Abstract
Chromosome 6p22 was identified recently as a neuroblastoma susceptibility locus, but its mechanistic contributions to tumorigenesis are as yet undefined. Here we report that the most highly significant single nucleotide polymorphism (SNP) associations reside within CASC15, a long non-coding RNA that we define as a tumor suppressor at 6p22. Low-level expression of a short CASC15 isoform (CASC15-S) associated highly with advanced neuroblastoma and poor patient survival. In human neuroblastoma cells, attenuating CASC15-S increased cellular growth and migratory capacity. Gene expression analysis revealed downregulation of neuroblastoma-specific markers in cells with attenuated CASC15-S, with concomitant increases in cell adhesion and extracellular matrix transcripts. Altogether, our results point to CASC15-S as a mediator of neural growth and differentiation, which impacts neuroblastoma initiation and progression.
Keywords: neuroblastoma, lncRNA, CASC15, LINC00340, FLJ22536
INTRODUCTION
Neuroblastoma, a cancer of the developing autonomic nervous system, is the most common malignancy diagnosed in the first year of life and accounts for approximately 10% of all pediatric cancer mortality1-3. While the majority of low-risk neuroblastoma patients are cured with surgery alone, 50% of patients have the high-risk form of the disease, and only about half of these children survive despite highly intensive therapy1. Neuroblastomas are thought to develop from cells derived from the neural crest committed to the sympathicoadrenal lineage (peripheral autonomic nervous system) 1-4. Because malignant transformation can occur at any point during sympathetic development, tumors may arise throughout the developing sympathetic nervous system (most commonly the adrenal gland), contributing to the hallmark heterogeneity observed in this disease4. To address the etiology of sporadic neuroblastoma, we conducted the first pediatric cancer genome-wide association study (GWAS), leading to the identification of numerous validated susceptibility loci in several populations5-12. Moreover, we showed that many of these susceptibility alleles are specifically associated with disease phenotype and patient outcomes. The majority of these SNPs act in cis to influence expression of protein coding genes at these loci, and several of these transcripts, such as LMO1, BARD1 and LIN28B, appear to play an oncogenic role in established tumors 5-12.
The first identified neuroblastoma susceptibility locus identified by GWAS, and the one that remains most significant, mapped to chromosome 6p22.3 and robustly replicated in three independent cohorts (rs6939340: p = 9.33 × 10−15; Allelic Odds Ratio 1.97, 95% C.I.: 1.58-2.45) 5. Like other subsequently identified loci, we observed a highly significant association with neuroblastoma susceptibility and clinically aggressive presentation. The minor allele (G) was over-represented in neuroblastoma cases compared to controls, and presence of the G allele was further enriched in the high-risk subset of neuroblastoma (p = 0.007), tumors with MYCN amplification (p = 0.002) and stage 4 disease (p = 0.025), implying the risk alleles were associated with a more malignant neuroblastoma phenotype. Based on HapMap data available at the time of this initial discovery, the associated SNPs tagged a 94.2-kb linkage disequilibrium (LD) block; this LD block overlapped two hypothetical genes (FLJ22536 and FLJ44180) 5. However, both FLJ22536 and FLJ44180 lacked protein coding potential, impeding further characterization of this region in neuroblastoma initiation.
Recent data obtained from whole genome sequencing studies of neuroblastoma have illustrated far fewer recurrent mutations in protein-coding genes than previously predicted 13-16; however it is now clear that as much as 70% of the genome is transcribed into products other than traditional protein-coding mRNAs 17,18. Although many of these transcriptionally active loci produce RNA species involved in translation (i.e. ribosomal and transfer RNAs), several other RNA classes have been functionally validated as bona-fide regulatory molecules. The recently identified long non-coding RNAs (lncRNAs), defined as RNA species >200nt in length that lack a functional open reading frame, have been increasingly implicated in a wide variety of cellular functions 19. LncRNAs share several transcriptional features in common with mRNAs - they are often spliced, demonstrate RNA polymerase II occupancy, contain a 5’ methylguanosine cap, and are commonly (though not always) polyadenylated 20,21. Although lncRNA function is highly context dependent, they commonly play a prominent role in the spatiotemporal regulation of gene expression during developmental processes 22-24, and therefore exhibit a tendency to be located throughout the genome, in sites proximal to developmentally critical protein-coding genes 25. Indeed, several lncRNAs reside near protein-coding genes known to regulate lineage commitment in neural crest cells 26, serving as an attractive hypothesis to explain the etiology of embryonal cancers such as neuroblastoma.
As might be expected, lncRNAs have been increasingly implicated in a variety of oncogenic processes through association with epigenetic complexes and modification of chromatin accessibility - ultimately influencing gene expression 27-30. To date, there are few reports concerning the role of lncRNAs in the initiation and progression of solid pediatric neoplasms, despite the fact that many childhood cancers are fundamentally defects of normal human development 31. Here we describe the identification and characterization of a novel lncRNA, CASC15, which contributes to the GWAS association signal on 6p22.3 by functioning as a tumor suppressor in neuroblastoma.
METHODS
Genome Wide Association Study (GWAS) and Imputation
In an effort to refine the association signal and search for a causal variant at the 6p22 locus, we performed genotype imputation in a previously described European ancestry cohort of 2,101 neuroblastoma cases and 4,202 controls10 using the 1000 Genomes Phase I Release 3 as a reference. Genotyping and quality control methods have been previously published5. GWAS imputation and statistical tests are detailed in the Supplementary Methods section.
Data Sources
The human February 2009 (GRCh37/hg19) genome assembly was used throughout the study. Transcript structures and annotations were obtained from GENCODE version 19. Details on the various data sources used are available in the Supplementary Methods section.
Neuroblastoma data
The neuroblastoma RNAseq, SNP profiling and HuEx datasets are part of the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) initiative, supported by NCI Grant U10 CA98543. The low-level sequence data have been deposited in the Sequence Read Archive (SRA) at the National Center for Biotechnology Information (NCBI), and are further accessible through the database of genotypes and phenotypes (dbGAP, accession number phs000218). The gene expression and copy number data, as well as clinical information on the NBL cases studied, is available via the TARGET Data Matrix32.
5’/3’ Rapid Amplification of cDNA Ends (RACE)
5’ and 3’ RACE was performed via the First Choice RLM RACE kit (Ambion) using 10 μ g of RNA obtained from fetal brain or NB69 neuroblastoma cells following the manufacturers protocol. Specificity for CASC15-S was achieved by nested PCR using the following gene specific primer (GSP) pairs:
5’ RACE: Outer GSP: 5’-CTAGCCCATCAGTTCCTTCG -3’
5’ RACE: Inner GSP: 5’-TTCACCCTGTCCTCCAAGTC-3’
3’ RACE: Outer GSP: 5’-TGGTTACCTGAGCTGCTCCT-3’
3’ RACE: Inner GSP: 5’-CTCAGCCAGTGCAACACAAC-3’
Gene products were cloned into a pCR4-TOPO vector for sequencing.
RNA Sequencing (RNASeq)
Neuroblastoma
PolyA selected RNA libraries obtained from 108 high-risk neuroblastoma patients as part of the NCI TARGET project were prepared using TruSeq v3 (Illumina) for RNA sequencing on Illumina HiSeq2000 sequencers. The 101bp paired-end reads generated were aligned to the hg19 build of the human reference genome using TopHat v2.2.0, yielding a median of 115 million total aligned reads per patient sample (range of 48 - 250 million total aligned reads). The HTSeq package (v0.6.1) was used to map aligned reads to the VISTA-annotated enhancer region, hs1335, as well as all transcripts annotated in Ref-Seq (v66) and/or the UCSC Genome Browser. Transcript expression values were normalized and quantified using the metric of reads per kilobase per million reads (RPKM). For isoform-specific quantitation of CASC15 and CASC15-S, only exons and exon-exon junctions that were unique to each isoform were used in computing RPKM. Other: Mapped RNA-seq reads from 16 primary tissues (Illumina Human Body Map) and 51 cell lines (Human ENCODE) were used to quantify gene- and transcript-level expression, based on GENCODE v19 annotations, using Cufflinks v.2.2.0 with default parameters and “--min-frags-per-transfrag 0 --compatible-hits-norm --min-isoform-fraction 0.0”. Expression data heatmaps were generated using the heatmap.2 function of the gplots R package.
RNA Fluorescent In Situ Hybridization (RNA-FISH)
We performed RNA FISH and counted RNA in single cells as described in Raj et al., 2008. Fluorescently labeled, non-overlapping oligonucleotide probes (20-mers) were designed to tile RNA transcripts, including CASC14 (LOC729177), hs1335, CASC15 and CASC15-S. Probes were then divided into odd and even pools and hybridized to neuroblastoma cells. Details of image acquisition are available in the Supplementary Methods section.
Quantification of CASC15-S in neuroblastoma cells
Quantification of RNA transcripts was performed on a panel of neuroblastoma cancer lines using Taqman RT-PCR. For quantification of CASC15-S, we developed a custom primer/probeset spanning exons 1-2 and consisting of the following primers:
forward: GCTGTCGACGAAGGAACTGAT
reverse: GTCCAAGTCAAAAGTCTCATCCAAGA
reporter: CCTGTGAGCCTAGCCC
Primer/probe sets for CASC15 (Hs01371949_m1) and CASC14 (Hs04275511_s1) are commercially available (Life Technologies). Quantification was normalized to the geometric mean of housekeeping genes TBP and GUSB.
Cell growth and siRNA assays
ON-TARGETplus SmartPool siRNAs containing four constructs per target (Thermo Scientific) were used for GAPDH and PLK-1 knockdown. ON-TARGETplus Non-targeting Control Pool (containing 4 constructs) was used as a control. Two constructs were used for each lncRNA, and were purchased as Silencer Select siRNA or custom siRNAs from Life Technologies.
siRNA constructs were transfected into neuroblastoma cells in triplicate, using 50nM of siRNA in 0.1-0.2% DharmaFECT 1 (Thermo Scientific). Knockdown was assessed by Taqman RT-PCR, comparing levels 72h post-transfection to non-targeting control transfected cells. For all siRNA experiments, the minimum knockdown achieved was 71.5% (avg. = 78.6, max = 92.0).
For growth assays, while both tested, the siRNA with the best knockdown efficiency for each target is shown. Cell growth assays were conducted using the xCELLigence real-time growth (RT-CES, ACEA Biosciences) and/or Cell-TiterGlo (Promega) assays according to manufacturer protocols.
CASC15-S add-back experiments
For CASC15-S addback experiments, expression plasmids containing the cDNA for either CASC15-S or GFP were transfected in triplicate in a 96-well plate using 250ng of DNA and 3ul of Lipofectamine 2000 per well. These experiments were conducted using the growth assays described above, and the expression levels of the CASC15-S transcript were confirmed by Taqman RT-PCR to be highly expressed in the addback condition. Transfection efficiency was estimated to be ~90% based on the GFP transfected condition.
shRNA expression and lentiviral constructs
The siRNA construct for CASC15-S (targeting exon 1) was used to create a double-stranded shRNA construct and was subsequently cloned into the pLenti-GFP DEST lentiviral vector (Addgene). 5×106 293T cells were transfected with 15μg of the pLenti transfer vector, 15μg of pLP1, 6μg of pLP2 and 3μg of pVSV-G vectors. Lentiviral particles were collected at 48 and 72 hours post transfection. Lentiviral transduction of neuroblastoma cells was carried out overnight at 37C using 3μg/ml of polybrene. For each neuroblastoma line used, serial dilutions of lentiviral particles in basal media were carried out in a 6-well plate, and cells were assessed at 48h post-transduction for GFP expression. The minimum lentiviral concentration resulting in >95% GFP expression was chosen for expansion and knockdown was validated by Taqman PCR. For all shRNA experiments, knockdown of at least 70.3% (avg. = 82.6, max = 91.8) was achieved. Each neuroblastoma line with confirmed knockdown was then frozen at low passage (< 5) and thawed as needed for subsequent experiments.
Luciferase Reporter Assay
The respective risk (A_A) and non-risk (T_T) genotypes of Lan-5 and CHP-134 cells at rs9295534 were verified by PCR and Sanger sequencing. Subsequently, a 1500bp fragment was cloned from each cell line (750bp on either side of the rs9295534) and was purified using the Qiaquick Gel Extraction kit (Qiagen). The fragment was inserted into the pGL4.23 vector upstream of a minimal promoter used to drive firefly luciferase expression. Fragment-containing vectors were then transfected into HEK-293 cells, along with a Renilla luciferase vector (pGL4.75) to control for transfection efficiency. 72 hours following transfection, luciferase activity was assayed by Dual-Glo reporter assay (Promega) using the GloMax Multi Detection System (Promga). Firefly luciferase was normalized to Renilla luciferase and a minimum of three replicates was used for each condition.
In Vitro Transcription/Translation Assay
CASC15-S was cloned into a T7-promoter containing plasmid (T7-CFE-Chis) and subsequently verified by Sanger sequencing. Coding potential of CASC15-S was assayed using the TnT® Quick Coupled Transcription/Translation System according to the manufacturers protocol. Incorporation of biotinylated lysine residues was visualized via western blot using a 1:10,000 anti-biotin HRP labeled antibody (Cell Signaling) and chemiluminescent detection (Thermo Scientific).
Wound Healing Assays
Scratch assays were carried out on SK-N-BE2 and SK-N-SH cells stably depleted of CASC15-S plated at 85% confluence in 60mm dishes, and were scratched using a sterile 200μl pipette tip. Cells were photographed at regular intervals using a previously calibrated 5× light microscope (Nikon). Assessment of cell migration was carried out by measuring scratch closure as a percentage of initial scratch size in ImageJ, and was compared to control cells using a linear regression function in GraphPad Prism 6.
Cell Culture
Neuroblastoma cell lines were obtained from the neuroblastoma cell line bank maintained at the Children's Hospital of Philadelphia. Cell line identity is routinely confirmed via AmpFLSTR Identifiler (Applied Biosystems), last done in November 2013. Non-neuroblastoma cell lines were purchased from ATCC and all cell lines are routinely tested for mycoplasma. All cell lines are maintained in basal media (either RPMI1640 or DMEM) supplemented with 10% FBS and 1% gentamycin and cultured at 5% CO2.
Statistics
All group comparisons were conducted using non-parametric testing (Mann-Whitney-U) in Graphpad Prism.
For Kaplan Meier analysis: optimal cutoff was determined by employing a scanning approach to the Kaplan-Meier method by iteratively splitting the ordered genes expression values across samples into two groups and calculating the p-value by the Mantel-Haenszel log-rank test. The lowest p-value corresponds to the optimal breakpoint. A Benjamini-Hochberg correction was applied to reflect the presence of multiple hypotheses testing.
For multivariate analyses, a Cox Proportional Hazard model was used to evaluate the effect of each gene expression on overall survival, adjusting for clinical factors such as age (as a continuous variable), MYCN amplification, and 1p/11q LOH status. Proportional hazard assumption was evaluated by the log-log plot and there was no evidence of violation of this assumption.
RESULTS
Fine mapping of the 6p22.3 neuroblastoma susceptibility locus
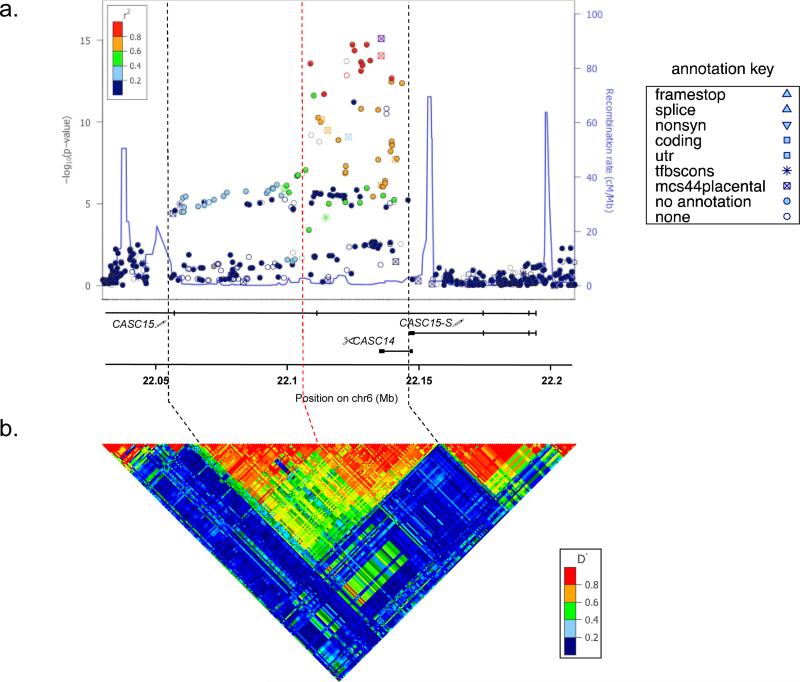
The initial GWAS study illustrating 6p22.3 as a neuroblastoma susceptibility locus identified three common polymorphisms clustered on chromosome 6p22.3 that were highly associated with aggressive disease and a significantly increased risk of neuroblastoma development 5. These SNPs were shown to tag a 94.2-kb LD block overlapping two hypothetical genes and flanked upstream by SOX4. To map this region with finer detail, and identify putative causal/functional SNPs, we performed genotype imputation in our recently published discovery cohort of 2,101 cases and 4,202 controls 10. We first applied SHAPEIT33 to infer haplotypes, and then utilized IMPUTE234 with default parameters and Ne=20000, along with a multi-population reference panel from the world-wide 1000 Genomes Project Phase 1 release to impute genotypes across the region. Genotyped and imputed variants were tested for association with neuroblastoma using the frequentist association test under the additive model using the “score” method implemented in SNPTEST35. Variants with minor allele frequency (MAF) < 1% and/or IMPUTE2-info quality score < 0.8 were excluded for quality control purposes. We identified 32 polymorphisms with p-values less than 1×10−10 (p = 8.26×10−10 – 1.88×10−15), 12 of which were in high LD (r2 > 0.8) and localized to an intronic region of an annotated gene locus formerly titled FLJ22536 / LINC00340 and more recently renamed cancer associated susceptibility candidate 15 (CASC15; Fig. 1a, Supplementary Table 1). These 32 SNPs identify a 34.9-kb linkage disequilibrium (LD) block based on the 1000 Genomes Northern and Western European (CEU) population (Fig. 1b), significantly refining this relatively small locus.
Figure 1.
Fine mapping of 6p22 identifies CASC15-S as a candidate cis-acting neuroblastoma susceptibility gene. (a) Regional association plot of single nucleotide polymorphisms (SNPs) generated with LocusZoom software using genome wide imputation data from 2,817 neuroblastoma cases and 7,473 controls. The pair-wise linkage disequilibrium (r2) for each SNP is denoted by color, and the log-transformed p-values for each SNP are shown on the y-axis. Thirty-two SNPs with highly significant p-values (< 1×10−10) ranging from 4.67×10−10 to 4.81×10−17 were found to cluster as a narrow peak within a genomic region containing the lncRNAs CASC14 and CASC15. (The exons and transcribed regions of genes are shown as solid vertical lines.) (b) The haplotype structure of this region in Northern European (CEU) population demonstrates a signal that overlaps with these SNPs, refining and initial 94.2kb LD block (boundaries denoted by black vertical dashed lines) down to 34.9kb, demonstrated by the dashed red vertical line and rightmost black vertical dashed line. (SNP annotations: color = representative of LD r2 value (purple = reference SNP);  predicted coding or 3’UTR region;
predicted coding or 3’UTR region;  = nonsynonymous; * = tfbccons, conserved motif at transcription factor binding site,
= nonsynonymous; * = tfbccons, conserved motif at transcription factor binding site,  = mcs44placental, highly conserved region in placental mammals).
= mcs44placental, highly conserved region in placental mammals).
Identification of CASC15 isoform expression
CASC15 is annotated as a long noncoding RNA (lncRNA), prompting us to examine the difference in lncRNA transcript levels between 220 high-risk and 30 low-risk primary tumors for which we possess microarray expression data. This analysis revealed that only 3.5% of annotated lncRNAs (hg19: 8/229) appear significantly differentially regulated between high- and low-risk tumors (Table 1). Furthermore, high-risk neuroblastomas exhibit a 4.4-fold lower level of CASC15 expression as compared to low-risk disease - the most highly significant (p = 3.60×10−17) differentially expressed lncRNA we observed. Several computationally predicted lncRNAs map to the 6p22.3 locus, including multiple CASC15 isoforms and an antisense-strand lncRNA, CASC14 (formerly LOC729177) (Supplementary Fig. 1a).
Table 1.
Differential expression analysis of long noncoding RNAs between high- and low-risk neuroblastomas.
| Gene | Ratio | Disease State | p-value | Location |
|---|---|---|---|---|
| LINC00340 | 4.4 -fold lower in | High Risk | 3.60E-17 | chr6:21666675-22194616 |
| LINC00174 | 2.4 -fold lower in | High Risk | 2.28E-15 | chr7:65841031-65865395 |
| LINC01296 | 8.9 -fold higher in | High Risk | 5.80E-15 | chr14:19880209-19925329 |
| LINC00260 | 2.8 -fold lower in | High Risk | 1.75E-13 | chr1:203699705-203700979 |
| LINC00221 | 2.4 -fold higher in | High Risk | 7.04E-12 | chr14:106938445-106951529 |
| LINC00478 | 2.5 -fold higher in | High Risk | 1.74E-04 | chr21:17442842-17982094 |
| LINC00514 | 2.4 -fold lower in | High Risk | 3.70E-04 | chr16:3039055-3044510 |
| LINC00355 | 2.9 -fold higher in | High Risk | 5.01E-04 | chr13:64560504-64650144 |
Differential gene expression analysis (> 2.0-fold, p < 0.05, FDR < 0.05) was conducting using 220 high- and 30 low-risk primary neuroblastoma tumors, focusing on differences in lncRNA expression. The top differentially regulated lncRNA was CASC15 (annotated here as LINC00340), which was significantly lower in high-risk disease (4.4-fold decrease, ANOVA, p = 3.60×10−17).
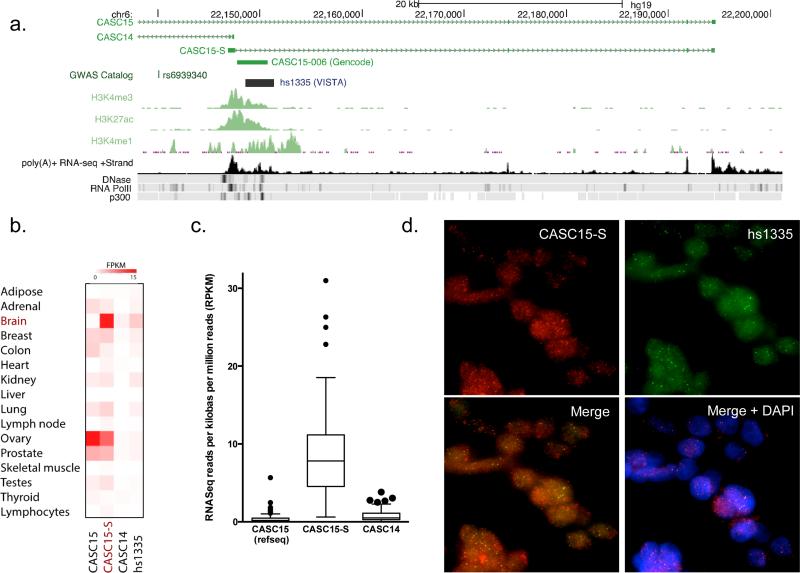
We therefore first utilized RNA sequencing (RNASeq) and RNA-paired end tagged (RNA-PET) data available from the ENCODE project to identify the products transcribed from this locus in SK-N-SH and SK-N-BE2 neuroblastoma cell lines. These data demonstrated the existence of two capped and polyadenylated nuclear CASC15 transcripts, a long (hg19, chr6: 21,666,675-22,194,616; Ensembl: CASC15-003) and a short isoform (hg19, chr6: 22,146,883-22,194,616; Ensembl: CASC15-004) (Fig. 2a, Supplementary Figs. 1b, 2a). These nuclear noncoding transcripts are highly conserved in vertebrates, and readily detected in several brain regions and neuroblastoma cell lines (Supplementary Fig. 2), with putative promoter regions separated by 480kb indicative of independent transcriptional regulation.
Figure 2.
CASC15-S is the predominant lncRNA isoform expressed in neuroblastoma. (a) Graphical representation of the lncRNA transcripts observed to originate from the 6p22.3 locus via RNA sequencing. This locus includes two predominant transcripts on the positive strand: a 1.9kb CASC15 transcript (current RefSeq for this lncRNA) and a novel shorter (1.2kb) transcript, CASC15-S that shares the last 3 exons with the long isoform of CASC15. These transcripts were validated using 5’ and 3’ RACE. Epigenetic marks support active transcription (RNA PolII occupancy) and an enhancer like function (H3K27Ac, H3K4Me3) of this region, including a proximal VISTA enhancer element (hs1335). RNA sequencing, RNA PolII, DNAse, and P300 ChIP-Seq data were obtained from SK-N-SH neuroblastoma cells (via ENCODE), whereas enhancer track ChIP-Seq data was taken from MGG8 human glioblastoma stem cells 47. (b)Expression levels of CASC15, CASC15-S, CASC14 and hs1335 were normalized and quantified across a panel of 16 normal primary tissues (Human Body Map 2.0 project), indicating predominant expression of the CASC15-S isoform in brain. (c) RNA sequencing performed on 108 primary neuroblastoma tumors, analyzed for unique isoform expression, exhibited a predominance of the short CASC15 isoform. This short isoform was expressed at 20-fold and 40-fold higher levels than CASC14 and or full-length CASC15, respectively. (d) RNA fluorescent in-situ hybridization (RNA-FISH) was conducted in NGP neuroblastoma cells for several transcripts known to exist at this locus. To ensure probe specificity, odd and even pools of fluorescently labeled oligonucleotide probes were used to tile CASC15-S and VISTA hs1335. The yellow fluorescence observed in the “merge” panel was obtained from overlap of the hs1335 and CASC15-S fluorescent signals and indicate colocalization of these two transcripts. Consistent with the majority of described lncRNAs, these transcripts appear to be predominately nuclear as evidenced in the “merge + DAPI” panel. (40× magnification)
We next examined RNA expression data from a panel of 16 primary human tissues as part of the Illumina Human Body Map project, where we found that the short CASC15 isoform was expressed in abundance in the brain, but at modest to low levels in most other tissues (Fig 2b). We subsequently generated RNA sequencing data from 108 primary neuroblastoma tumors using non-overlapping, transcript-specific reads to investigate the expression of lncRNAs transcribed from this locus. We augmented these findings with our own strand-specific RNA sequencing data from two primary tumors, confirming nearly complete alignment to the plus strand (avg. 92.6%, min. 86.9%, max 98.6%). Together, these RNA sequencing data identify the short CASC15 isoform as the predominant transcript expressed from this locus in neuroblastoma, with expression values 20- to 40-fold higher than CASC14 and full-length CASC15, respectively (Fig. 2c). Lastly, RNA sequencing data identified a third unspliced transcript, spanning part of exon 1 of the short CASC15 isoform through a downstream noncoding element (hs1335) with a validated enhancer function in the developing murine neural tube, further supporting the role of this locus in neural development 36.
To validate the isoforms identified from RNA sequencing data, we performed 5’ and 3’ RACE for CASC15, subsequently cloning and sequencing these transcripts from two neuroblastoma cell lines and fetal brain tissue. We verified the sequence of both the 12-exon 1.9kb CASC15 transcript (NR_015410.1, Ensembl: CASC15-003) and the 4-exon short (1.2kb) variant (Ensembl: CASC15-004), hereafter referred to as CASC15-S. CASC15-S resembles a known cDNA clone (GenBank: AK094718), containing a unique first exon, yet sharing its remaining sequence with the last three exons of CASC15 (Fig. 2a, Supplementary Fig. 3a). Despite several attempts, we were unable to isolate a 5’-capped product for the intron-less transcript (Ensembl: CASC15-006) predicted to overlap exon 1 of CASC15-S and extend to the noncoding enhancer element (hs1335). Indeed, although the presence of this isoform is indicated on the SK-N-SH RNASeq track (Fig. 2a), it is absent in RNA-PET data (Supplementary Fig. 1b), supporting this result. Finally, RNA fluorescent in situ hybridization (RNA-FISH) experimentally confirmed the presence, relative abundance and cellular localization of these transcripts in both NB-69 and NGP neuroblastoma cells; using non-overlapping, strand-specific probes to label CASC15, CASC15-S, CASC14 and hs1335. These studies revealed exclusively nuclear CASC15-S and hs1335 transcripts (Fig. 2d) and virtually no CASC14 or CASC15 expression (Supplementary Fig. 3b,c). Taken together, these experimental results confirm our predictive bioinformatic data identifying CASC15-S as a bona-fide lncRNA transcript in neuroblastoma.
Identification of candidate functional SNPs at 6p22.3
Because risk alleles at several other neuroblastoma susceptibility loci have been shown to function in cis to influence mRNA expression levels of nearby transcripts 6,9,10, we attempted to associate our previously published, highly significant polymorphism, rs6939340 (p = 1.67×10−14, odds ratio: 1.80, 95% confidence interval: 1.55 – 2.10)5 with expression of the CASC14, CASC15, and CASC15-S gene products at this locus. However, we did not observe a significant correlation between risk alleles and transcript expression levels in either a set of 250 primary neuroblastomas (Supplementary Fig. 4a) or a representative panel of 20 neuroblastoma cell lines (Supplementary Fig. 4b). Furthermore, all three of our previously published polymorphisms lie in regions devoid of DNase hypersensitivity or other epigenetic marks indicative of transcriptional activity. In fact, eQTL analysis of all imputed genotypes with CASC15-S expression failed to reach significance after multiple comparison testing, leading us to postulate that polymorphisms contributing marginal effects may aggregately impact function at this locus.
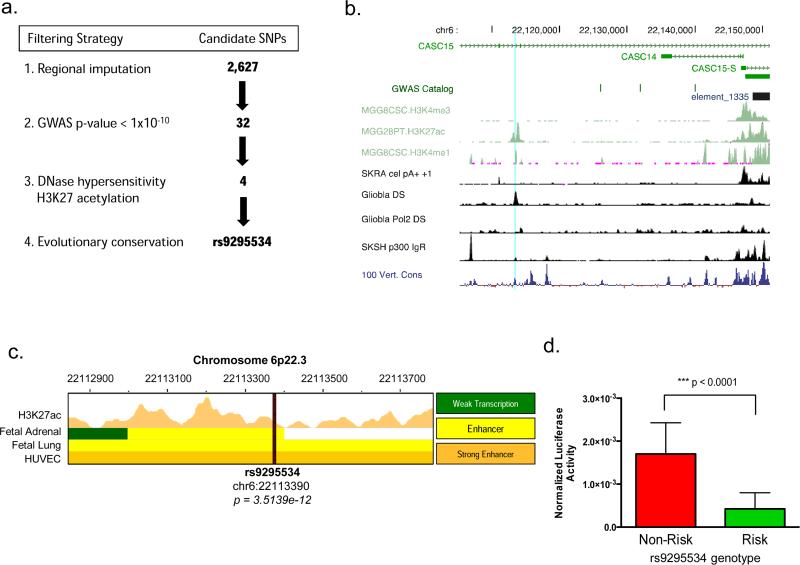
We therefore took advantage of our genome wide imputation data to identify other highly significant polymorphisms lying within putative regulatory regions. As demonstrated in other post-GWAS follow-up studies 37, we employed the following workflow (Fig. 3a) to narrow the field of potentially functional polymorphisms: (1) we first chose polymorphisms with a GWAS p-value < 1×10−10, resulting in 32 candidates. (2) We further refined this list by selecting only those SNPs within regions of DNaseI hypersensitivity (indicating open chromatin) and H3K27 acetylation marks (indicative of enhancer activity) leaving us with four candidates: rs1543310, rs6905441, rs9295534 and rs9368402. (3) Lastly, we looked for SNPs with evolutionary conservation, resulting in a single candidate polymorphism, rs9295534 (p=3.51×10−12, odds ratio: 1.63, 95% confidence interval: 1.4 – 1.89) upstream of CASC15-S, and this variant localizes to an expanse of regulatory chromatin and dense transcription factor binding sites in several cell lines (Fig. 3b). Moreover, this region exhibits enhancer activity, evidenced by H3K27Ac CHIP-Seq data in several fetal tissues available from the NIH Roadmap Epigenomics Mapping Consortium (Fig. 3c).
Figure 3.
Identification of rs9295534 as a functional polymorphism. (a) Filtration strategy employed to refine list of imputed SPSs at the 6p22.3 locus for functional variants. We identified SNPs with robust p-values (< 1×10−10) that fell within regions of regulator chromatin (DNase hypersensitivity) and putative enhancer activity (H3K27 acetylation) in SK-N-SH neuroblastoma cells. This resulted in four highly significant polymorphisms: rs1543310, rs6905441, rs9295534 and rs9368402. Further refinement based on evolutionary conservation identified rs9295534 as the only SNP to fit all criteria. (A full table of SNP attributes is given in Supplementary Table 1) (b) Evidence from glioblastoma cells revealing that rs9295534 (light blue vertical line) maps to the most proximal enhancer site to CASC15-S as evidenced by DNaseI sensitivity (DS), H3K4me1 and H3K27ac marks48. (c) Further characterization of rs9295534 was indicated due to a robust p-value (p=3.51×10−12), as well as the inclusion of this SNP within a region of predicted enhancer activity (H3K27Ac peaks) in several fetal tissues, including fetal adrenal gland. (d) Functional demonstration of rs9295534 was accomplished by insertion of 1500bp risk (A_A) or non-risk (T_T) fragments (cloned from Lan5 and Chp134 neuroblastoma cell lines, respectively) into a luciferase reporter vector (pGL4.23) upstream of a minimal promoter. Luciferase activity was normalized to a contransfected Renilla luciferase vector (pGL4.75). Expression of the risk fragment resulted in significantly attenuated transcriptional activity than was observed with the non-risk fragment following quantification of luciferase activity in HEK-293 cells (p < 0.0001).
We next verified rs9295534 genotypes in Chp134 (homozygous non-risk) and Lan5 (homozygous risk) neuroblastoma cells by Sanger sequencing of a 1.5kb region encompassing this SNP, and subsequently cloned risk and non-risk fragments from these lines. To assess the impact of rs9295534 genotype on transcriptional activity, we inserted risk (A/A) and non-risk (T/T) fragments into luciferase reporter constructs upstream of a minimal promoter. Results from these experiments demonstrated significantly decreased transcriptional reporter activity following insertion of the risk genotype fragment (Fig. 3d), suggesting this region possesses an enhancer-like function that is disrupted following the inclusion of a neuroblastoma risk allele.
CASC15-S is differentially expressed in neuroblastoma and highly associated with disease outcome
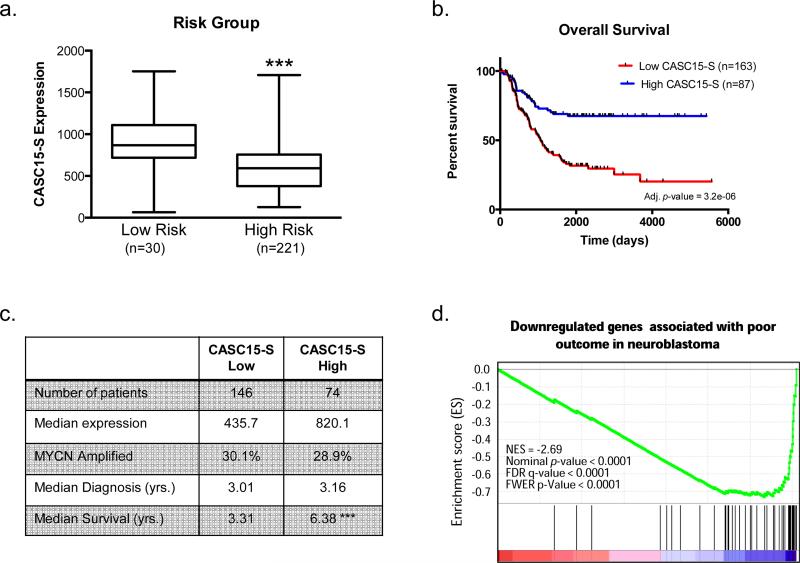
Because the rs9295534 homozygous risk genotype, by virtue of its linkage with rs6939340, is associated with aggressive neuroblastoma, poor survival5, and demonstrates decreased transcriptional activity in a minimal promoter assay, we postulated that patients with high-risk disease would have reduced CASC15-S expression. Indeed, we observed significantly lower CASC15-S expression in high-risk patient tumors (n=230) compared to low-risk patient tumors (n=30, Fig. 4a). This finding appeared independent of MYCN amplification - a known oncogenic driver in neuroblastoma (p = 0.29, Supplementary Fig. 5a). In addition, patients with tumors enriched in CASC15-S expression exhibited superior survival when compared to patients harboring neuroblastoma with low CASC15-S expression, both when low- and high-risk patients were included in the analysis (adj. p = 3.2×10−06, Fig. 4b) but also when comparing expression from only high-risk patients (adj. p = 0.002, Supplementary Fig. 5b). Furthermore, this finding remained significant (p = 0.0084) after multivariate analysis adjusting for clinical factors such as age, MYCN amplification, and 1p/11q deletion status – all known prognostic variables in this disease (Supplementary Table 2). Although expressed at much lower levels than CASC15-S, the long isoform of CASC15 demonstrated a similar pattern in patient tumors (Supplementary Fig. 5c-e). Taken together, these data indicate that low CASC15-S expression correlates with a more aggressive phenotype in neuroblastoma and decreased overall survival probability.
Figure 4.
Low CASC15-S expression correlates with poor clinical prognosis. (a) Microarray expression data from clinically annotated primary neuroblastoma tumors (n=250) obtained at diagnosis reveals that high-risk (stage 4) neuroblastomas (n=220) demonstrate significantly lower expression of CASC15-S than low-risk (stage 1) tumors (n=30). (b) Kaplan-Meier analysis demonstrates significantly poorer overall survival for children with tumors expressing low levels of CASC15-S (n=163 for group “low”, n=87 for group “high”, adj. p = 3.2×10−06). (c) Relevant metrics for selection of high-risk patient tumors used for differential gene expression analyses between patients with high (n=74) and low (n=146) CASC15-S levels. CASC15-S was expression was increased 1.9-fold in the “High” group, and these patients exhibited a significant increase in overall survival. (d) The most highly significantly regulated pathway using GSEA was identified to be a set of genes downregulated in poor outcome neuroblastoma (Asgharzadeh neuroblastoma poor survival down). Patients with high CASC15-S expression demonstrated enrichment of these genes, suggesting that CASC15-S acts in a protective manner. (*** p < 0.0001).
In order to understand the contribution of CASC15-S in the initiation and progression of neuroblastoma, we performed gene set enrichment analysis (GSEA) restricted to high-risk neuroblastoma samples (n=220), where we utilized the same 1.9-fold difference in median CASC15-S expression as the Kaplan Meier analysis to define 146 low- and 74 high-expressing CASC15-S samples (Fig. 4c). The top differential gene expression profile (normalized enrichment score = −2.69, nominal p-value < 0.0001, FDR q-value < 0.0001) that emerged from these analyses consists of a 55-gene signature previously shown by Asgharzadeh and colleagues to be downregulated in neuroblastoma patients with poor outcomes38. This result indicates that tumors with high CASC15-S expression are enriched in the expression of these genes, and again support a protective role for CASC15-S, resulting in less aggressive disease within even this subgroup of high-risk cases (Fig. 4d).
CASC15 depletion in neuroblastoma cell lines enhances proliferation and invasive capabilities
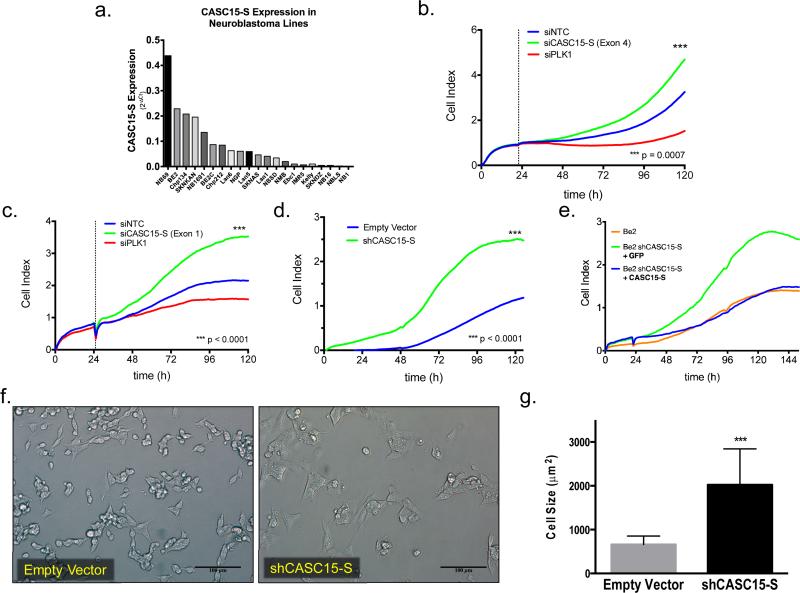
Having demonstrated a clinically relevant association with patient outcome for CASC15-S in neuroblastoma patients, we next sought functional validation for a role in tumorigenesis. We first assessed CASC15-S levels by qRT-PCR across a well-characterized panel of neuroblastoma cell lines (n=21) where we observed differential expression similar to our primary tumor panel (Fig. 5a). To investigate the functional role of CASC15-S in neuroblastoma, we utilized an siRNA construct targeting exon 4 near the 3’ end of the gene. Depletion of CASC15-S resulted in a highly reproducible increase in neuroblastoma proliferation as evidenced by real-time cell growth and viability assays (Fig. 5b), and we were able to recapitulate these results by subsequently targeting CASC15-S within its unique first exon in several neuroblastoma lines (Fig. 5c, Supplementary Fig. 6a,b). Depletion of full-length CASC15 or CASC14 had no observable impact on cell growth or viability, supporting our initial findings of CASC15-S as the functional isoform in neuroblastoma (Supplementary Figs. 6c-d, 7a-d). We next derived neuroblastoma cell lines stably depleted of CASC15-S (Supplementary Fig. 7e) and found that these cells also exhibited a substantial increase in cellular proliferation identical to what we observed in our transient siRNA-based CASC15-S depletion experiments (Fig. 4d, Supplementary Figs. 6e-f, 8). Furthermore, rescue experiments, conducted by ectopically expressing CASC15-S in these cells, were able to revert the accelerated growth (Fig. 5e). Microscopic examination revealed overt morphological changes in shCASC15-S SK-N-BE2 cells; including striking changes in cell shape and size, with a resultant three-fold increase in cell area (659.5 ± 50.2μm2 in control vs. 2024 ± 211.1 μm2 in shCASC15 cells, p<0.0001) (Fig. 5f, g). Furthermore, both SK-N-BE2 (Fig. 6a, b) and SK-N-SH neuroblastoma cells (Fig. 6c) stably depleted of CASC15-S exhibited an increased migratory capacity and invasiveness as evidenced by wound-healing assays (SK-N-SH: p=0.0006, SK-N-BE2: p<0.0001). Taken together, these data suggest that CASC15-S loss promotes increased cellular growth and a more migratory phenotype in neuroblastoma.
Figure 5.
CASC15-S depletion induces a more aggressive phenotype in neuroblastoma cells. (a) CASC15-S expression was investigated by qRT-PCR in a panel of neuroblastoma cell lines (n=21) and normalized relative to the geometric mean of GUSB, HPRT and TBP housekeeping genes. CASC15-S demonstrated a wide range of expression across neuroblastoma cell lines. (b) SK-N-BE2 neuroblastoma cells transiently transfected with siRNA targeting an exon common to both CASC15 and CASC15-S isoforms (exon 12 or exon 4, respectively), or (c) specifically targeting only the unique exon of CASC15-S (exon 1), show a significant increase in proliferative rate. (d) Stable depletion of CASC15-S in SK-N-BE2 cells was achieved with lentiviral transduction of shRNA, and recapitulated the increased growth observed with transient knockdown. (e) Forced ectopic expression of CASC15-S cDNA was able to rescue the growth characteristics of SK-N-BE2 shCASC15-S cells, reverting their growth pattern to that of wild-type cells (f) Morphological observation of SK-N-BE2 cells stably depleted of CASC15-S showed that cells were substantially larger than control cells (scale bar = 100μm). (g) Cell area was measured in biological triplicate (n=10 for each replicate) and quantified in ImageJ, where the area of shCASC15 cells was found to be 3.1-fold increased over controls (*** p < 0.001). siNTC = non-targeting control (negative control), siPLK1 = polo-like kinase 1 (positive control)
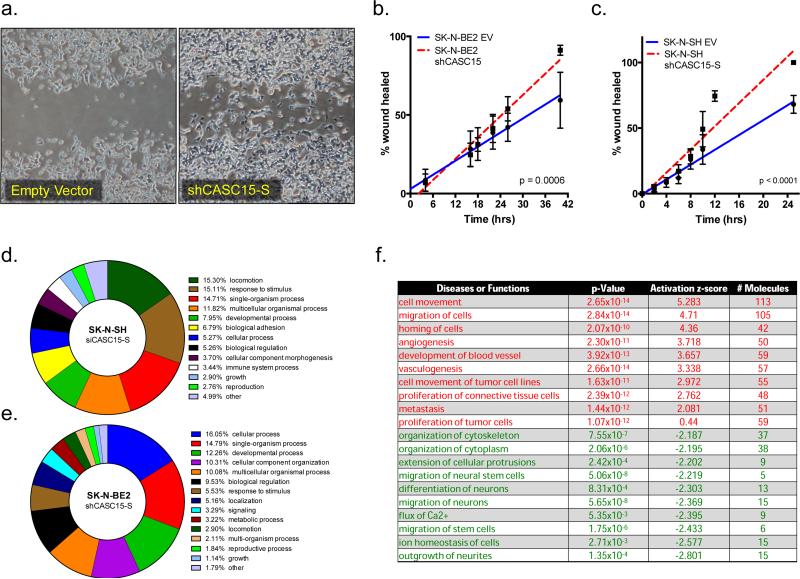
Figure 6.
CASC15-S regulates a subset of genes involved in neural differentiation and neuroblastoma tumorigenesis. (a) SK-N-BE2 cells constitutively depleted of CASC15-S demonstrated an increased migratory capacity in wound healing assays (t=24h). Linear regression comparison of wound closure at regular intervals demonstrates a clear enhancement of migration in silenced SK-N-BE2 (b) or SK-N-SH (c) cells (EV = Empty Vector). (d) Gene ontology (GO) analysis of SK-N-SH cells following CASC15-S depletion via siRNA at 48 hours. The top-level biological processes are shown by percentage of enrichment signal, with both locomotion and cellular adhesion gene sets exhibiting over representation. (e) A similar analysis was carried out for SK-N-BE2 cells stably expressing an shRNA construct targeting CASC15-S. The top enrichment observed in these cells, “cellular process” was primarily the result of the cell differentiation gene subset. (f) Most significantly altered pathways in SK-N-SH and SK-N-BE2 neuroblastoma cells following depletion of CASC15-S and subjected to Ingenuity pathway analysis. Pathways shown were the top gene signatures to arise from differential analysis, and indicate activation of cellular programs of proliferation, migration and metastasis (shown in red), as well as downregulation of several pathways known to modulate neural-specific development (shown in green).
CASC15-S regulates a subset of genes involved in neural crest development
To better characterize the phenotypic changes we observed following CASC15-S depletion, we surveyed gene expression signatures of neuroblastoma cells depleted of CASC15-S. For initial studies, SK-N-SH neuroblastoma cells were transfected in triplicate with either a non-targeting construct or siRNA specific for CASC15-S, and microarray gene expression signatures were examined at 48-hours following transfection. We observed substantial upregulation of several known cell adhesion genes, most notably entactin (NID1, p = 3.7×10−4) and activated leukocyte cell adhesion molecule (ALCAM, p = 1.05×10−7), after CASC15-S depletion. These gene expression data were used for gene ontology (GO) analysis to examine enrichment top-level biological processes. In support of the increased migratory phenotype we observed in wound-healing assays, both locomotion and cellular adhesion pathways were found amongst the top differentially regulated processes as a result of CASC15-S depletion (Fig. 6d).
We next assayed the result of persistent CASC15-S depletion on gene expression changes by comparing SK-N-BE2 neuroblastoma cells stably silenced for CASC15-S compared to control vector-transfected cells. We again examined gene expression signatures via microarray analysis, where we observed downregulation of several proneural gene family members with known roles in neurogenesis and differentiation such as neurogenic differentiation 1 (NEUROD1, p=1.1×10−4), neural precursor cell-expressed, developmentally down-regulated gene 9 (NEDD9, p=5.8×10−4) and neurogenin 2 (NEUROG2, p=5.3×10−4). In a manner identical to the SK-N-SH gene expression comparison, we utilized GO analysis of these differentially regulated genesets, with the top pathway, “cellular process” arising due to enrichment of the cell differentiation pathway node contained within this subset (Fig. 6e).
Lastly, we submitted the differentially expressed gene lists from siCASC15-S SK-N-SH and shCASC15-S SK-N-BE2 cells to Ingenuity pathway analyses (IPA). Neuroblastoma cells depleted of CASC15-S demonstrated highly significant upregulation of pathways involved in cell migration, proliferation and metastasis (Fig. 6f, top), and substantial decreases in proneural gene signatures (Fig. 6f, bottom). Taken together, these data suggest that loss of CASC15-S shifts the neuroblastoma gene expression away from a well-differentiated neural phenotype and promotes increased expression of cellular adhesion and migratory genes - a finding consistent with our phenotypic and morphological observations.
DISCUSSION
High-risk neuroblastoma remains a major challenge due to a relative paucity of somatic mutations hindering the development of targeted therapies1. The identification of mutations in ALK and PHOX2B has helped explain the origin of familial neuroblastoma; however, an understanding of the basis of sporadic disease has only recently begun to come into focus 39,40. Here we identify and demonstrate CASC15-S as a neuroblastoma suppressor gene via a post-GWAS mechanistic evaluation of a complex region of the human genome. Despite this robust association, however, eQTL analyses correlating risk genotypes with transcript expression failed to reach statistical significance - a likely consequence of an underpowered patient data set and/or additional mechanisms capable of impacting expression (such as additional SNPs affecting expression, post-translational modification/degradation, etc.). To address this shortcoming, we fine mapped this locus, using orthogonal methodologies to identify the likely disease causal SNP, rs9295534, which localizes to the closest upstream enhancer of CASC15-S. Moreover, expression of the rs9295534 risk allele disrupts the enhancer function of this region, proving that genotype can indeed impact transcriptional ability at this locus. More importantly, we provide evidence for a potent effect of this lncRNA on neuroblastoma differentiation and migratory capacities, yielding mechanistic insights into why the GWAS signal at this locus is associated with metastatic disease and poor survival probability.
A growing body of work supports a defined role of lncRNAs as spatiotemporal-specific regulators of gene expression critical for ensuring proper differentiation during development. Thus, predominant expression of CASC15-S in brain (but not other tissues), the derivation of several cDNA clones from brain regions, and its proximity to a validated enhancer element (hs1335) strongly suggest that this lncRNA is uniquely involved in neural tube development. The role of lncRNA-mediated tumorigenesis in embryonal cancers provides a logical hypothesis to contribute to the understanding of the etiology of neuroblastoma tumors, which are typically devoid of activating somatic mutations1.
A preliminary working model of how this transcript functions in neuroblastoma biology can be proposed from the functional and expression data we have demonstrated. CASC15-S expression strongly correlates with disease stage and overall survival, and patient tumors with high CASC15-S levels are enriched in genes typically lost in poor outcome neuroblastoma, demonstrating a protective role for CASC15-S. Furthermore, ablation of CASC15-S in neuroblastoma cell lines results in increased proliferative and migratory capacities, upregulation of adhesion and migration gene pathways and a concomitant decrease in neural-specific transcripts. Therefore, reduced CASC15-S expression as the result an inherited polymorphism would impact neural crest cellular lineage commitment and predispose these cells to undergo malignant transformation.
The phenotypic and gene signatures changes we observed signify that CASC15-S is responsible for maintaining a more differentiated and benign cell state, with CASC15-S loss leading to a poorly differentiated phenotype and expression of genes associated with transformed cells. It has been recently shown that CASC14 (renamed NBAT-1), although very lowly expressed, exerts a similar phenotype41, suggesting that many lncRNAs in this region may cooperate. The precise mechanism by which CASC15-S (and potentially other transcripts near this locus) exerts its effect is currently under investigation, although it is likely that the observed transcriptional changes may be the result of modulation of cis elements. One possibility, given its proximity to a validated enhancer (hs1335) with neural tube expression and its position downstream of the developmental regulatory gene SOX4, is that CASC15-S functions as an enhancer RNA42. Future studies to identify direct interaction partners of CASC15-S will undoubtedly strengthen our understanding of lncRNA function and yield key insights into neuroblastoma tumorigenesis.
In summary, our findings support a recent and growing body of evidence that convincingly demonstrates involvement of the noncoding genome in the tumorigenesis of pediatric cancers in general, and neuroblastoma in particular 41,43-46. While we certify CASC15-S as neuroblastoma suppressor gene, we still have not elucidated all of the stochastic and/or epigenetic events that select for CASC15-S repression. Future studies focused on engineering CASC15-S depletion in vivo will further explore mechanisms for tumor initiation and progression as well as screen for synthetic lethal interactions to be leveraged therapeutically. Finally, this work provides the first highly significant GWAS-supported identification of lncRNAs in neuroblastoma, thus further stimulating exploration of this class of regulatory RNAs in human cancer etiology and clonal evolution.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Children's Oncology Group (U10-CA98543) for providing blood and tumor specimens from neuroblastoma patients.
Grant Support: K08CA136979 (KAC) and Alex's Lemonade Stand Foundation (KAC and MRR)
Footnotes
There are no conflicts of interest to disclose.
REFERENCES
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Cheung N-KV, Dyer M. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi Y, Sipp D, Enomoto H. Tissue interactions in neural crest cell development and disease. Science. 2013;341:860–3. doi: 10.1126/science.1230717. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Mosse YP, Bradfield JP, Hou C, Monni S, Scott RH, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585–93. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capasso M, Devoto M, Hou C, Asgharzadeh S, Glessner JT, Attiyeh EF, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41:718–23. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diskin SJ, Hou C, Glessner JT, Attiyeh EF, Laudenslager M, Bosse K, et al. Copy number variation at 1q21.1 associated with neuroblastoma. Nature. 2009;459:987–91. doi: 10.1038/nature08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen le B, Diskin SJ, Capasso M, Wang K, Diamond MA, Glessner J, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 2011;7:e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Diskin SJ, Zhang H, Attiyeh EF, Winter C, Hou C, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–20. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diskin SJ, Capasso M, Schnepp RW, Cole KA, Attiyeh EF, Hou C, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet. 2012;44:1126–30. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latorre V, Diskin SJ, Diamond MA, Zhang H, Hakonarson H, Maris JM, et al. Replication of Neuroblastoma SNP Association at the BARD1 Locus in African-Americans. Cancer Epidemiol Biomarkers Prev. 2012;21:658–63. doi: 10.1158/1055-9965.EPI-11-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capasso M, Diskin SJ, Totaro F, Longo L, De Mariano M, Russo R, et al. Replication of GWAS-identified neuroblastoma risk loci strengthens the role of BARD1 and affirms the cumulative effect of genetic variations on disease susceptibility. Carcinogenesis. 2013;34:605–11. doi: 10.1093/carcin/bgs380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–93. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 14.Sausen M, Leary RJ, Jones S, Wu J, Reynolds CP, Liu X, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45:279–84. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung N, Zhang J, Lu C, Parker M. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062–71. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306:2242–46. doi: 10.1126/science.1103388. [DOI] [PubMed] [Google Scholar]
- 19.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 20.Mercer T, Dinger M, Mattick J. Long non-coding RNA insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 21.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JT. Epigenetic Regulation by Long Noncoding RNAs. Science. 2012;338:1435–9. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 23.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–7. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 24.Chu C, Qu K, Zhong F, Artandi S, Chang H. Genomic maps of lincRNA occupancy reveal principles of RNA chromatin interactions. Mol Cell. 2011;44:667–78. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponjavic J, Oliver PL, Lunter G, Ponting CP. Genomic and transcriptional co-localization of protein-coding and long non-coding RNA pairs in the developing brain. PLoS Genet. 2009;5:e1000617. doi: 10.1371/journal.pgen.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauss JL, Sun T. Regulatory mechanisms of long noncoding RNAs in vertebrate central nervous system development and function. Neuroscience. 2013;3:200–14. doi: 10.1016/j.neuroscience.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. CancerDiscovery. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genetics. 2013;45:1392–8. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. CancerRes. 2013;73:1180–9. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Federico S, Brennan R, Dyer M. Childhood Cancer and Developmental Biology: A Crucial Partnership. Top Dev Biol. 2011;901:1–10. doi: 10.1016/B978-0-12-380916-2.00001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. http://target.nci.nih.gov/dataMatrix/TARGET_DataMatrix.html.
- 33.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9:179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 34.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genetics. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–13. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 36.Visel A, Minovitsky S, Dubchak I, Pennacchio LA. VISTA Enhancer Browser-a database of tissue-specific human enhancers. Nucleic Acids Res. 2007;35:D88–92. doi: 10.1093/nar/gkl822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Bailey SD, Lupien M. Laying a solid foundation for Manhattan--’setting the functional basis for the post-GWAS era'. Trends Genet. 2014;30:140–9. doi: 10.1016/j.tig.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asgharzadeh S, Pique-Regi R, Sposto R, Wang H, Yang Y, Shimada H, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN amplification. JNCI. 2006;98:1193–203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 39.Mossé YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosse YP, Laudenslager M, Khazi D, Carlisle AJ, Winter CL, Rappaport E, et al. Germline PHOX2B mutation in hereditary neuroblastoma. Am J Hum Genet. 2004;75:727–30. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, et al. The Risk-Associated Long Noncoding RNA NBAT-1 Controls Neuroblastoma Progression by Regulating Cell Proliferation and Neuronal Differentiation. Cancer Cell. 2014;26:722–37. doi: 10.1016/j.ccell.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnhill LM, Williams RT, Cohen O, Kim Y, Batova A, Mielke JA, et al. High Expression of CAI2, a 9p21-Embedded Long Noncoding RNA, Contributes to Advanced-Stage Neuroblastoma. Cancer Res. 2014;74:3753–63. doi: 10.1158/0008-5472.CAN-13-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu PY, Erriquez D, Marshall GM, Tee AE, Polly P, Wong M, et al. Effects of a novel long noncoding RNA, lncUSMycN, on N-Myc expression and neuroblastoma progression. J Natl Cancer Inst. 2014;106:1–11. doi: 10.1093/jnci/dju113. [DOI] [PubMed] [Google Scholar]
- 45.Tee A, Ling D, Nelson C, Atmadibrata B, Dinger M, Xu N, et al. The histone demethylase JMJD1A induces cell migration and invasion by up-regulating the expression of the long noncoding RNA MALAT1. Oncotarget. 2014;5:1793–1804. doi: 10.18632/oncotarget.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atmadibrata B, Liu PY, Sokolowski N, Zhang L, Wong M, Tee AE, et al. The Novel Long Noncoding RNA linc00467 Promotes Cell Survival but Is Down-Regulated by N-Myc. PLoS One. 2014;9:e88112. doi: 10.1371/journal.pone.0088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suvà ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–94. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rheinbay E, Suvà ML, Gillespie SM, Wakimoto H, Patel AP, Shahid M, et al. An aberrant transcription factor network essential for Wnt signaling and stem cell maintenance in glioblastoma. Cell Reports. 2013;3:1567–1579. doi: 10.1016/j.celrep.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.