SUMMARY
The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) was developed in a single center as a weighted scoring system to predict risks of non-relapse mortality (NRM) following allogeneic hematopoietic cell transplantation. Information on the performance of the HCT-CI in multi-center studies is lacking in the literature. To that end, a collaborative multicenter retrospective study was initiated. Comorbidity data from 2523 consecutive recipients of human leukocyte antigen-matched grafts from five different US institutions were analyzed. Among all patients, HCT-CI scores of 0 vs 1–2 vs ≥3 were associated with 2-year NRM rates of 14%, 23% and 39% (p<0.0001), respectively, and 2-year overall survival (OS) of 74%, 61% and 39%, respectively (p<0.0001). Using regression models, increasing HCT-CI scores were independently associated with increases in hazard ratios for NRM and worse survival within individual institutions. The HCT-CI retained independent capacity for association with outcomes within different age as well as conditioning intensity groups. C-statistic estimates for the prognostic power of the HCT-CI for NRM and OS were 0.66 and 0.64, respectively. The estimates within each institution were overall similar. The HCT-CI is a valid tool for capturing comorbidities and predicting mortality after hematopoietic cell transplantation across different institutions.
Keywords: HCT-CI, comorbidities, allogeneic, hematopoietic cell transplantation, validation
INTRODUCTION
Appropriate decision making about efficacy versus futility of allogeneic hematopoietic cell transplantation (HCT) and about the most suitable regimen intensity could minimize risks of non-relapse mortality (NRM) and improve survival. Hence, there is a continued need to develop and validate risk-assessment measures that could guide physicians and patients throughout the decision making process. This is in particular given the wide variability in the intensity of currently available conditioning regimens that allows selecting a regimen that can control malignancy with the lowest risk of NRM for each patient (Bacigalupo et al, 2009). Historically, the decision to offer allogeneic HCT has relied on disease-specific characteristics such as remission status and patient-specific characteristics such as age, without the inclusion of risks associated with pretransplant comorbidities (Gratwohl, 2012).
The introduction of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in 2005 made it possible to integrate an assessment of pretransplant comorbidities into the decision-making process (Sorror et al, 2005). The HCT-CI was developed as a sensitive tool to measure the burden of comorbidities before HCT and to predict the risks of NRM and the probabilities of survival after HCT (Kerbauy et al, 2005; Sorror et al, 2007b). The HCT-CI scores effectively stratified outcomes for patients undergoing allogeneic HCT using different conditioning regimens for a wide variety of malignancies (Farina et al, 2009; Lim et al, 2010; Ratan et al, 2013; Sorror et al, 2007a). However, the prognostic significance of the HCT-CI has been confirmed in some studies (Barba et al, 2010; Farina et al, 2009; Fujimaki et al, 2008; Raimondi et al, 2012) but not in others (Birninger et al, 2011; Majhail et al, 2008). Here, we report results of a large multi-institutional retrospective study designed to assess the discriminant validity of the HCT-CI across different institutions in predicting NRM and overall mortality as well as its prognostic capacity across different ages and conditioning regimens. Based on the findings, we also discuss the possible reasons for lack of agreement on the significance of the HCT-CI in different studies.
METHODS
Patients
We have designed a large retrospective multi-center study that included data collected from five different U.S. transplant centers for patients receiving first allogeneic HCT between January 1, 2000, and December 31, 2006. Cord blood recipients were excluded. The study was approved by the internal Review Boards (IRB) of all five institutions. The study was designed to address three major aims 1) Validation of the HCT-CI across different institutions, 2) assessing the independent role of age in predicting HCT outcomes and whether age could be combined with the HCT-CI in a composite model, and 3) the associations between the HCT-CI and specific post-transplant morbidities such as acute graft-versus host disease (GVHD). The last two aims were recently addressed in two different publications (Sorror et al, 2014a; Sorror et al, 2014b). Here, we are focusing on validating the discriminant validity of the HCT-CI across institutions as well as its convergent validity across institutions, ages, and conditioning regimens. Details on inclusion criteria, data collection, and assessment of pre-transplant comorbidities were previously described (Sorror et al, 2014a; Sorror et al, 2014b). The five institutions were randomly assigned letters A, B, C, D, E for description of results.
Definitions
NRM was defined as post-transplantation death that was not preceded by disease progression or relapse. Progression was defined as >50% increase in the burden of primary disease compared to pre-transplant disease status or development of disease at new sites. Relapse was defined as reappearance of primary disease following complete remission (CR) after HCT. Low disease risk included acute leukemia in first CR, chronic myeloid leukemia in first chronic phase, chronic lymphocytic leukemia in CR, myelodysplasia-refractory anemia or refractory anemia with ringed sideroblasts, and lymphoma in CR at the time of HCT. All remaining other diagnoses were considered high-risk. Non-malignant diseases were considered as a separate category for disease risk. Definitions of comorbidities are described in Table I and further details were published previously (Sorror, 2013). Conditioning regimens were classified into high-dose, reduced-intensity, or nonmyeloablative intensity based on previously published criteria (Bacigalupo et al, 2009).
Table I.
Definitions of comorbidities included in the HCT-CI and the corresponding HCT-CI scores
| Comorbidity | Definitions of comorbidities included in the new HCT-CI | HCT-CI weighted scores |
|---|---|---|
| Arrhythmia | Any type of arrhythmia that necessitates treatment at any time point during past medical history. | 1 |
| Cardiac | Coronary artery disease,§ congestive heart failure, myocardial infarction, low EF ≤ 50% for adults or SF ≤ 26% for children (lack of EF or SF doesn’t preclude calculation of a total score) | 1 |
| Inflammatory bowel disease | Documented Crohn’s disease or ulcerative colitis in the past medical history (endoscopy± biopsy) requiring treatment. | 1 |
| Diabetes | Diagnosis of diabetes mellitus or steroid induced hyperglycemia requiring continuous treatment with insulin or oral hypoglycemics but not diet alone during the instantaneous period of 4 weeks before day −10. | 1 |
| Cerebrovascular disease | Transient ischemic attack or cerebrovascular accident at any time point of past medical history. | 1 |
| Psychiatric disturbance | Requiring treatment during the instantaneous period of 4 weeks before day −10. | 1 |
| Hepatic, mild | Prior diagnosis of an infection with hepatitis B or C at any time point of past medical history, bilirubin > ULN to 1.5 × ULN, or AST/ALT> ULN to 2.5 × ULN (at least two values at two different days between day −24 and day −10 or day −40 and day −10 if only one value is available). | 1 |
| Obesity | Patients with a body mass index > 35 kg/m2 for patients >18 years or BMI for age ≥ 95th percentile for patients ≤18 years. | 1 |
| Infection | Fever of unknown origin, suspected pulmonary fungal infection by imaging, or documented infection resulting in the start of a specific antimicrobial therapy before the start of conditioning regimen and the continuation of the same antimicrobial therapy during as well as after completion of conditioning regimen | 1 |
| Rheumatologic | Any rheumatologic or autoimmune disease requiring specific treatment in the patient’s past medical history. | 2 |
| Peptic ulcer | Prior endoscopic or radiologic diagnosis of peptic ulcer in the patient’s past medical history. | 2 |
| Moderate/severe renal | Chronic renal failure requiring weekly dialysis during the instantaneous period of 4 weeks before day −10, history of renal transplant or serum creatinine > 2 mg/dL (at least two values at two different days between day −24 and day −10 or day −40 and day −10 if only one value is available) | 2 |
| Moderate pulmonary | DLco and/or FEV1 66%–80% or dyspnea on slight activity that is attributed to pulmonary disease and cannot be corrected by blood transfusion for anemia, as assessed during a clinic visit within the immediate period of two weeks before day −10 (a total score cannot be calculated in the absence of pulmonary function tests except if it wasn’t done due to technical difficulties e.g. pediatric patients) | 2 |
| Prior solid tumor | Treated at any time point in the patient’s past history, excluding nonmelanoma skin cancer. Same lineage prior malignancies are not scored for this comorbidity e.g. diagnosis of a non-Hodgkin lymphoma that was preceded by Hodgkin lymphoma or acute myeloid leukemia preceded by myelodysplastic syndromes. | 3 |
| Heart valve disease | Moderate to severe valve stenosis or insufficiency as assessed by echocardiogram, prosthetic mitral or aortic valve, or symptomatic mitral valve prolapse. | 3 |
| Severe pulmonary | DLco and/or FEV1 ≤ 65% or dyspnea at rest or requiring intermittent or continuous oxygen administration during the instantaneous period of 4 weeks before day −10. | 3 |
| Moderate/severe hepatic | Liver cirrhosis, bilirubin > 1.5 × ULN, or AST/ALT > 2.5 × ULN (at least two values at two different days between day −24 and day −10 or day −40 and day −10 if only one value is available) | 3 |
Abbreviations: EF = ejection fraction; SF= shortening fraction; ULN= upper limit of normal; SLE= systemic lupus erythmatosis; RA= rheumatoid arthritis; CTD = connective tissue disease; DLco= diffusion capacity of carbon monoxide.
One or more vessel-coronary artery stenosis requiring medical treatment, stent, or bypass graft.
Statistical methods
Cumulative incidence estimates were used to evaluate NRM, while Kaplan Meier estimates were used to assess survival. Relapse or progression of the primary disease was treated as a competing risk for NRM. Proportional hazards models were used to estimate the hazard ratios (HRs) for NRM and overall mortality associated with HCT-CI scores in each of the five institutions. The models were calculated for all available events over time and they were adjusted for patient-related risk factors: age, Karnofsky performance status score, and cytomegalovirus (CMV) serology results; disease-related risk factors: diagnosis category, disease risk, and number of prior regimens; and transplant-related risk factors: donor type, stem cell source, degree of conditioning intensity, and inclusion of anti-thymocyte globulin (ATG) in conditioning. Multivariate p-values for each variable were based on adjustment for all other variables in the model. All p-values were derived from likelihood ratio statistics and were two-sided. Tests of homogeneity of HRs across institutions were used to evaluate the convergent validity, with any p-value <0.05 indicating lack of homogeneity. Correlation between age and HCT-CI scores were evaluated by Spearman rank correlation. Correlation coefficients ≤0.30 were considered weak (Fleiss, 1999). Discriminant validity of the HCT-CI among all patients and within individual institutions was done by computing c-statistic estimates (Harrell, Jr. et al, 1984) for 2-year NRM and OS with the same interpretation of results as previously described (Sorror et al, 2005). The c-statistic was computed based on time to event using the entire follow-up period.
RESULTS
Patient characteristics
Among 3334 patients who met the study criteria, 811 were excluded from the analyses due to lack of comorbidity data (n=267), lack of data on patient CMV serology results (n=34), or participation in the design of the original model (n=510) yielding a final sample size of 2523. Institutions differed significantly in their patient-, disease-, and transplant-related characteristics (Table II).
Table II.
Patient, transplant and disease characteristics.
| Characteristics | All patients (n=2523) | Institutions
|
p value
|
|||||
|---|---|---|---|---|---|---|---|---|
| A (n is not shown) | B (n is not shown) | C (n is not shown) | D (n is not shown) | E (n is not shown) | <0.001 | |||
| % | % | % | % | % | % | |||
| HCT-CI scores | 0 | 31 | 32 | 29 | 31 | 30 | 41 | |
| 1 | 14 | 17 | 16 | 8 | 12 | 16 | ||
| 2 | 16 | 12 | 18 | 15 | 16 | 13 | ||
| 3 | 18 | 16 | 16 | 24 | 23 | 15 | <0.001 | |
| 4 | 10 | 12 | 10 | 12 | 8 | 6 | ||
| 5 | 5 | 7 | 5 | 5 | 5 | 3 | ||
| ≥6 | 6 | 4 | 6 | 5 | 6 | 6 | ||
| Age, years | 0.2–19 | 14 | 1 | 13 | 0 | 17 | 37 | |
| 20–39 | 26 | 28 | 23 | 18 | 29 | 27 | ||
| 40–49 | 23 | 25 | 22 | 33 | 25 | 15 | <0.001 | |
| 50–59 | 25 | 32 | 26 | 34 | 21 | 15 | ||
| ≥60 | 12 | 14 | 16 | 15 | 8 | 6 | ||
| KPS | > 80% | 73 | 70 | 70 | 75 | 82 | 62 | |
| ≤ 80% | 27 | 30 | 30 | 25 | 18 | 38 | <0.001 | |
| Prior regimens | 0–3 | 77 | 76 | 76 | 69 | 78 | 80 | |
| ≥ 4 | 23 | 24 | 24 | 31 | 22 | 20 | <0.001 | |
| Prior Autologous | 13 | 11 | 11 | 24 | 11 | 6 | <0.0001 | |
| HCT | ||||||||
| Donor | Related | 56 | 49 | 50 | 69 | 62 | 60 | |
| Unrelated | 44 | 51 | 50 | 31 | 38 | 40 | <0.001 | |
| Stem cell source | Marrow | 22 | 24 | 19 | 10 | 19 | 55 | |
| PBMC | 88 | 76 | 81 | 90 | 81 | 45 | <0.001 | |
| Conditioning regimens | High-dose | 62 | 80 | 53 | 46 | 67 | 66 | |
| Reduced-intensity | 18 | 10 | 13 | 31 | 29 | 13 | <0.001 | |
| Nonmyeloablative | 20 | 10 | 34 | 23 | 4 | 21 | ||
| ATG in conditioning | Yes | 9 | 3 | 11 | 14 | 4 | 15 | |
| No | 91 | 97 | 89 | 86 | 96 | 85 | <0.001 | |
| Diagnoses* | Myeloid | 61 | 60 | 65 | 52 | 59 | 60 | |
| Lymphoid | 34 | 38 | 28 | 46 | 41 | 25 | ||
| Non-malignant diseases | 5 | 2 | 7 | 4 | 0 | 15 | <0.001 | |
| Disease risk† | High | 61 | 67 | 59 | 67 | 62 | 51 | |
| Low | 39 | 33 | 41 | 33 | 38 | 49 | <0.001 | |
| Patient CMV sero-status | Positive | 64 | 70 | 56 | 51 | 73 | 65 | <0.001 |
| Negative | 36 | 30 | 44 | 49 | 27 | 35 | ||
HCT-CI indicates hematopoietic cell transplantation-comorbidity index; KPS, Karnofsky performance status; PBMC, peripheral blood mononuclear cells; ATG, anti-thymocyte globulin; and CMV, cytomegalovirus.
Myeloid malignancies included acute myeloid leukemia, biphenotypic leukemia, chronic myeloid leukemia, and myelodysplastic syndromes; lymphoid/plasma cell malignancies, acute lymphocytic leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma, and plasma cell leukemia; and non-malignant disease, aplastic anemia, sickle cell anemia, autoimmune disease, and other hematological non-malignant diseases.
Low-risk diseases included acute leukemia in first complete remission; chronic myeloid leukemia in first chronic phase; myelodysplastic syndromes with refractory anemia or refractory anemia with ringed sideroblasts; chronic lymphocytic leukemia, lymphoma, or multiple myeloma in complete remission and nonmalignant diseases. High-risk diseases included all other disease status.
Performance of the HCT-CI in predicting NRM among different institutions
Among all patients, increasing HCT-CI scores of 0, 1, 2, 3, 4, 5, and ≥6 were statistically significantly associated with step-wise increased 2-year NRM (12%, 21%, 25%, 33%, 45%, 43%, and 46%, respectively) and decreased 2-year survival (75%, 63%, 59%, 46%, 32%, 35%, and 28%, respectively). When the scores were collapsed into HCT-CI risk groups of 0, 1–2, and ≥3 the results for NRM were 14%, 23%, and 39% (p<0.0001) and the results for survival were 74%, 61%, and 39% (p<0.0001), respectively. Sepsis and pulmonary complications constituted the two most common causes of 2-year NRM among all patients (31% and 20%, respectively) (Table III).
Table III.
Causes of 2-year NRM among the total population.
| Cause of NRM | Total NRM (n=768) | |
|---|---|---|
|
| ||
| % | ||
| Sepsis without GVHD | 31 | |
| Pulmonary complications‡ | 20 | |
| GVHD† | 19 | |
| Sepsis with aGVHD | 11 | |
| Multiple organ failure | 7 | |
| Vascular complications§ | 3 | |
| Others* | 9 | |
Abbreviations: aGVHD= acute graft versus host disease; NRM= non-relapse mortality.
Includes acute respiratory distress syndrome (ARDS); idiopathic pneumonia syndrome (IPS); and Bronchiolitis obliterans organizing pneumonia (BOOP).
Includes acute and chronic GVHD.
Includes hemorrhagic or thrombotic complications.
For example: graft rejection or failure, second malignancy, sinusoidal-obstructive syndrome.
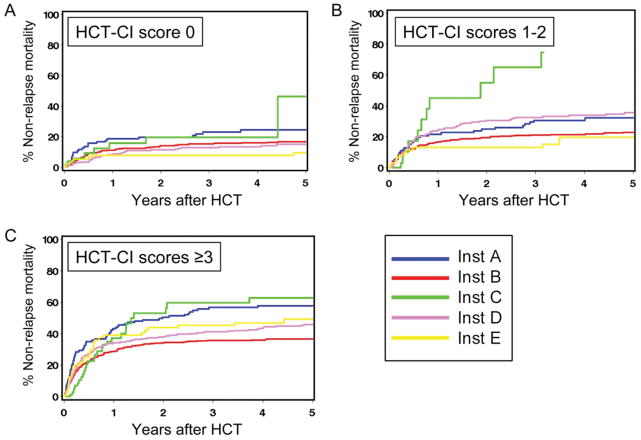
Among individual institutions, higher HCT-CI scores were consistently associated with an increasing cumulative incidence of NRM and decreasing survival (Table IV). In multivariate regression models adjusted for the previously mentioned risk factors, increasing HCT-CI scores were statistically significantly (p<0.001) associated with increasing HRs for NRM and survival across different institutions except for institution C (p=0.09 for NRM and survival), which had the smallest sample size (n=154), although increases in HRs were in the same direction as with other institutions.
Table IV.
Cox regression models for assessment of associations between HCT-CI risk groups and NRM and overall mortality*
| HCT-CI scores Non-relapse mortality, HR (2-year incidence, %)
|
HCT-CI scores Overall mortality, HR (2-year rate, %)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–2 | ≥3 | p value † | 0 | 1–2 | ≥3 | p value† | ||
| Institutions | A | 1.0 (20) | 1.30 (25) | 3.62 (51) | <0.001 | 1.0 (71) | 1.33 (57) | 3.28 (28) | <0.0001 |
| B | 1.0 (14) | 1.40 (20) | 2.50 (34) | <0.0001 | 1.0 (75) | 1.36 (61) | 2.23 (39) | <0.0001 | |
| C | 1.0 (20) | 1.76 (55) | 2.66 (53) | 0.09 | 1.0 (56) | 1.13 (27) | 2.28 (22) | 0.09 | |
| D | 1.0 (12) | 2.88 (31) | 4.15 (38) | <0.0001 | 1.0 (77) | 1.88 (61) | 2.77 (45) | <0.0001 | |
| E | 1.0 (8) | 1.65 (14) | 6.89 (44) | <0.0001 | 1.0 (86) | 1.84 (69) | 5.81 (31) | <0.0001 | |
Patients treated with allogeneic hematopoietic cell transplantation (HCT) at five institutions.
HR indicates hazard ratio.
The Cox regression models were calculated for all available events over time and they were adjusted for diagnosis category, disease risk, age, donor type, stem cell source, KPS score, number of prior regimens, degree of conditioning intensity, inclusion of anti-thymocyte globulin (ATG) in conditioning, and CMV sero-status.
p values were calculated for the adjusted HRs.
However, within the same HCT-CI score strata, degrees of increase in HRs for NRM and survival were variable across different institutions (Table IV). Additionally, we found that the cumulative incidence estimates of NRM differed among patients at the five institutions when compared within a single HCT-CI risk group of 1–2 or ≥3 (Fig 1). In order to confirm this observation, we performed a unified cox regression model for NRM that included all institutions. The model showed a statistically significant lack of homogeneity across institutions for HRs of NRM that were associated with scores 1–2 (p=0.03), and ≥3 (p=0.01) compared to score 0, respectively. Similarly, there was a statistically significant lack of homogeneity across institutions for overall mortality that was associated with scores ≥3 (p=0.01) but not with scores 1–2 (p=0.18) compared to score 0, respectively.
Figure 1.
Non-relapse mortality (NRM) according to HCT-CI scores at different institutions. Cumulative incidences of NRM are shown for patients who received allogeneic HCT at institutions A, B, C, D and E, respectively. HCT-CI scores were stratified as (A) 0, (B) 1–2, and (C) ≥3.
Given the above observations of variable magnitudes of impacts of the HCT-CI across centers, we decided to investigate the presence of a center effect. Further multivariate analysis confirmed the presence of an independent center effect, where the HRs of NRM at institutions B, C, D, and E relative to institution A were 0.71, 1.12, 0.81, and 0.69, respectively, for NRM (p=0.001) and the HRs for survival were 0.81, 1.25, 0.76, and 0.81, respectively (p<0.001).
C-statistic estimates were calculated for prediction of NRM and survival by the HCT-CI scores as a continuous predictor. C-statistic estimates were roughly comparable among institutions with a range of 0.602 – 0.722 for NRM and 0.625 – 0.708 for survival. These estimates were in general comparable to the overall estimates of 0.66 for NRM and 0.64 for OS (Table V).
Table V.
c -statistic estimates for 2-years NRM and OS across different institutions.
| c-statistic estimates for NRM | c-statistic estimates for OS | ||
|---|---|---|---|
| Overall | 0.665 | 0.640 | |
| Sites | A | 0.659 | 0.645 |
| B | 0.659 | 0.633 | |
| C | 0.602 | 0.625 | |
| D | 0.662 | 0.633 | |
| E | 0.722 | 0.708 |
NRM and OS indicate non- relapse mortality and overall survival, respectively
The use of the HCT-CI among conditioning regimens of different intensities
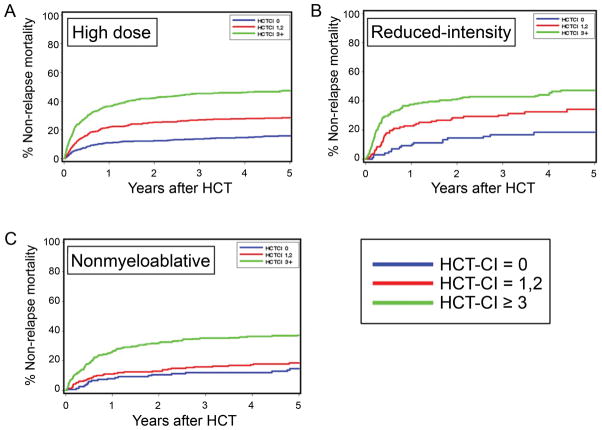
Overall and with few exceptions, increasing HCT-CI scores stratified cumulative incidences of NRM and survival rates consistently among recipients treated with high-dose, reduced-intensity, and nonmyeloablative regimens, respectively (Fig 2 and Table VI). Patients with HCT-CI scores of 1–2 had statistically significant higher risks for NRM in pairwise comparisons with those who had HCT-CI score of 0 after high-dose and reduced-intensity conditioning (p≤0.0001 and p=0.02, respectively), but not after nonmyeloablative conditioning (p=0.13) (Table VI). Among patients with HCT-CI scores of ≥3, the magnitude and the significance of increased risks of NRM compared to those with score 0 were comparable among recipients of all three types of conditioning regimens.
Figure 2.
NRM according to HCT-CI scores and conditioning intensity. Cumulative incidences of NRM are shown for patients who had HCT-CI scores of 0, 1–2, and ≥3 and received (A) high-dose, (B) reduced-intensity, and (C) nonmyeloablative conditioning regimens before allogeneic HCT.
Table VI.
Pairwise comparisons of non-relapse mortality between the three comorbidity risk groups and within each conditioning intensity.
| Univariate
|
Multivariate
|
|||||
|---|---|---|---|---|---|---|
| HCT-CI | Cumulative incidence, % 2years | HR (95% CI) | P | HR (95% CI) | P | |
| Myeloablative (n=1548) | 0 | 12 | 1.0 | 1.0 | ||
| 1–2 | 25 | 2.01 (1.6–2.6) | <0.0001 | 1.80 (1.4–2.3) | <0.0001 | |
| ≥3 | 42 | 4.07 (3.2–5.2) | <0.0001 | 3.33 (2.6–4.3) | <0.0001 | |
| Reduced intensity (n=465) | 0 | 15 | 1.0 | 1.0 | ||
| 1–2 | 29 | 2.05 (1.2–3.6) | 0.01 | 1.98 (1.1–3.5) | 0.02 | |
| ≥3 | 41 | 3.83 (2.3–6.3) | <0.0001 | 3.80 (2.2–6.5) | <0.0001 | |
| Nonmyeloablative (n=510) | 0 | 11 | 1.0 | 1.0 | ||
| 1–2 | 13 | 1.42 (0.8–2.5) | 0.24 | 1.61 (0.9–3.0) | 0.13 | |
| ≥3 | 32 | 3.31 (2.0–5.5) | <0.0001 | 3.42 (2.0–5.8) | <0.0001 | |
The Cox regression models were calculated for all available events over time and they were adjusted for diagnosis category, disease risk, age, donor type, stem cell source, KPS score, number of prior regimens, inclusion of anti-thymocyte globulin (ATG) in conditioning, and CMV sero-status.
The use of the HCT-CI among different age groups
Overall, the correlation between increasing age and increasing HCT-CI scores was weak (r=0.26). Higher HCT-CI score groups were associated with increased 2-year cumulative incidences of NRM and worsening of survival rates consistently in the five separate age groups (Table VII) including the pediatric population. In a proportional hazards model, tests of homogeneity showed no statistically significant age-related differences in the associations of HCT-CI scores with risks of NRM (p=0.66 and p=0.86, respectively) or survival (p=0.76 and p=0.24, respectively), indicating consistency of performance of the HCT-CI at different age intervals.
Table VII.
2-year NRM and OS by HCT-CI scores across different age groups.
| Cumulative percent incidence of NRM (2-yr) | Percent overall survival (2-yr) | |||||||
|---|---|---|---|---|---|---|---|---|
| HCT-CI scores | HCT-CI scores | |||||||
| Age groups, years | 0 | 1–2 | ≥3 | p | 0 | 1–2 | ≥3 | p |
| 0–19, malignant diseases | 8 | 26 | 28 | <0.001 | 73 | 61 | 41 | <0.001 |
| 0–19, non-malignant diseases | NE | NE | NE | 84 | 57 | 40 | <0.001 | |
| 20–39 | 11 | 20 | 39 | <0.001 | 80 | 62 | 33 | <0.001 |
| 40–49 | 12 | 26 | 43 | <0.001 | 75 | 56 | 39 | <0.001 |
| 50–59 | 21 | 31 | 39 | <0.001 | 60 | 48 | 33 | <0.001 |
| ≥60 | 7 | 27 | 38 | <0.001 | 63 | 47 | 27 | <0.001 |
NA indicates not evaluable since patients with non-malignant diseases could not be evaluated for non-relapse mortality
DISCUSSION
Allogeneic HCT is a potentially curative treatment for many patients with hematological malignant or non-malignant diseases. The success of allogeneic HCT depends, in part, on patient’s overall health status. The HCT-CI has been shown to be a simple tool to evaluate the burden of comorbidities before and to risk-stratify outcomes after allogeneic HCT (Sorror et al, 2005). Here, we demonstrated that the HCT-CI has a convergent validity as a comorbidity instrument across different transplant centers, different conditioning intensities, and different age groups. The predictive capacities as captured by the c-statistic in the whole cohort and within each institution were overall comparable to those initially reported with the design of the original model (0.66 vs 0.64 and 0.64 vs 0.62 for NRM and OS, respectively) (Sorror et al, 2005). This finding clearly demonstrates reproducibility of the index.
Results of the current study show that the HCT-CI serves as a discriminant predictor of mortality. Overall, each digit increase in the score of the HCT-CI was associated with increases in the unadjusted rates as well as the adjusted HRs for NRM and overall mortality. The three risk groups of the HCT-CI retained the unadjusted and adjusted associations with NRM and survival at each of the five institutions. The ability to demonstrate statistically significant differences between groups categorized by the HCT-CI depends on two factors: the magnitude of the difference between groups and the size of the cohort. Results of a recent study suggested that a sample of at least 200 patients is required to detect differences in outcomes between comorbidity subgroups (Sorror et al, 2011a). In the current study, the only institution with a total sample size <200 patients was the one with a marginal statistical significance in the associations between HCT-CI scores and outcomes, even though those associations were in the same directions as those in other institutions’ results. Statistically significant associations between HCT-CI and NRM have been shown in studies with ~200 patients or more (Barba et al, 2010; Farina et al, 2009; Pavlu et al, 2010; Smith et al, 2011), but not in those with <200 patients (Bokhari et al, 2012; Guilfoyle et al, 2009; Terwey et al, 2010). Small numbers of patients in validation studies could result in random variation in the significance of statistical associations and in the difficulty to appropriately adjust for confounding factors. Findings suggest that a sample size of at least 200 patients is needed for appropriate validation analyses of the performance of the HCT-CI.
Variation in mortality rates associated with higher HCT-CI scores across centers could be explained, in part, by differences in baseline risk factors that are not included in the HCT-CT, such as CMV serology results, diagnosis category, disease risk, number of prior regimens, donor type and stem cell source (Sorror et al, 2011b). However, variation across centers persisted even after adjustment for these known risk factors, revealing undefined “center effect” that also influenced outcomes. This observation clearly demonstrates the value of the HCT-CI in revealing differences in outcomes across institutions. Variability of outcomes across institutions could reflect differences in the continuity of care, the availability of consult services, or other confounders known to influence outcomes such as the proximity to the specialized treatment centers (Abou-Nassar et al, 2012) or other socio-demographic characteristics (Rao et al, 2007). Therefore, while HCT-CI proves to be an important tool to predict NRM and overall mortality after allogeneic HCT, accountability for other baseline risk factors and for center effect is crucial when comparing trial results across institutions. The Center for International Blood and Marrow Transplantation Research (CIBMTR) has incorporated comorbidities and other variables into Center-Outcome Analyses designed to compare outcomes across transplant centers and to provide this information to patients, insurance companies, and academic investigators.
The HCT-CI calibrates the tolerability of conditioning regimens for patients with given comorbidity risks. For example, patients with scores of 1–2 had non-significantly different 2-year incidence of NRM compared to those with score 0 when given nonmyeloablative regimens but they had statistically significant higher NRM incidence when given higher intensity regimens, respectively. The importance of intensity in conditioning regimens for allogeneic HCT remains controversial. Accrual in a clinical trial randomizing patients between high-dose and reduced-intensity conditioning allogeneic HCT (NCT00322101) was stopped earlier than planned after an interim analysis showed a benefit from high-dose regimens. This clinical trial used the HCT-CI as a stratification factor between the two arms and served as a key inclusion criterion to avoid offering high-dose regimens to patients with scores >3. Our results support continuation of this practice. However, these decisions need continuous evaluation given that clinical practice is constantly changing as new conditioning regimens are explored with the aim of improving disease control while reducing NRM. The HCT-CI could help assess the tolerability of new regimens as compared to current regimens.
Patients with HCT-CI scores of ≥3 had at least three-fold higher risks for NRM compared to those with scores of 0 consistently across the three categories of regimen intensity (adjusted HRs, 3.42, 3.80, and 3.33, respectively). The 2-year incidences of NRM for these patients were in the range of 32–42% regardless of the regimen intensity and in the range of 34–53% regardless of the center. Thus far, this information has been used only in counseling patients before allogeneic HCT. Recent findings suggest that comorbidities might also be associated with the risk of common post-HCT complications such as severe acute GVHD (Sorror et al, 2014a). These findings could pave the way for future intervention studies aimed at ameliorating the impact of comorbidities on post-HCT morbidity and hence, decreasing mortality associated with HCT. Likewise, Higher HCT-CI scores were associated with worsening of survival and NRM consistently in all 5 age groups. A proportional hazard model test for homogeneity showed no statistically significant age related differences in the associations of HCT-CT with risks of NRM or survival. These results suggest suitability of the HCT-CI for risk-stratification among older as well as children and young adult patients.
The retrospective design of the current study is a limitation, but strengths come from the recruitment of consecutive patients from five different centers and the low amount of missing comorbidity data (8%). Comorbidity research in HCT is still at an early stage of development. Comorbidity data are being used by collaborative research groups and centralized data collection systems such as the CIBMTR to open new avenues of biomedical outcome research. Comorbidity scores could be integrated into decision-making on the type of conditioning regimen, donor graft, and hematopoietic cell source appropriate for individual patients. How comorbidities interact with each other and result in post-HCT cause-specific morbidity and mortality is another important question. Comorbidity data could be used in genetic epidemiology studies evaluating toxicities after HCT (Chien et al, 2012) in order to understand whether specific genetic polymorphisms contribute to a given pre-HCT comorbidity and a given outcome after HCT. Current results showing the prognostic validity of the HCT-CI coupled with previous evidence on excellent reliability of the index (Sorror, 2013) will support its use as a platform for future investigations into the independent roles of plasma biomarkers (Sorror et al, 2009), patient quality of life (Sorror et al, 2013), and frailty measures on HCT outcomes (Muffly et al, 2014).
Acknowledgments
We are grateful to Gary Schoch, Gresford Thomas, Cara Hanby, Kayo Togawa, Nan Subbiah, Rachel Frires, Juli Murphy, Araki Kristen, and Jennifer Sheldrake for their tremendous help in protocol approval and acquisition of data from computerized databases at the different institutions. We also thank Bonnie Larson and Helen Crawford for their help with manuscript preparation. We are grateful to the many physicians, nurses, research nurses, physician assistants, nurse practitioners, pharmacists, data coordinators, and support staff who cared for our patients, and to the patients who allowed us to care for them and who participated in our ongoing clinical research. This work was supported by Grants No. HL088021, CA018029, HL036444, and CA078902 from the National Institutes of Health; by Research Scholar Grant No. RSG-13-084-01-CPHPS from the American Cancer Society; by Patient-Centered Outcome Research Institute Contract No. CE-1304-7451 (M.L.S.); and by grant No. JS2865 from the Egyptian ministry of higher education (M.E.).
Footnotes
Prior Presentation: Part of the study results were presented at the Annual Meeting of the American Society of Hematology 2011 in San Diego, California.
AUTHORSHIP AND DISCLOSURES
MLS, RFS, and BES designed research; MLS collected data and obtained funding for the study; SB, RTM, MAP, MBM coordinated the study at respective centers; SB, RTM, MAP and MBM contributed data to the study; BES performed statistical analysis; ME, RTM, MAP, KLS, PJM, DGM, BMS, RFS, MLS analyzed and interpreted data; ME and MLS wrote the manuscript; RTM, MAP, MBM, KLS, PJM, BMS, and RFS edited the manuscript. The authors have no conflicts of interest.
References
- Abou-Nassar KE, Kim HT, Blossom J, Ho VT, Soiffer RJ, Cutler CS, Alyea EP, Koreth J, Antin JH, Armand P. The impact of geographic proximity to transplant center on outcomes after allogeneic hematopoietic stem cell transplantation. Biology of Blood & Marrow Transplantation. 2012;18:708–715. doi: 10.1016/j.bbmt.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperly J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, Blaise D, Lowski R, Horowitz M. Defining the intensity of conditioning regimens: working definitions. Biology of Blood and Marrow Transplantation. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba P, Piñana JL, Martino R, Valcárcel D, Amorós A, Sureda A, Briones J, Delgado J, Brunet S, Sierra J. Comparison of two pretransplant predictive models and a flexible HCT-CI using different cut points to determine low-, intermediate-, and high-risk groups: the flexible HCT-CI is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biology of Blood and Marrow Transplantation. 2010;16:413–420. doi: 10.1016/j.bbmt.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Birninger N, Bornhäuser M, Schaich M, Ehninger G, Schetelig J. The hematopoietic cell transplantation-specific comorbidity index fails to predict outcomes in high-risk AML patients undergoing allogeneic transplantation--investigation of potential limitations of the index. Biology of Blood and Marrow Transplantation. 2011;17:1822–1832. doi: 10.1016/j.bbmt.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Bokhari SW, Watson L, Nagra S, Cook M, Byrne JL, Craddock C, Russell NH. Role of HCT-comorbidity index, age and disease status at transplantation in predicting survival and non-relapse mortality in patients with myelodysplasia and leukemia undergoing reduced-intensity-conditioning hemopoeitic progenitor cell transplantation. Bone Marrow Transplantation. 2012;47:528–534. doi: 10.1038/bmt.2011.138. [DOI] [PubMed] [Google Scholar]
- Chien JW, Zhang XC, Fan W, Wang H, Zhao LP, Martin PJ, Storer BE, Boeckh M, Warren EH, Hansen JA. Evaluation of published single nucleotide polymorphisms associated with acute graft versus host disease. Blood. 2012;119:5311–5319. doi: 10.1182/blood-2011-09-371153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina L, Bruno B, Patriarca F, Spina F, Sorasio R, Morelli M, Fanin R, Boccadoro M, Corradini P. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23:1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. The Design and Analysis of Clinical Experiments. John Wiley & Sons, Inc; New York, NY: 1999. [Google Scholar]
- Fujimaki K, Sakai R, Fujisawa S, Fujita H, Tanaka M, Hagihara M, Koharazawa H, Miyazaki T, Tomita N, Kanamori H, Maruta A, Ishigatsubo Y. Usefulness of hematopoietic cell transplantation-specific comorbidity index after allogeneic hematopoietic stem cell transplantation [Japanese] Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 2008;35:87–91. [PubMed] [Google Scholar]
- Gratwohl A. The EBMT risk score (Review) Bone Marrow Transplantation. 2012;47:749–756. doi: 10.1038/bmt.2011.110. [DOI] [PubMed] [Google Scholar]
- Guilfoyle R, Demers A, Bredeson C, Richardson E, Rubinger M, Szwajcer D, Seftel MD. Performance status, but not the hematopoietic cell transplantation comorbidity index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplantation. 2009;43:133–139. doi: 10.1038/bmt.2008.300. [DOI] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Statistics in Medicine. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- Kerbauy DMB, Chyou F, Gooley T, Sorror ML, Scott B, Pagel JM, Myerson D, Appelbaum FR, Storb R, Deeg HJ. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biology of Blood and Marrow Transplantation. 2005;11:713–720. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, Marsh J, Ho AY, Mufti GJ, Pagliuca A. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leukemia Research. 2010;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Majhail NS, Brunstein CG, McAvoy S, Defor TE, Al-Hazzouri A, Setubal D, Arora M, Le CT, Wagner JE, Weisdorf DJ. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes? A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biology of Blood and Marrow Transplantation. 2008;14:985–992. doi: 10.1016/j.bbmt.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, Kline J, Liu H, Odenike OM, Larson RA, van Besien K, Artz AS. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlu J, Kew AK, Taylor-Roberts B, Auner HW, Marin D, Olavarria E, Kanfer EJ, MacDonald DH, Milojkovic D, Rahemtulla A, Rezvani K, Goldman JM, Apperley JF, Szydlo RM. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010;115:4018–4020. doi: 10.1182/blood-2010-01-263624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A, Fanin R, Rambaldi A, Bosi A. Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood. 2012;120:1327–1333. doi: 10.1182/blood-2012-03-414573. [DOI] [PubMed] [Google Scholar]
- Rao K, Darrington DL, Schumacher JJ, Devetten M, Vose JM, Loberiza FR., Jr Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Biology of Blood & Marrow Transplantation. 2007;13:1508–1514. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Ratan R, Ceberio I, Hilden P, Devlin SM, Malloy MA, Barker JN, Castro-Malaspina H, Jakubowski AA, Koehne G, Papadopoulos EB, Ponce DM, Sauter CS, van den Brink MRM, Young JW, O’Reilly RJ, Giralt S, Goldberg JD, Perales MA. The Hematopoietic Cell Transplant-Co-Morbidity Index (HCT-CI) predicts outcomes after T cell depleted (TCD) allogeneic HCT for AML and MDS. Blood. 2013;122:2045. (Abstract) [Google Scholar]
- Smith AR, Majhail NS, MacMillan ML, Defor TE, Jodele S, Lehmann LE, Krance R, Davies SM. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- Sorror M. How I assess comorbidities prior to hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Giralt S, Sandmaier BM, de Lima M, Shahjahan M, Maloney DG, Deeg HJ, Appelbaum FR, Storer B, Storb R. Hematopoietic cell transplantation-specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: Combined FHCRC and MDACC experiences. Blood. 2007a;110:4608–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Martin PJ, Storb R, Bhatia S, Maziarz RT, Pulsipher MA, Maris MB, Davis C, Deeg HJ, Lee SJ, Maloney DG, Sandmaier BM, Appelbaum FR, Gooley T. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014a;124:287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Ostronoff F, Storb R, Bhatia S, Maziarz RT, Pulsipher MA, Maris M, Deeg HJ, Martin PJ, Appelbaum FR, Maloney DG, Sandmaier BM, Storer B. Multi-institutional validation of the predictive power of the hematopoietic cell transplantation comorbidity index (HCT-CI) for HCT outcomes. Blood. 2011a;118:145. (Abstract) [Google Scholar]
- Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, Scott BL, Deeg HJ, Appelbaum FR, Storb R. Comorbidity and disease status-based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology. 2007b;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, Bhatia S, Ostronoff F, Deeg HJ, Syrjala KL, Estey E, Maloney DG, Appelbaum FR, Martin PJ, Storer BE. Comorbidity-age index: a clinical measure of biological age before allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology. 2014b;32:3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorror ML, Storer B, Storb R. Assignment of scores for the Hematopoietic Cell Transplantation-Comorbidity Index: integer vs exact weights (Letter to the Editor) Bone Marrow Transplantation. 2011b;46:464–466. doi: 10.1038/bmt.2010.131. [DOI] [PubMed] [Google Scholar]
- Sorror ML, Storer BE, Schoch G, Sandmaier BM, Martin PJ, Scott BL, Deeg HJ, Appelbaum FR, Maloney DG, Storb RF. Low albumin, high ferritin, and thrombocytopenia before transplant predict non-relapse mortality (NRM) independent of the hematopoeitic cell transplantation comorbidity index (HCT-CI) Blood. 2009;114:271, 651. (Abstract) [Google Scholar]
- Sorror ML, Yi JC, Storer BE, Rock EE, Artherholt SB, Storb R, Martin PJ, Syrjala KL. Association of Pre-Transplant Comorbidities with Long-Term Quality of Life (QOL) Among Survivors After Allogeneic Hematopoietic Cell Transplantation (HCT) Biology of Blood and Marrow Transplantation. 2013;19:S153. (Abstract) [Google Scholar]
- Terwey TH, Hemmati PG, Martus P, Dietz E, Vuong LG, Massenkeil G, Dörken B, Arnold R. A modifed EBMT risk score and the hematopoietic cell transplantation-specific comorbidity index for pre-transplant risk assessment in adult acute lymphoblastic leukemia. Haematologica. 2010;95:810–818. doi: 10.3324/haematol.2009.011809. [DOI] [PMC free article] [PubMed] [Google Scholar]