Abstract
Obesity and inactivity have been with associated advanced stage prostate cancer, and poor prostate cancer outcomes, though the underlying mechanism(s) is unknown. To determine if telomere shortening, which has been associated with lethal prostate cancer, may be a potential underlying mechanism, we prospectively evaluated the association between measures of adiposity, physical activity and telomere length in 596 participants in the Health Professionals Follow-up Study, who were surgically treated for prostate cancer. Using tissue microarrays, we measured telomere length in cancer and benign cells using a telomere-specific fluorescence in situ hybridization assay. Adiposity and activity were assessed via questionnaire within 2 years of diagnosis. Adjusting for age, pathologic stage and grade, the median and standard deviation of the per cell telomere signals were determined for each man for stromal cells and cancer cells by adiposity and activity categories. Overweight/obese men (54%) were similar to normal weight men on most factors, but had higher Gleason sum and lower activity levels. Overweight/obese men had 7.4% shorter telomeres in stromal cells than normal weight men (P=0.06). The least active men had shorter telomeres in stromal cells than more active men (P-trend=0.002). Men who were overweight/obese and the least active had the shortest telomeres in stromal cells (20.7% shorter; P=0.0005) compared to normal weight men who were the most active. Cancer cell telomere length and telomere length variability did not differ by measures of adiposity or activity. Telomere shortening in prostate cells may be one mechanism through which lifestyle influences prostate cancer risk and outcomes.
Keywords: Obesity, Inactivity, Telomere Length, Prostate Cancer, Stromal Cells
Introduction
Obesity and physical inactivity have been associated with the development of prostate cancer, with a worse prognosis at diagnosis and poorer prostate cancer-specific outcomes (1–11). Despite this overall body of evidence, the biological mechanism(s) underlying the associations between obesity, physical inactivity and prostate cancer related outcomes remain unknown. Telomere shortening is one mechanism by which obesity and physical inactivity could influence prostate cancer risk and progression. Telomeres are repetitive DNA sequences that protect the ends of chromosomes from degradation and recombination. Telomeres can be shortened, and ultimately become dysfunctional, by incomplete replication during DNA synthesis, alterations of telomere-binding proteins involved in telomere maintenance, or by oxidative stress leading to DNA damage (12–14). However, in cancer cells the ability to maintain telomeres enhances viability (15). Variability in telomere length from cancer cell to cancer cell may reflect or lead to more generalized genetic instability. Variability in phenotypic, genetic, and epigenetic markers in multiple cancer types, including prostate cancer, tends to be related to a more aggressive phenotype (16).
Individuals with excess body fat tend to have a greater oxidative stress (e.g. from inflammation) and produce a greater amount of growth factors (e.g., insulin), both of which may influence telomere shortening. For example, increased circulating growth factors could lead to increased cell proliferation and, thus, increased telomere shortening. Several, but not all, studies have observed an inverse association between adiposity and telomere length in peripheral blood leukocytes (17, 18), whereas physical activity has been associated with longer telomere length in peripheral blood leukocytes (19, 20). One small study reported blood leukocyte telomere lengthening after a comprehensive lifestyle change (21). We recently reported that shorter telomeres in prostate cancer associated stromal cells were associated with higher risk of poor prostate cancer outcomes, including death, in men surgically treated for prostate cancer (22). Collectively, this evidence suggests that one possible way that obesity and inactivity may influence prostate cancer risk is through telomere shortening, though the influence of obesity and inactivity on telomere length in prostate tumor or normal tissue is unknown.
Thus, we prospectively evaluated the association between measures of adiposity, physical activity and telomere length in 596 participants in the Health Professionals Follow-up Study, who were surgically treated for clinically-localized prostate cancer. We hypothesized that men with greater adiposity and inactive men would have shorter prostate tissue telomere length, especially in the stroma, whereas in cancer tissue we did not expect telomere associations. Telomere length was measured using a state of the art telomere-specific fluorescence in situ hybridization (FISH) assay that, unlike other methods, provides single cell resolution of telomere length while maintaining tissue architecture.
Materials and Methods
Study Population
We conducted a prospective study of 596 men surgically treated for prostate cancer (median year of diagnosis =1994) who participated in the Health Professionals Follow-up Study (HPFS) (22). The HPFS began in 1986 with 51,529 men, aged 40–75 years, who completed a mailed questionnaire on demographics, lifestyle factors and medical history. The men have been asked to complete questionnaires every two years since baseline, and on diet every four years; response among men eligible to receive them is 94%. The conduct of the HPFS was approved by the Human Subjects Committee of the Harvard School of Public Health. The study on telomere length in prostate tissue was additionally approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health.
Measures of Obesity and Physical Activity
Self-reported height was collected on the baseline questionnaire. Self-reported weight was reported on the baseline questionnaire and every biennial questionnaire. We selected the weight reported on the questionnaire immediately preceding the year in which the man was diagnosed with prostate cancer. Men were categorized into normal weight (BMI <25 kg/m2), overweight (25≤ BMI <30 kg/m2), and obese (BMI ≥30 kg/m2); the latter two categories were later combined. Waist circumference was reported on the 1987 and 1996 questionnaires; the men were provided with a measuring tape and a standardized protocol for measuring their waist circumference. Self-reported weight and self-measured waist circumference were validated against the measurements of trained technicians in the cohort (23). We selected the waist circumference measured closest in time, but before the prostate cancer diagnosis. Men were then categorized into those with ≤40 inch waists and those with >40 inch waists. Men also reported their weight at age 21 years on the 1987 questionnaire. Weight change was calculated as the difference between weight at age 21 and weight reported on the questionnaire preceding the one in which the man was diagnosed with prostate cancer. Men were then categorized into those who had lost weight, maintained weight within 5 lbs, gained >5 lbs to <25 lbs, or gained ≥25 lbs.
On the baseline questionnaire and then again every two years, men reported their average time per week spent on specified leisure time activities, as well as number of flights of stairs climbed each day and their usual walking pace. In 1988, questions on heavy outdoor work, and in 1990 questions on weight training, were added. Activity-specific metabolic equivalent task (MET) hours were summed across all activities per week, vigorous or high intensity activities (running, jogging, biking, swimming, tennis, racquetball/squash, rowing/calisthenics, heavy outdoor work, and weight training) and non vigorous activities (flights of stairs climbed and walking), for total physical activity, and across high intensity activities (>6 METs) for vigorous activities (8). Walking pace-specific METs were used. As additional activities were added to the HPFS questionnaires over time, they were incorporated into the METs sum, as previously done in this cohort. The final activity scores were expressed as MET-hours per week. Occupational activity was not assessed, because all of the participants are in health professions, which have generally low levels of occupational activity. This method of physical activity assessment has been previously validated in this cohort (24). Total and vigorous activity were both categorized into tertiles.
Tissue Collection and Tissue Microarray Construction
The ascertainment of prostate cancer in the HPFS has been described previously (22). With participant permission, tissue blocks of the prostatectomy specimens were obtained from the original pathology departments. H&E-stained tissue sections were re-reviewed by study pathologists and assigned a standardized Gleason sum (25). For this project, we used five tissue microarrays (TMAs) that had been constructed (22), sampling at least three areas of the tumor focus that was the largest and/or had the highest Gleason sum.
Measurement of Telomere Length
Tissue microarray sections containing areas of adenocarcinoma and benign tissue were stained using a telomere-specific fluorescence in situ hybridization (FISH) probe and 4',6-diamidino-2-phenylindole (DAPI) for labeling total nuclear DNA (22). Image analysis was used to quantify telomeric signals in individual cancer cells, and in these same TMA spots with cancer, stromal cells (lymphocytes excluded), basal epithelial cells and luminal epithelial cells in non-cancer areas. For each cell type, 30 to 50 individual cells per man were analyzed, but not all cell types were available for evaluation for some men; exact cell counts for each cell type and analysis are provided in Supplemental Tables 1 and 2 (22).
Statistical Analysis
Means and proportions for demographic and other factors by BMI and physical activity categories were calculated; differences across BMI and physical activity categories were evaluated using t-tests and chi-square tests, respectively. Median (telomere length) and standard deviation (telomere length variability) of the telomere signal normalized to DAPI were determined for each man for cancer cells and non-cancer cells by categories for the measures of obesity and physical activity. We evaluated the associations between the measures of obesity and physical activity with telomere length and variability in telomere length using linear regression. All analyses were adjusted the potential confounders: age at diagnosis (continuous), and known prognostic factors, prostatectomy Gleason sum (categorical: ≤6, 3+4, 4+3, ≥8), and pathologic TNM stage (categorical ≥T3b, or N1, or M1). Adjustment for age only did not change inferences (data not shown). We tested for trend in the associations by entering into the models a continuous variable for each measure of obesity and physical activity, the coefficient for which was evaluated by the Wald test. To address possible influential observations for telomere length or variability in telomere length, we used two definitions for outliers, one more (3 standard deviations away from the mean and a DFFIT > |2×√(P+1)/N|) and one less (4 standard deviations away from the mean and a DFFIT > |3×√(P+1)/N|) conservative. DFFIT is a regression diagnostic that shows how influential a point is. It is the change in the predicted value for a point when that point is omitted from the regression and divided by the estimated standard deviation of the fit at that point. After excluding these observations, the inferences remained the same. All analyses were performed using SAS v 9.2 (SAS Institute, Cary, NC). All statistical tests were two-sided, with P<0.05 considered to be statistically significant.
Results
As shown in Table 1, overweight and obese men were similar to normal weight men on most characteristics, but they reported significantly less physical activity than normal weight men. Men were also similar on most characteristics across activity levels, but the least active men were significantly older and had significantly higher body mass index than the most active men. Overweight and obese men were also less likely to have a Gleason sum of 6 or lower (17.9% vs. 25.3%), though this difference was not statistically significant.
Table 1.
Characteristics of men surgically treated for clinically localized prostate cancer by pre-diagnostic* body mass index and total physical activity level, HPFS.
| Body Mass Index, kg/m2 | Total Physical Activity, MET-hours/wk | ||||||
|---|---|---|---|---|---|---|---|
| Normal, <25 (n=273) |
Overweight/Obese, ≥25 (n=323) |
P- value** |
Low, Tertile 1 (n=199) |
Tertile 2 (n=198) |
High, Tertile 3 (n=199) |
P- trend*** |
|
| Mean age at diagnosis, years (std) | 65.2 ± 6.5 | 65.4 ± 5.8 | .58 | 64.3 ± 6.3 | 65.8 ± 5.8 | 65.8 ± 6.2 | .02 |
| White (%) | 91.9 | 90.4 | .51 | 92.5 | 91.4 | 89.5 | .29 |
| Mean pre-diagnostic* body mass index, kg/m2 (std) | 23.2 ± 1.3 | 27.8 ± 2.5 | - | 26.2 ± 3.5 | 25.8 ± 3.1 | 25.0 ± 2.4 | <.0001 |
| Mean height, inches (std) | 70.4 ± 2.8 | 70.4 ± 2.7 | .99 | 70.3 ± 2.8 | 70.3 ± 2.9 | 70.5 ± 2.6 | .41 |
| Mean pre-diagnostic* total physical activity, MET-hours/wk (std) | 38.1 ± 34.3 | 31.9 ± 34.9 | .03 | 6.9 ± 4.7 | 25.8 ± 6.8 | 71.5 ± 36.7 | - |
| Gleason sum (%) | |||||||
| ≤6 | 25.3 | 17.9 | 23.1 | 20.7 | 20.1 | ||
| 3+4 | 33.0 | 38.1 | .15 | 33.2 | 34.8 | 39.2 | .69 |
| 4+3 | 24.9 | 24.8 | 24.1 | 25.3 | 25.1 | ||
| ≥8 | 16.8 | 19.2 | 19.6 | 19.2 | 15.6 | ||
| Pathologic stage ≥T3b (%) | 12.1 | 13.0 | .74 | 8.5 | 16.7 | 12.6 | .23 |
| Mean PSA at diagnosis in ng/mL (std) | 10.3 ± 12.1 | 11.3 ± 12.4 | .35 | 12.1 ± 16.5 | 9.8 ± 8.6 | 10.8 ± 10.6 | .51 |
| % Missing | 13.6 | 12.7 | 16.1 | 11.1 | 12.1 | ||
Pre-diagnostic measures collected within 2 years prior to diagnosis;
T-test (means), Chi-square test (proportion),
Wald test from regression
Stromal Cells
Next, we evaluated the association of adiposity, physical activity and measures of telomere length in stromal cells. When men were categorized by their pre-diagnostic BMI, overweight (45.9% of the men) and obese (8.1% of the men) men had similarly shorter telomeres in stromal cells while normal weight men had longer telomeres. Given the relatively small proportion of men who were obese and given that obese men had similar telomere lengths as overweight men, we combined overweight and obese men for all subsequent analyses. As compared to normal weight men, overweight/obese men had shorter telomeres in stromal cells (Table 2). AS BMI increased, the variability in telomere length in stromal cells increased (Table 2). When men were categorized by their pre-diagnostic waist circumference, men with large waists had shorter telomeres in stromal cells (Table 2), and more variability in telomere length in stromal cells as compared to men with normal waists (Table 2). When men were categorized by their weight change since the age of 21, men who had gained 25 pounds or more had shorter telomeres in stromal cells (Table 2); and more variability in telomere length in stromal cells as compared to men who maintained weight (Table 2).
Table 2.
Association of pre-diagnostic BMI, waist circumference, and weight gain from age 21 with stromal cell telomere length in men surgically treated for clinically localized prostate cancer, HPFS
| N | Adjusted mean telomere length (95% CI) |
Difference in adjusted mean telomere length |
Adjusted mean telomere length variability (95% CI) |
Difference in adjusted mean telomere length variability |
|
|---|---|---|---|---|---|
| Pre-diagnostic BMI* | |||||
| Normal | 263 | 60.4 (56.2, 64.6) | Reference | 27.2 (24.3, 30.1) | Reference |
| Overweight/Obese | 308 | 55.9 (51.9, 60.0) | −7.4% | 27.6 (24.8, 30.3) | +1.4% |
| PDifference | 0.06 | 0.82 | |||
| Per 1 kg/m2 increase | −0.59 | 0.53 | |||
| PTrend | 0.12 | 0.04 | |||
| Pre-diagnostic waist circumference† | |||||
| ≤ 40 in | 401 | 58.0 (54.3, 61.7) | Reference | 26.4 (23.8, 29.0) | Reference |
| > 40 in | 127 | 55.9 (50.2, 61.6) | −3.7% | 31.1 (27.2, 35.1) | +18.0% |
| PDifference | 0.46 | 0.02 | |||
| Per 1 inch increase | −0.80 | 0.45 | |||
| PTrend | 0.03 | 0.08 | |||
| Weight change from age 21‡ | |||||
| Loss | 30 | 62.5 (51.8, 73.2) | 29.0 (21.7, 36.4) | ||
| Maintain | 89 | 61.8 (55.5, 68.2) | Reference | 25.0 (20.7, 29.4) | Reference |
| Gain < 25 lbs | 211 | 58.4 (53.7, 63.1) | 26.7 (23.5, 30.0) | ||
| Gain ≥ 25 lbs | 220 | 55.8 (51.1, 60.5) | −9.8% | 28.5 (25.3, 31.7) | +14.0% |
| Per 1 lb increase | −0.17 | 0.10 | |||
| PTrend | 0.01 | 0.02 | |||
Adjusted for age at diagnosis, pathologic stage and grade
Normal weight: <25 kg/m2; Overweight/Obese: ≥25 kg/m2
Additionally adjusted for height
Additionally adjusted for height and weight at age 21; difference in adjusted mean telomere length and variability only between reference and top category of weight gain.
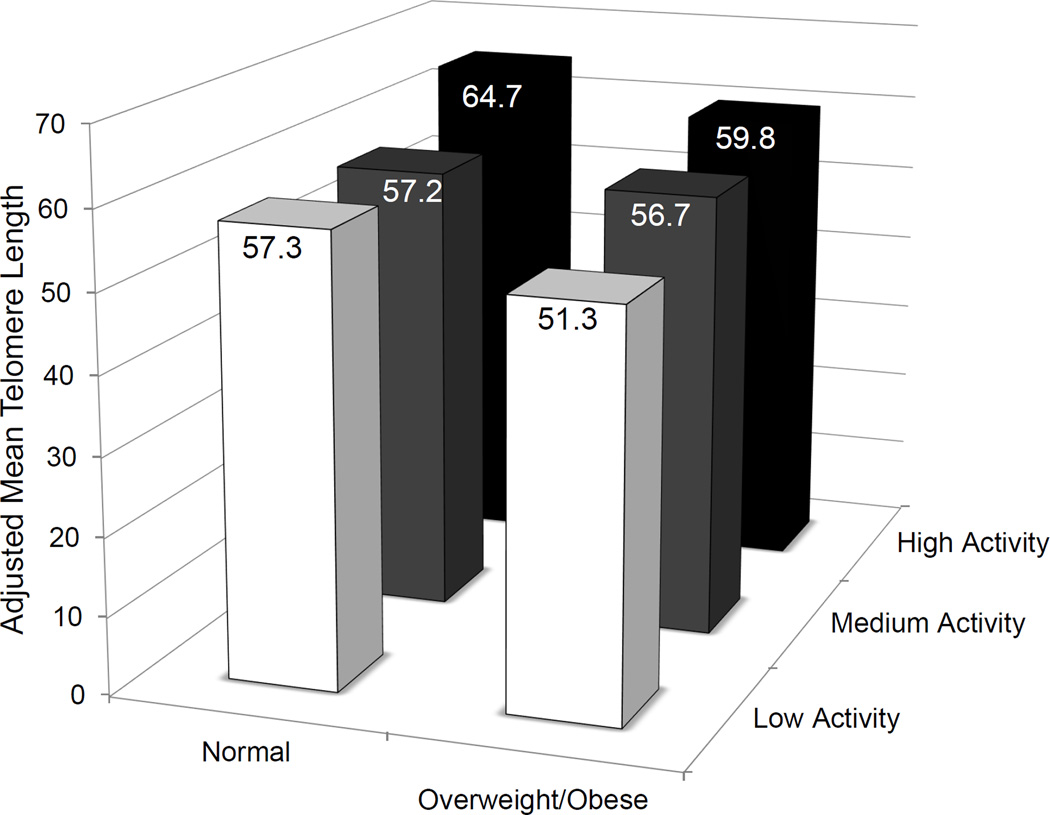
When men were categorized by total activity level, the least active men had shorter telomeres in stromal cells as compared to more active men (Table 3); this pattern persisted even after adjustment for BMI (P-trend=0.004). When men were jointly categorized by total activity level and weight, men who were the least active and overweight/obese had the shortest telomeres in stromal cells (20.7% shorter) compared to normal weight men who were the most active (Figure 1). Normal weight men who were the most active had the longest telomeres of all of the categories. Even when restricting to overweight/obese men, the least active men had 14.2% shorter telomeres than the most active men. When men were categorized by vigorous activity level, there was no significant difference in telomere length in stromal cells (Table 3). Whether evaluating by total or vigorous activity level, there was no significant difference in telomere length variability in stromal cells (Table 3).
Table 3.
Association of total and vigorous physical activity with stromal cell telomere length in men surgically treated for clinically localized prostate cancer, HPFS
| N | Adjusted mean telomere length (95% CI) |
Difference in adjusted mean telomere length |
Adjusted mean telomere length variability (95% CI) |
Difference in adjusted mean telomere length variability |
|
|---|---|---|---|---|---|
| Total physical activity (MET-hr/wk) | |||||
| 0 –15.4 | 187 | 53.9 (49.0, 58.9) | −13.8% | 27.3 (24.0, 30.7) | −4.0% |
| 15.5 – 38.2 | 190 | 56.9 (52.4, 61.5) | 26.4 (23.2, 29.5) | ||
| 38.3 – 352.8 | 194 | 62.5 (57.8, 67.2) | Reference | 28.5 (25.3, 31.7) | Reference |
| Per MET-hr/wk increase | 0.09 | 0.02 | |||
| PTrend | 0.002 | 0.45 | |||
| Vigorous physical activity (MET-hr/wk) | |||||
| 0 | 261 | 55.4 (51.2, 59.7) | −7.1% | 26.6 (23.7, 29.5) | −7.3% |
| >0 – 12.0 | 152 | 60.7 (55.5, 65.8) | 27.2 (23.7, 30.7) | ||
| >12.0 – 201.0 | 158 | 59.7 (54.7, 64.8) | Reference | 28.7 (25.2, 32.1) | Reference |
| Per MET-hr/wk increase | 0.13 | 0.01 | |||
| PTrend | 0.21 | 0.28 | |||
Adjusted for age at diagnosis, pathologic stage and grade; metabolic equivalent (MET) hours; difference in adjusted mean telomere length and variability only between extreme groups.
Figure 1.
Adjusted mean telomere length in cancer associated stromal cells in men surgically treated for clinically localized prostate cancer by body weight and physical activity level, HPFS.Men who were the least active and overweight/obese had the shortest telomeres in stromal cells (20.7% shorter) compared to normal weight men who were the most active (P-difference=0.0005). Normal weight men who were the most active had the longest telomeres of all of the categories. Even when restricting to overweight/obese men, the least active overweight/obese men had 14.2% shorter telomeres than the most active overweight/obese men (P-difference=0.03).
Basal Epithelial Cells and Luminal Epithelial Cells
Next, we evaluated the association of adiposity, physical activity and measures of telomere length in basal epithelial cells and luminal epithelial cells. There were no significant differences in telomere length or telomere length variability by any measure of adiposity or physical activity in basal epithelial cells or luminal epithelial cells (Supplemental Table 1; Supplemental Table 2) with two exceptions. Men with large waists and men who gained 25 pounds or more since the age of 21 had significantly shorter telomeres in luminal epithelial cells as compared to men with normal waist circumference (Supplemental Table 1) and men who maintained their weight since the age of 21 (Supplemental Table 1), respectively.
Cancer Cells
Next, we evaluated the association of adiposity, physical activity and measures of telomere length in prostate cancer cells. There were no significant differences in telomere length or telomere length variability by any measure of adiposity or physical activity in cancer cells (Supplemental Table 1; Supplemental Table 2) with one exception. Men who gained 25 pounds or more since age 21 had significantly less variability in telomere length in cancer cells as compared to men who maintained weight (P-trend=0.03; Supplemental Table 2).
Discussion
In this prospective study of men surgically treated for prostate cancer, we evaluated the associations of adiposity and physical activity with telomere length in specific populations of prostate cells. Our work differs from other studies in that we evaluated these risk factors in relation to telomere length in the target tissue itself, rather than in peripheral blood leukocytes. Men with increased measures of adiposity, especially large waist circumference and greater weight gain since the age of 21, had shorter telomere length in stromal cells. In addition, men who reported the least amount of total physical activity, even after adjustment for adiposity, also had shorter telomeres in stromal cells. Men who were overweight/obese and the least active had 20.7% shorter telomeres in stromal cells than the most active, normal weight men. Based on our previous work in which we estimated the association between short stromal cell telomere length and fatal prostate cancer (22), this relative decrement in telomere length translates to an approximately 29% increase in the relative risk of fatal prostate cancer for an overweight/obese, inactive man surgically treated for prostate cancer relative to a normal weight, active man surgically treated for prostate cancer.
Men who participated in some or more vigorous activity tended to have longer stromal cell telomere length than men who did not participate in any vigorous activity, though this association was not statistically significant. This pattern was similar in direction but weaker than the pattern observed for telomere length across categories of total physical activity. While we do not know why a stronger pattern was observed for total activity than for vigorous activity, we speculate that it is due, at least in part, to a difference in comparison groups in the two analyses. In the total physical activity analysis, the comparison group contained men who were inactive or reported very low total activity. In the vigorous analysis, the comparison group contained men who reported no vigorous activities, specifically; these men are not necessarily inactive (e.g. the comparison group in the total analysis). Thus, it is possible that we were not able to detect an association between vigorous physical activity and telomere length because there was insufficient contrast between men who were in the top category of vigorous physical activity and men who did not participate in vigorous activity, but may have participated in non-vigorous activity.
We also observed increased cell-to-cell telomere length variability in stromal cells by all measures of adiposity. In our previous study, we did not observe an association between increased telomere length variation in stromal cells and prostate cancer outcomes after adjustment for clinico-pathological factors (22). Nevertheless, there still may be a biologic influence of obesity on telomere length variability in prostate cells. Physical activity level was not associated with telomere length variability in any cell type.
We also evaluated telomere length and telomere length variability in luminal epithelial and basal epithelial cells in the same TMA spots as the cancer. We observed similar patterns between measures of adiposity and telomere length and telomere length variability in luminal epithelial cells as in stromal cells. When the TMAs were constructed, the goal was to enrich for areas of adenocarcinoma. This process included the systematic sampling of stromal cells, which are consistently found near the cancer, but not luminal epithelial and basal epithelial cells. While luminal epithelial and basal epithelial cells were evaluated when present, their sample sizes were approximately one half of the stromal and cancer cell sample sizes.
We did not expect to observe associations of adiposity and activity with telomere length or telomere length variability in cancer cells for the following reason. While telomere dysfunction is causally related to genomic instability and promotes tumorigenesis, it is also a dynamic process that may be affected as a consequence of cancer development. Cancer cells, including prostate cancer cells (26–28), typically have significantly shorter telomeres than normal cells from the same tissue. At the point that a tumor is present and detectable, the profound genomic instability, a hallmark of carcinogenesis (29), would be the dominant influence on telomere dysfunction rather than lifestyle factors. We did observe that weight gain since age 21 was significantly associated with decreased telomere length variability in cancer cells, but this association is not in the expected direction.
To our knowledge this is the first study to investigate the influence of obesity and physical inactivity on telomere length and cell-to-cell telomere length variability in prostate tissue, including specific cell populations. As such, our study may inform the observation that obesity and inactivity are associated with an increased risk of poor outcomes among men with prostate cancer. However, it is unclear whether the results we observed for stromal cell telomere length would be similar in a cohort of men at risk for prostate cancer. We had several measures of adiposity and detailed information on leisure time activity. These measures have been validated in the HPFS (24). We used a validated, state-of-the-art method to measure telomere length that provided single cell resolution (26), allowing us to compare by compartment and to estimate cell-to-cell variability in telomere length.
The mechanisms that underlie the associations of obesity and physical inactivity with poor prostate cancer-specific outcomes are currently unknown. Because obesity and inactivity have been associated with telomere shortening in leukocytes (17–20), and telomere shortening in prostate stromal cells has been associated with poor prostate cancer outcomes (22), we investigated the association of obesity and inactivity with telomere length and cell-to-cell variability in telomere length in non-cancer prostate cell populations in the same TMA spot with cancer as well as in prostate cancer cells. We observed that adiposity and inactivity were associated with shorter telomeres in stromal cells. The findings from this study suggest that telomere shortening may be one mechanism by which adiposity and physical activity influence prostate cancer outcomes.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial Support: DOD W81XWH-05-1-0030, P50 CA58236, CA55075, CA72036, HL35464, CA133891, CA141298, UM1 CA167552, and DOD W81XWH-05-1-0562. CE Joshu, CM Heaphy, and SA Kenfield supported by the Prostate Cancer Foundation. CE Joshu also supported by The Seraph Foundation.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 4.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian journal of andrology. 2012;14:365–374. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60:1029–1044. doi: 10.1016/j.eururo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 9.Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2005;14:275–279. [PubMed] [Google Scholar]
- 10.Moore SC, Peters TM, Ahn J, Park Y, Schatzkin A, Albanes D, et al. Physical activity in relation to total, advanced, and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2458–2466. doi: 10.1158/1055-9965.EPI-08-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsen NF, Tjonneland A, Thomsen BL, Christensen J, Loft S, Friedenreich C, et al. Physical activity and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2009;125:902–908. doi: 10.1002/ijc.24326. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn EH. Telomeres. Trends Biochem Sci. 1991;16:378–381. doi: 10.1016/0968-0004(91)90155-o. [DOI] [PubMed] [Google Scholar]
- 13.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annual review of genetics. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nature reviews Molecular cell biology. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlo LM, Maley CC. The role of genetic diversity in cancer. J Clin Invest. 2010;120:401–403. doi: 10.1172/JCI42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 17.Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. "Is obesity linked to aging?": adipose tissue and the role of telomeres. Ageing research reviews. 2012;11:220–229. doi: 10.1016/j.arr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- 19.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 20.Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, et al. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol. 2012;175:414–422. doi: 10.1093/aje/kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 22.Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannucci E, et al. Prostate Cancer Cell Telomere Length Variability and Stromal Cell Telomere Length as Prognostic Markers for Metastasis and Death. Cancer discovery. 2013;3:1130–1141. doi: 10.1158/2159-8290.CD-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, et al. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Hiroi S, Nakanishi K, Meeker AK. Telomere length and telomerase expression in atypical adenomatous hyperplasia and small bronchioloalveolar carcinoma of the lung. Am J Clin Pathol. 2007;127:254–262. doi: 10.1309/91PY0RBD9W8Y5GNX. [DOI] [PubMed] [Google Scholar]
- 28.Hansel DE, Meeker AK, Hicks J, De Marzo AM, Lillemoe KD, Schulick R, et al. Telomere length variation in biliary tract metaplasia, dysplasia, and carcinoma. Mod Pathol. 2006;19:772–779. doi: 10.1038/modpathol.3800591. [DOI] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.