomic “bottlenecks”, such as the ostiomeatal complex, led to upstream obstruction and persistent inflammation.1 Surgeons are trained to focus on these anatomic narrowings to improve ventilation and drainage, and for a portion of patients with CRS minimal surgical correction of these anatomic bottlenecks can relieve symptoms associated with CRS. The etiology of CRS is not only secondary to impaired drainage pathways but also to a diffuse mucosal inflammatory response commonly seen in aspirin-exacerbated disease, allergic fungal sinusitis, superantigen-induced inflammation, and eosinophilic disease. In these patient populations a targeted minimal approach may not be as effective as complete marsupialization of the maxillary, sphenoid, ethmoid, and frontal sinuses. For instance, eosinophilic CRS is proving difficult to treat both medically and surgically as subjects with eosinophilic disease have been shown to have worse QOL outcomes after FESS.3,4,22,23 However, subjects with eosinophilic disease have been shown to achieve comparable outcomes when FESS when coupled with postoperative continued medical therapy.3 These findings suggest that improved topical access following surgery is critical. Furthermore, a common theme of distribution studies of topical therapies show that high-volume irrigations coupled with larger sinus openings translates into increased irrigation distribution.5 An improved understanding of the role of the eosinophil in pathogenesis of CRS, the recognition that ongoing medical therapy improve QOL outcomes, and that topical irrigations delivers these medications to the target best with maximized sinus openings is suggestive of a paradigm shift for endoscopic sinus surgeons.
Without truly comprehensive diagnostic tools to guide surgical extent, each rhinologist must weigh the risk-benefit profile of a targeted approach versus a complete intervention using those clinical instruments, which are readily available. Due to the fact that risk-stratification is difficult without preoperative pathology, the judgment surrounding surgical extent is really only a function of an individual surgeons’ individual experience and training. Greater surgical intervention may potentially expose patients to greater risk of frontal iatrogenic sinusitis or devastating complication to orbit or skull base. A theoretical benefit of a complete surgical intervention is improvement in facilitating postoperative debridements. In subjects undergoing complete FESS, a wider and more consistent ethmoid bed and frontal recess can facilitate more complete debridements. Regardless, in the present cohort, subjects who underwent complete surgery experienced a greater improvement in QOL suggesting that, regardless of underlying disease process, there was, although short of clinical perception, an increased response to complete surgery.
Surgical approach involving complete FESS, in many ways, is a surrogate (collinear) marker for the extent and severity of disease as determined by the surgeon. Therefore, we expected subjects being exposed to complete surgical intervention to have worse clinical markers of sinonasal inflammation and greater burdens of disease on endoscopy and CT (Table 1). Linear regression modeling (Table 7) was utilized to adjust for any measurable confounding and determined that there was still a greater mean improvement in subjects undergoing complete surgery with no evidence of perfect confounding by any proxy measure of disease severity. Given the non-randomized study design there remains the possibility that unmeasured confounding may differentially impact reported improvements in QOL. A viable future study direction includes randomization of surgical extent in subjects that elect FESS as the subsequent treatment option. Similarly, there is marked heterogeneity in the targeted surgical group ranging from a single maxillary antrostomy, to a unilateral spheno-ethmoidectomy with frontal and maxillary sinus interventions as well as a nearly complete FESS missing a unilateral frontal. Investigating how targeted sinus surgery influences disease-specific QOL and severity outcomes will need to be better characterized in future studies.
The present study has some important limitations worth discussing. The reported differential mean improvement may be a result of a greater likelihood for improvement in subjects undergoing complete FESS given higher (worse) baseline SNOT-22 total scores. Linear regression models could not account for baseline SNOT-22 scores as baseline scores are a function of the measured postoperative change score. The assessment of relative improvement has been suggested as one approach to avoid bias associated with differential baseline scores.21 Relative improvement for each surgical subgroup was calculated and reported in Table 6 with greater improvement still found in the rhinologic and extra-nasal rhinologic domains of the SNOT-22 in subjects experiencing complete FESS. Due to the multi-institutional and observational study design, there is an inherent possibility for observation bias in surgical subgroup classification due to differential surgical philosophies of each enrolling physician and their knowledge of symptom reporting during preoperative clinical evaluations. Likewise, misclassification bias of disease factors may exist and result in an underestimation of true effect estimates, however this strengthens the assertion that observed effects between surgical subgroup differences are not spurious. The clinical significance of a 5.9 unit difference in SNOT-22 total scores is debatable as prior studies have established an 8.9 difference as the minimal clinically detectable.16 Although the surgical extent did not single-handedly achieve clinically significant change, it is the only variable under the control of the surgeon and is an independent predictor of SNOT-22 score improvement following FESS in this investigation, and therefore elevates the importance of these findings. Finally, these results are derived from subjects at four academic referral centers with high-volume rhinology practices and may not be externally generalizable to subjects in the community setting.
Conclusion
Subjects undergoing complete surgery are more likely to have clinical characteristics associated with worse sinonasal inflammation compared to subjects undergoing targeted surgery. Regardless, subjects undergoing complete surgery experience greater mean improvements in QOL. Although the greater QOL gains associated with complete surgery do not reach a minimal clinically detectable difference, extent of surgery represents one of the rare variables under the control of the surgeon. CRS is a heterogenous group of pathologies some of which may benefit more from complete marsupialization of the frontal, ethmoid, sphenoid and maxillary sinuses and may have persistent inflammation regardless of anatomic drainage bottlenecks. Unfortunately, there is no currently recognized paradigm to preoperatively identify subjects that would benefit from a complete surgery.
Acknowledgments
Financial Disclosures: Timothy L. Smith, MD, MPH, Jeremiah A. Alt, MD, PhD, and Jess C. Mace, MPH, CCRP were supported for this investigation by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (www.clinicaltrials.gov; ID# NCT01332136). Timothy L. Smith is also a consultant for IntersectENT, Inc (Menlo Park, CA.) which is not affiliated with this investigation.
Footnotes
There are no financial disclosures for Adam S. DeConde, MD or Jeffrey D. Suh, MD.
Conflicts of Interest: None to report
References
- 1.Stammberger H, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990;247(2):63–76. doi: 10.1007/BF00183169. [DOI] [PubMed] [Google Scholar]
- 2.Timperley D, Schlosser RJ, Harvey RJ. Chronic rhinosinusitis: An education and treatment model. Otolaryngol Head Neck Surg. 2010 Nov;143(5, Supplement 3):S3–8. doi: 10.1016/j.otohns.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(5):415–421. doi: 10.1002/alr.21047. [DOI] [PubMed] [Google Scholar]
- 4.Jang DW, Lachanas VA, Segel J, Kountakis SE. Budesonide nasal irrigations in the postoperative management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3(9):708–11. doi: 10.1002/alr.21189. [DOI] [PubMed] [Google Scholar]
- 5.Harvey RJ, Goddard JC, Wise SK, Schlosser RJ. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg. 2008;139(1):137–142. doi: 10.1016/j.otohns.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld RM, Andes D, Neil B, Cheung D, Eisenberg S, Ganiats TG, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 7.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–6. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(9):693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123(10):2364–70. doi: 10.1002/lary.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alt JA, Mace JC, Buniel MCF, Soler ZM, Smith TL. Predictors of Olfactory Dysfunction in Rhinosinusitis Using the Brief Smell Identification Test. Laryngoscope. 2014;124(7):E259–266. doi: 10.1002/lary.24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeConde AS, Mace JC, Alt JA, Soler ZM, Orlandi RR, Smith TL. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2014 Sep 18; doi: 10.1002/alr.21410. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeConde AS, Mace JC, Alt JA, Schlosser RJ, Smith TL, Soler ZM. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014 Sep;4(9):725–33. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140(8):712–9. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeConde AS, Barton MD, Mace JC, Smith TL. Can sinus anatomy predict quality of life outcomes and operative times of endoscopic frontal sinus surgery? Am J Otolaryngol. 2015;36(1):13–19. doi: 10.1016/j.amjoto.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeConde AS, Mace JC, Bodner T, Hwang PH, Rudmik L, Soler ZM, Smith TL. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 17.DeConde AS, Mace JC, Alt JA, Rudmik L, Soler ZM, Smith TL. Longitudinal improvement and stability of the SNOT-22 survey in the evaluation of surgical management for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014 doi: 10.1002/alr.21458. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doty RL. The Brief Smell Identification Test Administration Manual. Haddon Heights, NJ: Sensonics Inc.; 2001. [Google Scholar]
- 19.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 20.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 21.Stewart MG, Donovan DT, Parke RB, Bautista MH. Does the Severity of Sinus Computed Tomography Findings Predict Outcome in Chronic Sinusitis? Otolaryngol Head Neck Surg. 2000;123(1):81–84. doi: 10.1067/mhn.2000.105922. [DOI] [PubMed] [Google Scholar]
- 22.Sok JC, Ferguson BJ. Differential diagnosis of eosinophilic chronic rhinosinusitis. Curr Allergy Asthma Rep. 2006;6(3):203–214. doi: 10.1007/s11882-006-0036-1. [DOI] [PubMed] [Google Scholar]
- 23.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–61. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]


