SUMMARY
Hoyeraal-Hreidarsson (HH) syndrome is a multisystem genetic disorder characterized by very short telomeres and considered a clinically severe variant of dyskeratosis congenita (DC). The main cause of mortality, usually in early childhood, is bone marrow failure. Mutations in several telomere biology genes have been reported to cause HH in about 60% of the HH patients, but the genetic defects in the rest of the patients are still unknown. Understanding the aetiology of HH and its diverse manifestations is challenging because of the complexity of telomere biology and the multiple telomeric and non-telomeric functions played by telomere-associated proteins in processes such as telomere replication, telomere protection, DNA damage response and ribosome and spliceosome assembly. Here we review the known clinical complications, molecular defects and germline mutations associated with HH, and elucidate possible mechanistic explanations and remaining questions in our understanding of the disease.
Keywords: Hoyeraal-Hreidarsson syndrome, dyskeratosis congenita, telomere, immunodeficiency, cerebellar hypoplasia
INTRODUCTION
Hoyeraal-Hreidarsson (HH) syndrome is a multisystem genetic disorder typically caused by germline mutations in telomere biology genes. It is considered a clinically severe variant of dyskeratosis congenita (DC) and represents the extreme phenotype caused by aberrant telomere biology. Very short (<1st percentile for age) leucocyte telomere lengths are diagnostic of DC and HH. Patients with HH typically present early in childhood with the constellation of cerebellar hypoplasia, immunodeficiency, progressive bone marrow failure, and intrauterine growth retardation (IUGR). The DC-associated mucocutaneous triad of nail dysplasia, lacy skin pigmentation and oral leucoplakia may be present at diagnosis or develop over time in patients with HH. To date, X-linked recessive mutations in DKC1 (encoding dyskerin), autosomal dominant mutations in TINF2 (encoding TIN2, also termed TINF2) and autosomal recessive mutations in TERT, ACD (encoding TPP1, also termed ACD) and RTEL1, have been reported to cause HH. All HH-associated genes encode proteins with specialized telomeric functions: TERT and dyskerin are components of the telomerase ribonucleoprotein (RNP) complex, TIN2 and TPP1 are components of the telomeric shelterin complex, and RTEL1 is a helicase important in telomere biology. However, dyskerin, RTEL1 and TERT have also been reported to have non-telomeric functions. Therefore, the question remains whether non-telomeric defects contribute to the pathology of HH, perhaps distinguishing it from DC.
EARLY DESCRIPTIONS OF HOYERAAL-HREIDARSSON SYNDROME
The eponym “Hoyeraal-Hreidarsson syndrome” (HHS or sometimes referred to as HH) was first proposed by a 1995 case report describing a child presenting with progressive pancytopenia, cerebellar hypoplasia, prenatal growth retardation, microcephaly and developmental delay (Aalfs, et al 1995). These clinical features were noted as strikingly similar to the clinical description of the patients reported by Hoyeraal et al (1970) and Hreidarsson et al (1988). Since the initial description by Hoyeraal et al (1970), about 50 cases of HH have been reported (Ballew, et al 2013a, Berthet, et al 1994, Cossu, et al 2002, Deng, et al 2013, Knight, et al 1999a, Kocak, et al 2014, Lamm, et al 2009, Le Guen, et al 2013, Malbora, et al 2014, Revy, et al 2000, Sznajer, et al 2003, Touzot, et al 2012, Walne, et al 2008, Walne, et al 2013b). Progressive bone marrow failure (BMF), cerebellar hypoplasia, immunodeficiency and IUGR appear to comprise a majority of the clinical complications in patients with HH.
CLINICAL MANIFESTATIONS
Clinical Overlap with Dyskeratosis Congenita
In addition to HH-specific symptoms, DC-associated manifestations are also found in HH. DC is classically diagnosed by the presence of the mucocutaneous triad of nail dysplasia, lacy skin pigmentation and oral leucoplakia or by the presence of one feature of the triad in combination with BMF and two other DC-associated findings (Vulliamy, et al 2006) (Figure 1). Patients with DC are at very high risk of progressive BMF, pulmonary fibrosis, leukaemia and squamous cell cancer of the head, neck or anogenital regions (Ballew and Savage 2013). Other DC-associated medical problems include non-alcoholic, non-infectious liver fibrosis, stenosis of the oesophagus, lacrimal ducts, and urethra, avascular necrosis of the hips or shoulders and premature greying of the hair (Table I).
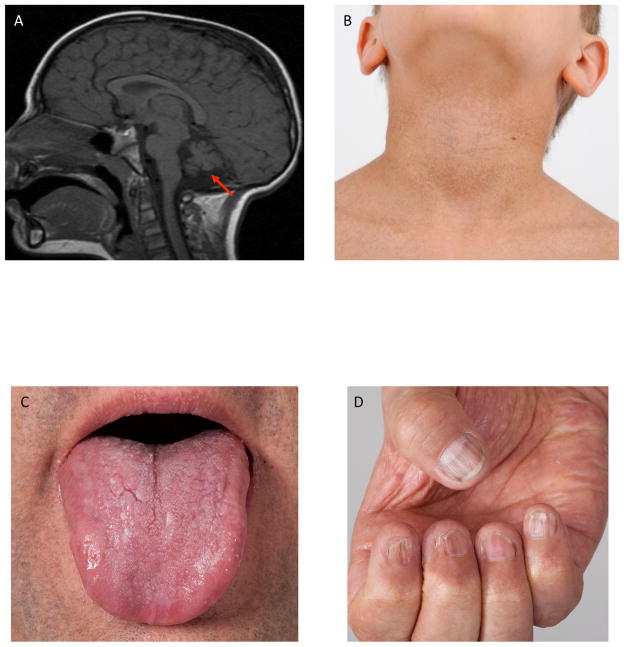
Figure 1. Clinical Features of Patients with Hoyeraal-Hreidarsson Syndrome.
A) cerebellar hypoplasia (arrow), B) skin pigmentation abnormalities, C) oral leucoplakia, D) nail dystrophy
Table I.
Clinical features associated with classical Hoyeraal Hreidarsson Syndrome
| Clinical feature/abnormality | Approximate % of reported patients^ |
|---|---|
| Classical signs | 96 |
| Intra-uterine growth retardation | 96 |
| Microcephaly | 96 |
| Cerebellar hypoplasia | 96 |
| Progressive bone marrow failure | 100 |
| Developmental delay | 92 |
| Associated features | |
| Mucocutaneous features | 71 |
| Immunodeficiency | 56 |
| Prematurity | 33 |
| Dysmorphology | 29 |
| Gastrointestinal features | 42 |
| Neurological symptoms | 13* |
Percentages based on a compilation of the following references (Ballew, et al 2013a, Ballew, et al 2013b, Berthet, et al 1994, Cossu, et al 2002, Deng, et al 2013, Knight, et al 1999a, Kocak, et al 2014, Lamm, et al 2009, Le Guen, et al 2013, Malbora, et al 2014, Revy, et al 2000, Sznajer, et al 2003, Touzot, et al 2012, Walne, et al 2008, Walne, et al 2013a)
Represents neurological features not necessarily related to cerebellar hypoplasia
Neurological Complications
The original description by Hoyeraal et al (1970) reported brothers with the co-occurrence of cerebellar hypoplasia and pancytopenia. The subsequent case by Hreidarsson et al (1988) also described a patient with cerebellar hypoplasia and progressive pancytopenia. When Aalfs et al (1995) proposed the term “Hoyeraal-Hreidarsson syndrome,” they also reported the presence of cerebellar hypoplasia in all cases and somewhat more variable presentations of IUGR, microcephaly, developmental delay, immunodeficiency and BMF. Consequently, cerebellar hypoplasia is now considered to be a requirement for the diagnosis of HH (Savage and Alter 2009, Savage and Bertuch 2010) (Figure 1). The underdevelopment of the cerebellum suggests a complex brain developmental abnormality that is probably the underlying cause of HH-associated microcephaly and developmental delay. Additional central nervous system involvement has also been reported in HH. Spastic paresis was reported in three of the four first reported cases (Aalfs, et al 1995). Specifically, one patient presented also with peripheral demyelinating neuropathy. Two patients were reported to have seizures (electroencephalogram was normal in one while displaying multifocal epileptiform abnormality in the other) (Ozdemir, et al 2004, Pearson, et al 2008). A hypoplastic corpus callosum and delayed myelination have also been reported (Kuwashima 2009). Occasionally, patients may also have multiple intracranial calcifications which expands the differential diagnosis to include congenital TORCH infection (Toxoplasmosis, Others Agents, Rubella, Cytomegalovirus and Herpes infection), Aicardi Goutieres syndromes (Knoblauch, et al 2003), or even the related telomere biology disorder, Coats plus (Anderson, et al 2012, Polvi, et al 2012, Savage 2012).
Immunodeficiency
Immunodeficiency is an under-recognized feature of HH even though it was specified by Berthet in the third case of HH published in 1994 (Berthet, et al 1994, Berthet, et al 1995, Cossu, et al 2002). HH patients are reported to have a progressive immune deficiency manifesting as an increased susceptibility to life-threatening infections. Lymphopenia is the most common immunological abnormality observed in patients with HH (approximately 56 % of patients). A decreased count of B cells and Natural Killer cells is often the most remarkable signature. In particular, a virtual absence of B lymphocytes from birth has been observed in HH (Berthet, et al 1994, Cossu, et al 2002, Hoyeraal, et al 1970, Hreidarsson, et al 1988, Jyonouchi, et al 2011, Le Guen, et al 2013, Revy, et al 2000, Touzot, et al 2010, Touzot, et al 2012). Hence, dysfunction of humoral immunity is the most consistent immunological feature of HH and consists of hypogammaglobulinaemia that can affect all immunoglobulin subtypes (IgG, IgM, or IgA). The antibody response toward specific antigens is sometimes impaired, rendering vaccination ineffective. The marked involvement of the B cell compartment is probably due to the additional cell proliferation that B lymphocytes undergo during their development (thus resulting in accelerated telomere shortening, see below) coupled with a shorter life span as compared with T lymphocytes (Hodes, et al 2002). The T cell compartment appears to be less frequently affected, with 16% of reported patients exhibiting a decrease in T cell counts (CD4 and/ or CD8 counts) and an inversion of CD4/CD8 ratio. Abnormalities of T cell proliferation in response to specific antigens (candida and tetanus) and, less frequently, to mitogens have also been observed, indicating reduced T cell function (Ballew, et al 2013b, Sznajer, et al 2003). Some cases presenting as severe combined immunodeficiency have also been reported (Cossu, et al 2002). Thus the diagnosis of HH should be considered in any child presenting with humoral deficiency or combined immunodeficiency associated with neurological features (such as microcephaly and/or cerebellar hypoplasia).
Bone Marrow Failure
In some cases, the development of BMF in a patient with developmental delay may be the first indication of HH. Subsequent clinical evaluation, including brain magnetic resonance imaging scan, can lead to the diagnosis of HH. Most patients with HH appear to develop some degree of BMF in the first decade of life, although the severity may be variable. Haematopoietic stem cell transplantation (HSCT) is the only curative therapy for both BMF and immune deficiency. Unfortunately, the outcome of the procedure is burdened by the occurrence of graft-versus-host disease and active post-transplant infections. Moreover, HSCT does not prevent the evolution of the disease in other organs, as HH patients can present with lethal pulmonary or liver fibrosis post-HSCT (Deng, et al 2013, Touzot, et al 2012).
Gastrointestinal Complications
Digestive tract anomalies and/or feeding difficulties have been documented in most reported cases and constitute one of the most important challenges in the supportive treatment of HH (Sznajer, et al 2003) (Borggraefe, et al 2009, Touzot, et al 2012). Initially, infants with HH and IUGR may present with feeding difficulties due to delayed development. Oesophageal strictures, sometimes requiring repetitive endoscopic dilations, are present in about 25% of reported cases. Severe enteropathy, manifesting as protracting noninfectious diarrhoea, is also reported in another quarter of cases. Digestives biopsies may show a change in the proliferative compartment of the digestive mucosa, leading to glandular atrophy (Sznajer, et al 2003). Colitis has also been described with non-specific lymphoplasmocytic infiltrate and chorionic oedema or more severe lesion consisting of granulomatous lesions associated with mononuclear and eosinophilic infiltrate (Borggraefe, et al 2009, Touzot, et al 2012).
Other Clinical Complications
There have been reports of skeletal malformations (Malbora, et al 2014, Touzot, et al 2012), urinary tract abnormalities including vesico-ureteral reflux and kidney duplication (Aalfs, et al 1995, Ballew, et al 2013b), and ophthalmological signs (enophthalmy, mild optic atrophy, and retinopathy) (Aalfs, et al 1995, Touzot, et al 2012). It can be challenging to differentiate retinopathy of prematurity in HH patients from those with Revesz syndrome. Revesz syndrome is characterized by the presence of bilateral exudative (“Coats”) retinopathy in the presence of DC-associated features including early-onset BMF, fine, sparse hair, nail dystrophy and intracranial calcifications (Revesz, et al 1992, Sasa, et al 2012, Walne, et al 2008).
Overall, HH is a severe multisystemic disorder with a high rate of early mortality linked to aplastic anaemia and immune deficiency. Notably, conversely to DC, in which very high incidence of cancers is observed (Alter, et al 2009), cancer has not been reported as a cause of early death of HH patients. This may be due to the early mortality caused by severe BMF and immunodeficiency.
THE AETIOLOGY OF HOYERAAL-HREIDARSSON SYNDROME
Telomere Biology
The telomeres correspond to the ends of linear chromosomes and are composed, in vertebrates, of hexameric TTAGGG repeats bound by specialized proteins (de Lange, et al 1990, Wright, et al 1997). A 3′ G-rich overhang is a conserved and essential feature of telomeres, and it was suggested to facilitate the formation of a protective telomere (t)-loop by invasion into the duplex part of the telomere (Griffith, et al 1999). During cell division, conventional DNA polymerases are unable to fully replicate the end of chromosomes, leading to the progressive loss of telomeric sequences (Olovnikov 1973, Watson 1972). In germ cells, some stem cells, and activated lymphocytes, telomere shortening is counteracted by telomerase, a reverse transcriptase that adds TTAGGG repeats to the 3′ overhang (Blackburn 2001). The telomerase RNP complex includes the telomerase RNA moiety (hTR; encoded by TERC), which contains the template for telomeric repeat synthesis, the telomerase reverse transcriptase protein (TERT) and other factors, such as dyskerin, NOP10, NHP2 and GAR1, which form a complex that binds to box H/ACA snoRNAs (including hTR) (Walne and Dokal 2008), and TCAB1 (also termed WRAP53), which directs the telomerase RNP to the Cajal bodies and facilitates telomere synthesis (Venteicher and Artandi 2009). The t-loop structure, formed and maintained by a specific telomeric protein complex called shelterin, hides the 3′ end of the telomeric overhang and prevents the telomeres from being recognized and processed as DNA-double strand breaks. The core shelterin complex is composed of TRF1 (TERF1), TRF2 (TERF2), RAP1 (TERF2IP), TIN2, TPP1 and POT1 (de Lange 2005). TRF1 and TRF2 bind the double-stranded telomeric DNA and are connected by TIN2 to each other and to the TPP1-POT1 heterodimer, which binds to the telomeric overhang. It protects the chromosome ends from degradation, activation of the DNA damage response (DDR), and fusion to other telomeres. Shelterin also regulates the activity of telomerase (de Lange 2005). Another complex, CST (CTC1-STN1-TEN1), also binds the telomeric overhang and functions in the regulation of telomerase and the coordination of the telomere lagging strand synthesis by Pola/primase (Chen, et al 2013). Other proteins, such as helicases, nucleases and DDR factors, play essential roles in telomere maintenance.
When telomere proteins fail to protect the telomeres, or telomerase fails to maintain their length, telomeres activate DDR and the cells stop proliferating and enter p53 (TP53)-induced replicative senescence or apoptosis (d’Adda di Fagagna, et al 2003, Zou, et al 2004). p53 activation in turn can induce the degradation of TRF2, exacerbating the telomere defect in a positive feedback loop (Donehower 2009, Fujita, et al 2010). Telomere dysfunction occuring on a large scale may cause a telomere biology disease (also termed telomeropathy) with severe manifestations, particularly in tissues with high turn over of cells (e.g., bone marrow).
Telomere Length in Hoyeraal-Hreidarsson Syndrome
Patients with HH have been included in studies of DC since the discovery of their common aetiology. Most of the DC and HH patients exhibit extremely short telomeres in comparison with age-matched controls. Givien that all the HH-causing genes identified so far participate in length regulation and/or protection of telomeres, HH is now recognized as a telomere biology disorder. Telomere lengths less than the 1st percentile for age, measured by flow cytometry with fluorescent in situ hybridization (flow FISH), are greater than 95% sensitive and specific for differentiating DC and HH patients from their healthy relatives or patients with other inherited BMF syndromes (Alter, et al 2007, Alter, et al 2015, Alter, et al 2012). Telomere lengths in HH patients are shorter than those of aged-matched patients with classic DC (Alter, et al 2012). This may account, in part, for the clinical severity seen in HH. Although severely short telomeres in the blood is a common feature of HH patients, the direct causality between critically short telomeres and the diverse clinical manifestations in various tissues is not clear. Some DC and HH patients have been reported to have normal or near-normal telomere lengths in tissues with clinical or molecular manifestations of the disease (Lamm, et al 2009, Touzot, et al 2012, Vulliamy, et al 2012). On the other hand, some individuals from HH families exhibit very short telomere lengths (< 1st percentile) without any clinical manifestation (Kocak, et al 2014).
Genetic anticipation, the worsening of a disease phenotype in successive generations, has been reported in DC and could conceivably also result in a child with HH (Savage and Bertuch 2010, Vulliamy, et al 2004). In addition to inheritance of a germline mutation (e.g., a TERT mutation), short telomeres are also inherited from the carrier parent, resulting in a more severe clinical phenotype. However, to our knowledge, only one case was reported where specific HH clinical features (IUGR, microcephaly, cerebellar hypoplasia) were associated with disease anticipation (Vulliamy, et al 2005). While exceedingly short telomeres are considered the main cause of HH, the contribution of other telomeric or non-telomeric defects to disease progression, and particularly to the HH-specific manifestations, remains to be understood.
Genetic and Molecular Defects in Hoyeraal-Hreidarsson Syndrome
One of the major breakthroughs in the understanding of HH aetiology came from two articles published in 1999. First, Knight et al (1999a) identified mutations in DKC1 in the patient described by Aalfs et al (1995) and in four additional HH patients. As DKC1 was previously found mutated in X-linked DC (Heiss, et al 1998), this suggested that HHS represents a severe variant of DC. Moreover, the same year, Mitchell and colleagues showed that dyskerin was a component of the telomerase RNP complex, which is involved in the maintenance of telomere length (Mitchell, et al 1999a), and further showed that DKC1-mutated patient cells exhibited short telomeres. These findings linked the DC and HH diseases to exceedingly short telomeres due to insufficient level of the telomerase RNP complex. Later, mutations in other telomere biology genes were identified, indicating other primary defects causing the disease.
Only a few in vitro phenotypic analyses have been reported in cells from HH patients: fibroblasts, lymphoblastoid cell lines (LCLs) and activated T cells. In addition to overall telomere shortening, these analyses have revealed diverse molecular phenotypes, such as shortening of the telomeric overhang, the activation of DDR at telomeric and non-telomeric sites, hypersensitivity to genotoxic stress, loss of hybridization signal from individual telomeres, telomere fragility, telomere-telomere fusions and anaphase bridges and the formation or the disappearance of extrachromosomal t-circles (Ballew, et al 2013b, Lamm, et al 2009, Le Guen, et al 2013, Touzot, et al 2010, Touzot, et al 2012). These diverse molecular phenotypes probably reflect diverse molecular functions and pathways implicated in HH (Figure 2). Some telomere biology genes, such as TERC, NHP2, NOP10, TCAB1 and CTC1, were implicated only in DC. Other genes, such as TERT and DKC1, are mostly implicated in DC, but when implicated in HH are likely to reflect a more severe dysfunction caused by homozygous or compound heterozygous mutations (TERT) or a more damaging mutation (DKC1). Mutations in other genes, such as the shelterin genes TINF2 (TIN2) and ACD (TPP1), tend to cause HH at higher frequency and recessive mutations in the helicase RTEL1 appear to only cause HH. These observations suggest that there might be a qualitative difference between DC and HH in the nature of the telomere biology dysfunction, and not only in its clinical severity. The genes and mutations implicated in HH are described below, in chronological order of discovery.
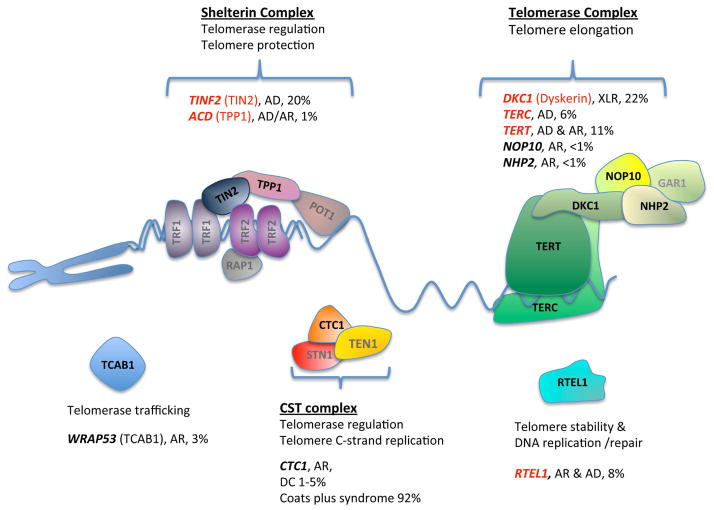
Figure 2. Factors involved in telomere maintenance and associated with telomere biology disorders.
Factors implicated in Hoyeraal-Hreidarsson syndrome (HH) are marked with red. The percentages are based on unpublished National Cancer Institute dyskeratosis congenita (DC) cohort data and review of the literature. They represent the combination of DC, HH, and Revesz syndrome. The shelterin complex binds along the telomeric DNA, protects the telomere end by forming a t-loop, suppressing DDR, and regulating telomerase action. TCAB1 regulates telomerase trafficking to the Cajal body. The telomerase ribonucleoprotein complex elongates the 3′ end of the telomere. The CTC1-STN1-TEN1 (CST) complex contributes to telomere replication and telomerase regulation. RTEL1 is an essential DNA helicase involved in telomere maintenance. Abbreviations: AD, autosomal dominant; AR, autosomal recessive; XLR, X-linked recessive.
Dyskerin (DKC1)
DKC1, encoding a protein named dyskerin after the name of the disease dyskeratosis congenita (DC), was the first gene implicated in DC and HH (Heiss, et al 1998, Knight, et al 1999a, Knight, et al 1999b, Knight, et al 1998, Vulliamy, et al 1999). Dyskerin is an essential nucleolar protein expressed in all tissues and highly conserved among living organisms. It comprises three main domains: a dyskerin-like domain with a yet unknown function at the N-terminus of the protein, TruB pseudouridine synthase catalytic domain, and PUA RNA binding domain. Dyskerin is part of the essential box H/ACA small nucleolar RNP (snoRNP) complex. In these snoRNPs, dyskerin and 3 other proteins, NHP2, NOP10 and GAR1, are associated with small nucleolar RNAs (snoRNA) through a box H and ACA consensus sequences (Angrisani, et al 2014). The snoRNAs guide the conversion of specific uridine residues to pseudouridines by base pairing to specific target RNAs, such as ribosomal RNAs, in the nucleolus. This modification is important for the folding and processing of the target RNAs (Kiss, et al 2010). A subset of box H/ACA snoRNPs, called small Cajal body RNPs (scaRNPs), are directed to the Cajal bodies by the protein TCAB1, which binds to a sequence called CAB box within the scaRNA (Tycowski, et al 2009). scaRNPs modify small nuclear RNAs (snRNAs), which are components of the spliceosome. Vertebrate telomerase RNAs contain H/ACA and CAB boxes, and assemble a typical box H/ACA scaRNP, which is important for the stability and recruitment of telomerase to telomeres (Mitchell, et al 1999b, Mitchell, et al 1999a, Venteicher and Artandi 2009).
Given that dyskerin is also important for ribosome biogenesis and function, DKC1 mutations were suggested to cause or contribute to X-linked DC/HH by compromising protein synthesis, and particularly internal ribosome entry site (IRES)-dependent translation (Jack, et al 2011, Ruggero, et al 2003). However, in human cells obtained from X-linked DC patients, ribosomes biogenesis and function were intact or only slightly affected (Carrillo, et al 2013, Thumati, et al 2013, Wong and Collins 2006) while the levels of hTR were significantly reduced, and some of the mutations were shown to disrupt the physical interaction with hTR (Ashbridge, et al 2009). Furthermore, TERC mutations in the box H/ACA motif found in DC patients had a similar effect on hTR levels. These observations support the notion that X-linked DC/HH is mainly caused by reduced levels of the telomerase RNP (Mitchell, et al 1999a, Mochizuki, et al 2004, Wong and Collins 2006, Zeng, et al 2011). Impaired protein synthesis does not appear to be a major factor causing the disease, but may still contribute to some of the symptoms, such as cancer predisposition (Bellodi, et al 2010). It is possible that protein translation is a more robust pathway that can tolerate some perturbations in dyskerin function, while telomere length homeostasis requires a delicate balance between telomere shortening and lengthening factors; even a minor decrease in telomerase activity may cause a disease over time.
DKC1 mutations cause DC and HH in an X-linked recessive manner. To date, over 50 different inherited and de novo DKC1 mutations have been found in association with DC, 13 of them also cause HH (P10L, I38T, T66A, T67I, H68Q, H68Y, S121G, R158W, K314R, A353V, R378Q, A386T and IVS12+1), and two mutations were only found in HH (T49M and S304N; Figure 3A; (Alder, et al 2013, Borggraefe, et al 2009, Cossu, et al 2002, Du, et al 2009, Heiss, et al 1998, Knight, et al 1999a, Knight, et al 1999b, Knight, et al 2001, Pearson, et al 2008, Sznajer, et al 2003, Vulliamy, et al 1999, Vulliamy, et al 2011, Vulliamy, et al 2006, Yaghmai, et al 2000). Most DKC1 mutations cluster into two regions outside the catalytic TruB domain: amino acids 2-72 (N-terminus of the protein) and 314–420 (PUA domain; (Knight, et al 1999b, Knight, et al 2001, Vulliamy, et al 2006). The three-dimensional structure of human dyskerin reveals that these domains are contiguous and probably form a binding site for additional factors (Rashid, et al 2006, Walbott, et al 2011). Some disease-causing mutations in both regions were shown to affect the binding of the H/ACA RNP assembly factor SHQ1 (Grozdanov, et al 2009). SHQ1 is known to stabilize the dyskerin protein and prevent its misfolding and degradation. Grozdanov et al (2009) suggested that both increase and decrease of SHQ1 binding reduces the dyskerin availability for RNP assembly. Recently, Brault et al (2013) reported that N-terminal DKC1 mutations fell into a consensus SUMOylation motif and showed that impaired dyskerin SUMOylation leads to reduced dyskerin and telomerase RNP levels, impaired telomerase activity and telomere shortening. Furthermore, forced SUMOylation of mutated dyskerin almost fully restored normal levels of telomerase. The authors suggested that SUMOylation regulates dyskerin stability and provide additional evidence that low dyskerin levels may contribute to DC (Brault, et al 2013). Finally, a growing body of evidence demonstrates that reduced expression level of dyskerin in the absence of mutations in the coding sequence may also cause the disease (Knight, et al 2001, Parry, et al 2011)
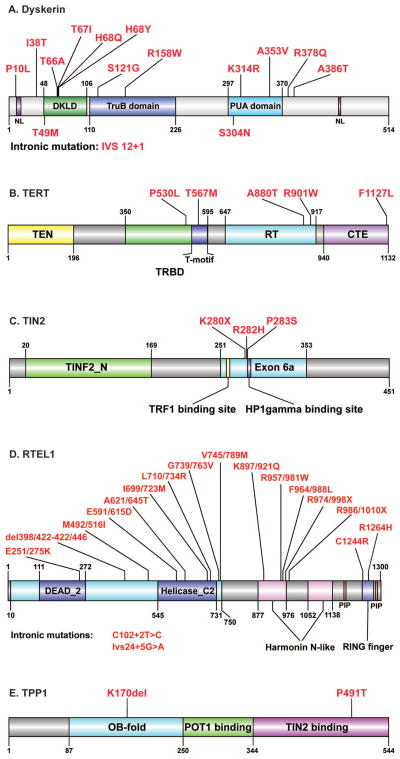
Figure 3. Hoyeraal-Hreidarsson syndrome-associated mutations.
A) Dyskerin (DKC1). Indicated are nuclear localization (NL) signals (purple), dyskerin-like domain (DKLD; green), TruB pseudouridine synthase catalytic domain (blue) and PUA RNA binding domain (cyan). B) TERT. Indicated are TEN domain (yellow), telomerase RNA binding domain (TRBD; green), T-motif within TRBD (blue), reverse transcriptase (RT) domain (cyan) and C-terminal extension (CTE; magenta). C) TIN2 (TINF2). Indicated are TIN2_N domain (green), exon 6a (cyan), TRF1 binding site (yellow) and HP1γ binding site (blue). D) RTEL1. Indicated are RAD3-related helicase domain (cyan) containing the helicase type 2 ATP binding and C-terminus domains (blue), PIP boxes (red), Harmonin N-like domains (pink) and RING-finger domain (light violet). Amino acid numbering refers to the main, 1,300aa, splice variant of RTEL1 (NM_001283009; left) and to a 1243aa splice variant, which includes an alternative 24aa exon close to the C-terminus (NM_032957; right). E) TPP1 (ADC). Indicated are OB fold (cyan), POT1 binding domain (green) and TIN2 binding domain (magenta). The schemes were illustrated using DOG 1.0 (Ren, et al 2009).
There is no clear correlation between the severity of the disease and the location of the mutation; some of the mutations cause only DC while others may cause DC or HH (Vulliamy, et al 2006). For example, the A353V mutation is associated with variable severity (from late onset DC to HH), even among siblings (Lai, et al 2011, Vulliamy, et al 2006). However, two mutations, T49M and S304N, were only found in HH probands (Knight, et al 1999a, Sznajer, et al 2003, Vulliamy, et al 2006). Interestingly, two HH-associated mutations (S121G and R158W) fall into the catalytic TruB domain, suggesting that the catalytic pseudouridinylation activity (and not merely the binding of dyskerin to hTR) is important for telomerase function (Knight, et al 1999a, Knight, et al 2001). Indeed, it has been shown that the TruB pseudouridine synthase domain of dyskerin alone is essential and sufficient for rescuing both hTR levels and telomerase activity in primary DC-affected cells (Machado-Pinilla, et al 2007). Interestingly, pseudouridylation of an essential stem loop (p6.1) of hTR was shown to affect the structure of p6.1 and the in vitro activity of telomerase (Kim, et al 2010). The in vivo pseudouridylation of uridine 307 within p6.1 was confirmed by transcriptome-wide mapping of pseudouridines, and the levels of pseudouridylated U307 were diminished in DC patient cells with DKC mutations (Schwartz, et al 2014). Altogether, these observations indicate that not only hTR is assembled as a snoRNA but it is also the target for pseudouridylation within one of its essential functional domains. Therefore, dyskerin may function not only by physically interacting with and stabilizing the telomerase RNP complex, as suggested above, but also by using its catalytic activity to pseudouridylate p6.1. The detailed molecular mechanism underlying the role of dyskerin in telomerase function and how DKC mutations cause a telomere disease remain to be further investigated.
Telomerase Reverse Transcriptase (TERT)
Most DC and HH cases examined to date share the feature of very short telomeres, which is thought to be the major defect causing the disease. More than 50 autosomal dominant (AD) or autosomal recessive (AR) mutations have been described in TERT, with diverse clinical presentations from pulmonary fibrosis and aplastic anaemia to DC and HH (Armanios, et al 2005, Basel-Vanagaite, et al 2008, Marrone, et al 2007, Tsakiri, et al 2007, Vulliamy, et al 2006, Vulliamy, et al 2005, Yamaguchi, et al 2005). Among them, only five mutations are implicated in HH (P530L, T567M, A880T, R901W, F1127L; Figure 3B), and four of them cause HH if homozygous (T567M and R901W, (Gramatges, et al 2013, Marrone, et al 2007) or compound heterozygous (P530L and A880T; (Vogiatzi, et al 2013). The heterozygous carriers of these mutations have short telomeres with minimal clinical consequences (Gramatges, et al 2013, Marrone, et al 2007, Vogiatzi, et al 2013).
The A880T and R901W mutations fall in the catalytic reverse transcriptase domain, and lead to extremely low levels of telomerase activity. The T576M substitution occurs in the T-motif, which lies within the telomerase RNA binding domain, and drastically affects telomerase processivity (Gramatges, et al 2013). However, the P530L substitution does not have an apparent effect on telomerase activity in vitro although it is associated with short telomeres even in heterozygous carriers, indicating impaired telomerase function in vivo (Vogiatzi, et al 2013). Heterozygous carriers of the mutations implicated in HH are healthy but have short telomeres, indicating that these heterozygous mutations may be better tolerated than the DC-causing autosomal dominant mutations. The only reported autosomal dominant mutation associated with HH, F1127L, was found also in the asymptomatic mother, indicating disease anticipation or the presence of another paternal mutation, yet to be found. The C-terminal domain of TERT, where this mutation is located, was shown to be involved in its interactions with TPP1, which is known to recruit telomerase to telomeres (Banik, et al 2002, Wang, et al 2007, Zaug, et al 2013, Zhong, et al 2012). Most reported DC patients with TERT mutations are monoallelic heterozygous, and the activity of telomerase is an average of the homozygous WT and mutant, suggesting that the disease is caused by haploinsufficiency (Armanios, et al 2005, Tsakiri, et al 2007, Xin, et al 2007, Yamaguchi, et al 2005, Zaug, et al 2013). Severe HH phenotypes associated with biallelic TERT mutations are distinguished from DC only by the severity of telomerase deficiency.
TERF1-interacting nuclear factor 2 (TINF2)
TINF2, encoding the shelterin component TIN2, was first associated with HH by Savage et al (2008). Over twenty TINF2 mutations have been described for DC, and three of them also for HH (K280X, R282H, P283S; Figure 3C) (Podlevsky, et al 2008, Savage, et al 2008, Touzot, et al 2012, Walne, et al 2008). Notably, several of these mutations are causative of Revesz syndrome, a related disorder characterized by the presence of bilateral exudative retinopathy in addition of features of DC (Podlevsky, et al 2008, Savage, et al 2008, Touzot, et al 2012, Walne, et al 2008). The reported TINF2 mutations are often de novo but may also be inherited in autosomal-dominant manner (Walne, et al 2008). All TINF2 mutations reported to date cluster in exon 6a (amino acids 269–298) of TINF2 (Sasa, et al 2012, Savage, et al 2008, Touzot, et al 2012, Vulliamy, et al 2012, Walne, et al 2008). Interestingly, despite the overall severe disease manifestations a few individuals were found to be clinically unaffected carriers at the time of evaluation with extremely short telomeres (Savage, et al 2008).
TIN2 is a shelterin protein, which links the double-stranded DNA binding proteins TRF1 and TRF2 to each other and to the single-stranded DNA binding heterodimer TPP1-POT1. The DC mutations cluster is located 8 amino acids downstream of the TRF1 binding domain (FxLxP) of the protein, but most of the mutations do not affect the ability of TIN2 to bind TRF1 (Sasa, et al 2012, Xin and Ly 2012). DC-associated mutations were suggested to compromise the ability of TIN2 to recruit telomerase to telomeres in a TPP1-dependent manner (Yang, et al 2011). TIN2 is also important for cohesion of sister telomeres. It interacts with heterochromatin protein 1 gamma (HP1γ) through the canonical binding site PTVML, which is located within the DC-associated mutation cluster (Walne, et al 2008). Several patient-derived cell lines displayed reduced associations between TIN2 and HP1γ and reduced sister telomere cohesion (Canudas, et al 2011). As the TIN2-associated DC and HH patients have very early onset and severely short telomeres, the authors suggested that severe telomere dysfunction might occur during embryogenesis because non-cohered telomeres were not efficiently elongated by telomerase. However, recent work in a mouse model suggested that telomere shortening in TINF2 mutation carriers is telomerase independent (Frescas and de Lange 2014).
Another possibility for the effect of TINF2 mutations is that TIN2-mediated telomere cohesion is important for recombinational telomere elongation during early embryogenesis. It was suggested that mice telomeres lengthen by a recombination-based mechanism in early embryogenesis, while only later in embryogenesis is telomerase activated to maintain telomere length (Liu, et al 2007). The ZSCAN4 family of proteins is essential for telomerase-independent telomere elongation in two-cell mouse embryos and ES cells (Falco, et al 2007, Zalzman, et al 2010). ZSCAN4 activates telomere sister chromatid exchange (T-SCE) and reduces non-telomeric SCE, ensuring the long-term genomic stability in mice ES cells (Zalzman, et al 2010). These data suggest that impaired sister telomere cohesion caused by TIN2-DC mutations compromises recombination-dependent telomere elongation in early embryogenesis, thus causing severe DC and HH. The severity of the HH phenotype caused by TINF2 mutations may be explained by its essential role in shelterin assembly and/or in cohesion-dependent telomere elongation during early embryogenesis.
Regulator of Telomere Elongation Helicase 1 (RTEL1)
RTEL1 is an essential DNA helicase that belongs to a small family of iron-sulfur-containing DNA helicases, together with XPD, FANCJ and DDX11/ChlR1, which are implicated in different genomic instability diseases (van der Lelij, et al 2010, White 2009, Wu, et al 2009). Mouse Rtel1 was genetically associated with telomere length and its deletion in embryonic stem cells led to the loss of telomeres and to chromosomal abnormalities, suggesting that it is required for both telomere maintenance and genomic stability (Ding, et al 2004). Mouse RTEL1 (mRTEL1) was found to associate with telomeres, transiently, during S phase (Uringa, et al 2012). Human RTEL1 was reported to interact with TRF1 and TRF2, indicating its recruitment to telomeres (Deng, et al 2013, Sarek, et al 2015, Sfeir, et al 2009). mRTEL1 was reported to have two distinct roles at telomeres: to prevent telomere fragility by resolving G-quadruplexes formed during telomere replication, and to disassemble t-loops to allow telomere replication and prevent t-loop excision (Sfeir, et al 2009, Uringa, et al 2012, Vannier, et al 2012). However, mRTEL1 also has important non-telomeric functions in DNA replication, DNA lesion repair and homologous recombination (Uringa, et al 2012, Vannier, et al 2013), and human RTEL1 was shown to unwind (CTG-CAG) trinucleotide repeat hairpins to inhibit repeat-mediated chromosome fragility (Frizzell, et al 2014) and to play a role in nuclear and cytoplasmic trafficking of pre-U2 RNA (Schertzer, et al 2015).
To date, 18 mutations in RTEL1 were described in 17 cases of HH from 14 families (Figure 3D; (Ballew, et al 2013b, Ballew, et al 2013a, Deng, et al 2013, Le Guen, et al 2013, Walne, et al 2013a): 15 missense mutations (E251K, M492I, E591D, A621T, I699M, L710R, G739V, V745M, K897Q, R957W, F964L, R974X, R986X, C1244R, R1264H), a deletion (del398-422) and two intronic splicing mutations (C102+2T>C, Ivs24+5G>A). Most of the RTEL1 mutations observed were biallelic, with either homozygous or (mostly) compound heterozygous autosomal recessive inheritance. A few apparently-monoallelic cases may actually have a second RTEL1 mutation yet to be identified, or may be explained by AD inheritance with genetic anticipation (Ballew, et al 2013a). The mutations were located mostly in the helicase domains, or in the harmonin-like or C-terminal RING finger domains, possibly involved in protein-protein interactions or ubiquitin transfer (Ballew, et al 2013b, Le Guen, et al 2013). The RTEL1 R1264H mutation was shown to compromise the interaction of RTEL1 with TRF2, causing t-loop excision and telomere length heterogeneity (Sarek, et al 2015). This mutation was determined to occur on a common haplotype found in individuals of Ashkenazi Jewish ancestry. Notably, the carrier frequency of this mutation was 1% in a collection of Orthodox Ashkenazi samples and 0.45% in the general Ashkenazi Jewish population (Fedick, et al 2014). This led to the clinical recommendation that this mutation be included in carrier screening panels in the Ashkenazi population.
All HH patients with RTEL1 mutations had similar clinical manifestations including IUGR, cerebellar hypoplasia, BMF and very short telomeres in white blood cells. Normal telomere length in fibroblasts was described for some HH patients, despite poor growth and the formation of DNA damage foci at telomeres (Lamm, et al 2009). Most patients developed complications as young children; none of them had significantly abnormal skin pigmentation and only some of them had nail dystrophy and leucoplakia (Ballew, et al 2013a, Ballew, et al 2013b, Deng, et al 2013, Le Guen, et al 2013, Walne, et al 2013a). RTEL1 is important for both telomere maintenance and genome stability (Ding, et al 2004, Frizzell, et al 2014, Uringa, et al 2012, Vannier, et al 2012). Some studies describe altered DNA replication, elevated levels of total DNA damage and defects in DNA repair in some HH-derived cell lines (Ballew, et al 2013b, Le Guen, et al 2013). However, most of the cells derived from HH patients harbouring RTEL1 mutations had significant defects only in telomere maintenance (Deng, et al 2013, Walne, et al 2013a). This suggests that either some of described mutations specifically affect the telomeric function of RTEL1, or that the non-telomeric functions are redundant and/or not essential in humans.
The suggested role of RTEL1 in resolving G-quadruplexes during telomere replication, is consistent with the telomere fragility observed in patients cells, presumably leading to telomere fusion, anaphase bridges and mitotic arrest (Deng, et al 2013, Le Guen, et al 2013). Another role was suggested for RTEL1 in resolving t-loops during telomere replication (Vannier, et al 2012). Defects in t-loop disassembly can induce t-loop excision yielding increased levels of t-circles. However, existing data about t-circle levels in RTEL1 deficient cells are conflicting (Ballew, et al 2013b, Deng, et al 2013, Uringa, et al 2012, Vannier, et al 2012, Walne, et al 2013a). The discrepancy may be explained by the different mutations having different effects on RTEL1 function or by the different assays used (a rolling circle amplification assay versus two-dimensional gel electrophoresis). RTEL1 is the first factor associated predominantly with HH rather than DC. Although it does not function only at telomeres, its implication in HH indicates that it is essential for telomere maintenance. Yet, its precise telomeric functions remain unclear. HH-causing mutations were described in different domains and likely affect different functions of RTEL1 (Figure 3D). Further characterization of the RTEL1 mutants will help distinguishing between different functions of RTEL1 and their implications in HH.
ACD (TPP1)
TPP1 (abbreviated from the former names TINT1, PTOP and PIP1; also termed ACD) is encoded by the Adrenocortical Dysplasia Homolog (ACD) gene and is one of the core components of the shelterin telomere protection complex. TPP1 has distinct domains with specific telomeric functions (Figure 3E). The C-terminal domain binds TIN2 to form a bridge between the single-stranded binding POT1 and the double-stranded binding TRF1 and TRF2 (Takai, et al 2011). The central domain of TPP1 is required for heterodimer formation with POT1 (Liu, et al 2004, Ye, et al 2004). In addition, an area on the surface of the N-terminal OB domain of TPP1, termed TEL patch, is required for the interaction of TPP1 with telomerase, telomerase processivity, and for the recruitment of telomerase to telomeres (Nandakumar, et al 2012, Zhong, et al 2012).
Whole exome sequencing of a patient with HH and his family members discovered a TEL patch mutation (delK170) in the proband and his father, and a missense mutation (P491T) in the proband and his mother (Kocak, et al 2014). The TEL patch single amino acid deletion resulted in significant reduction of telomerase processivity and recruitment to telomeres. The P491T missense mutation was less clearly deleterious but may have an effect on TIN2 binding. Similarly, the same TEL patch deletion was identified in a family with aplastic anaemia and other features consistent with DC (Guo, et al 2014). This family showed autosomal dominant inheritance of disease.
Apollo
Some cases of HH were reported in which telomere lengths in both blood cells and fibroblasts were normal (Touzot, et al 2010, Touzot, et al 2012). The disease causing mutation is unknown, but in one patient, the cells were found to express an aberrant splice variant of Apollo, a nuclease that plays a role in the 3′ overhang formation at leading-strand telomeres (Touzot, et al 2010, Wu, et al 2012). The authors suggested that this variant, lacking the TRF2-binding domain, has a dominant-negative effect and impairs telomere function. However, the germline mutation(s) that cause the aberrant splicing of Apollo is yet to be identified.
Genes implicated in Dyskeratosis Congenita but not in Hoyeraal-Hreidarsson Syndrome TERC (hTR)
AD mutations in the telomerase RNA component, TERC, were identified through linkage analysis of a large family (Vulliamy, et al 2001). To date 50 TERC mutations are described, resulting in spectrum of diseases, from mild aplastic anaemia to DC (Podlevsky, et al 2008). Among 14 DC-associated mutations that include large and small deletions and nucleotide substitutions, 9 fall into the pseudoknot-containing telomerase template domain (Podlevsky, et al 2008). Most of TERC mutations implicated in DC significantly affect telomerase activity (Vulliamy, et al 2011). One HH-associated TERC mutation (242C>T) was found in a patient whose mother and sister were both asymptomatic carriers. The telomerase activity of this allele was surprisingly higher than for the rest of the DC-causing alleles (46.8%), indicating that HH in this patient was probably caused by another unidentified mutation, either alone or in combination with the TERC mutation described (Vulliamy, et al 2011).
NOP10 and NHP2
In just a few cases of AR DC, mutations in two additional components of the box H/ACA snoRNPs, NOP10 and NHP2, have been described. Homozygous R34W mutations in NOP10 impaired hTR binding and blocked pre-RNP formation (Trahan, et al 2009, Walne, et al 2007). Homozygous Y139H substitution in NHP2 was described in a patient with severe DC, and compound heterozygous V126M and a stop-loss mutation X154R was found in a patient with classic DC (Vulliamy, et al 2008). Patients with mutations in NOP10 or NHP2 had decreased hTR levels and short telomeres, similar to those having mutations in dyskerin (Vulliamy, et al 2008, Walne, et al 2007). No disease-causing mutations were so far identified in other proteins that are involved in snoRNP assembly and function (Trahan, et al 2009).
WRAP53 (TCAB1)
Another telomerase holoenzyme component found mutated in AR DC is TCAB1 (encoded by WRAP53; (Zhong, et al 2011). TCAB1 binds a consensus sequence termed CAB box in scaRNAs, including hTR, and is required for telomerase trafficking to Cajal bodies and telomere elongation (Stern, et al 2012, Venteicher and Artandi 2009). The DC-causing mutations were compound heterozygous (F164L and R398W; H376Y and G345R) and disrupted telomerase localization to the Cajal bodies, leading to hTR accumulation in the nucleoli and impairing telomere elongation (Zhong, et al 2011).
CTC1
Two different groups identified compound heterozygous mutations in CTC1 (conserved telomere maintenance component 1) and short telomeres in patients with Coats plus syndrome (Anderson, et al 2012, Polvi, et al 2012). Later, compound heterozygous CTC1 mutations were found in DC patients (Keller, et al 2012, Walne, et al 2013b). The connection of CTC1 to telomere biology and the clinical overlap suggests that Coats plus is also a telomere biology disorder. To date more then 20 disease-causing mutations are found in CTC1, 10 of them associated with DC (Walne, et al 2013b). Although CTC1 mutations have various effects on telomere length, all of them significantly affect semiconservative replication of telomeric DNA (Chen, et al 2013). These findings demonstrated that telomerase deficiency leading to excessive telomere shortening is not the only pathway causing a telomere biology disorder.
CONCLUSIONS
The clinical consequences of abnormal telomere biology are most significant in HH. However, there is substantial clinical overlap between HH, Revesz Syndrome, Coats plus, DC and other apparently isolated illnesses (e.g., pulmonary fibrosis, or aplastic anaemia). These disorders represent different extremes of the illnesses caused by germline mutations in key telomere biology genes. The presence of cerebellar hypoplasia in the setting of immunodeficiency in a young child should prompt a clinical evaluation for HH. The clinical differences caused by germline mutations in genes in the same pathway are probably due to differences in the functional consequence of a specific mutation in a protein, as well as incomplete penetrance and variable expressivity of the mutation in different individuals. The genetic overlap of HH with DC and related telomere biology disorders is well established. De novo and inherited (AD, AR and X-linked) mutations have been identified in about 60% of the HH patients. Many HH-associated mutations also cause DC. In some cases (e.g. ACD/TPP1) monoallelic mutations caused DC and biallelic mutations caused HH (Guo, et al 2014), suggesting that HH is a severe form of DC. On the other hand, mutations in the telomerase core genes TERC and TERT cause only or mostly DC, while mutations in genes with additional non-telomeric functions, such as RTEL1 and DKC1, cause only or mostly HH, suggesting that non-telomeric defects may contribute to the HH-specific manifestations.
Novel mutations causing HH and other telomere biology disorders are likely to be identified soon with the increasing availability of exome and whole genome sequencing. Some of these mutations may be found in telomere biology genes not yet implicated in DC or HH, or in other genes not known to be involved in telomere maintenance. Since p53 activation can induce degradation of TRF2 (Fujita, et al 2010), genetic defects activating p53 may contribute to the progression of telomere biology diseases by combining increased senescence or apoptosis and telomere dysfunction (Donehower 2009). Studying the aetiology of telomere biology diseases is invaluable for understanding the mechanisms involved in normal telomere maintenance and function. Such understanding will facilitate the design of therapeutic approaches, not only for telomere biology diseases but also for other clinical manifestations of telomere dysfunction, such as cancer and aging-associated pathologies.
Acknowledgments
This work was supported by the intramural research program of the Division of Cancer Epidemiology, National Cancer Institute, National Institutes of Health, by a grant from the Israel Science Foundation (1729/13) and a project grant from the Israel Cancer Research Fund to Y.T., and by institutional grants from INSERM, Ligue Nationale contre le Cancer (Equipe Labellisée La Ligue), GIS-Institut des maladies rares, ARC, INCa/Cancéropôle Ile de France, Institut Imagine, and the European Research Council [PIDIMMUN grant no. 249816]. P.R. is a scientist from Centre National de la Recherche Scientifique (CNRS).
Footnotes
G.G., F.T, P.R, Y.T, and S.A.S. reviewed the literature and wrote the manuscript.
BIBLIOGRAPHY
- Aalfs CM, van den Berg H, Barth PG, Hennekam RC. The Hoyeraal-Hreidarsson syndrome: the fourth case of a separate entity with prenatal growth retardation, progressive pancytopenia and cerebellar hypoplasia. Eur J Pediatr. 1995;154:304–308. doi: 10.1007/BF01957367. [DOI] [PubMed] [Google Scholar]
- Alder JK, Parry EM, Yegnasubramanian S, Wagner CL, Lieblich LM, Auerbach R, Auerbach AD, Wheelan SJ, Armanios M. Telomere Phenotypes in Females with Heterozygous Mutations in the Dyskeratosis Congenita 1 (DKC1) Gene. Human Mutation. 2013;34:1481–1485. doi: 10.1002/humu.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS. Telomere length in inherited bone marrow failure syndromes. Haematologica. 2015;100:49–54. doi: 10.3324/haematol.2014.114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, Urquhart JE, Daly SB, Dickerson JE, O’Sullivan J, Leibundgut EO, Muter J, Abdel-Salem GM, Babul-Hirji R, Baxter P, Berger A, Bonafe L, Brunstom-Hernandez JE, Buckard JA, Chitayat D, Chong WK, Cordelli DM, Ferreira P, Fluss J, Forrest EH, Franzoni E, Garone C, Hammans SR, Houge G, Hughes I, Jacquemont S, Jeannet PY, Jefferson RJ, Kumar R, Kutschke G, Lundberg S, Lourenco CM, Mehta R, Naidu S, Nischal KK, Nunes L, Ounap K, Philippart M, Prabhakar P, Risen SR, Schiffmann R, Soh C, Stephenson JB, Stewart H, Stone J, Tolmie JL, van der Knaap MS, Vieira JP, Vilain CN, Wakeling EL, Wermenbol V, Whitney A, Lovell SC, Meyer S, Livingston JH, Baerlocher GM, Black GC, Rice GI, Crow YJ. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nature Genetics. 2012;44:338–342. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- Angrisani A, Vicidomini R, Turano M, MF Human dyskerin: beyond telomeres. Biological Chemistry. 2014;395:593–610. doi: 10.1515/hsz-2013-0287. [DOI] [PubMed] [Google Scholar]
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge B, Orte A, Yeoman JA, Kirwan M, Vulliamy T, Dokal I, Klenerman D, Balasubramanian S. Single-Molecule Analysis of the Human Telomerase RNA·Dyskerin Interaction and the Effect of Dyskeratosis Congenita Mutations. Biochemistry. 2009;48:10858–10865. doi: 10.1021/bi901373e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Review of Hematology. 2013;6:327–337. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
- Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, Alter BP, Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Human Genetics. 2013a;132:473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew BJ, Joseph V, De S, Sarek G, Vannier JB, Stracker T, Schrader KA, Small TN, O’Reilly R, Manschreck C, Harlan Fleischut MM, Zhang L, Sullivan J, Stratton K, Yeager M, Jacobs K, Giri N, Alter BP, Boland J, Burdett L, Offit K, Boulton SJ, Savage SA, Petrini JHJ. A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, RTEL1, Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome. PLoS Genetics. 2013b;9:e1003695. doi: 10.1371/journal.pgen.1003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik SSR, Guo C, Smith AC, Margolis SS, Richardson DA, Tirado CA, Counter CM. C-Terminal Regions of the Human Telomerase Catalytic Subunit Essential for In Vivo Enzyme Activity. Mol Cell Biol. 2002;22:6234–6246. doi: 10.1128/MCB.22.17.6234-6246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Dokal I, Tamary H, Avigdor A, Garty BZ, Volkov A, Vulliamy T. Expanding the clinical phenotype of autosomal dominant dyskeratosis congenita caused by TERT mutations. Haematologica. 2008;93:943–944. doi: 10.3324/haematol.12317. [DOI] [PubMed] [Google Scholar]
- Bellodi C, Kopmar N, Ruggero D. Deregulation of oncogene, induced senescence and p53 translational control in X-linked dyskeratosis congenita. The EMBO Journal. 2010;29:1865–1876. doi: 10.1038/emboj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet F, Caduff R, Schaad UB, Roten H, Tuchschmid P, Boltshauser E, Seger RA. A syndrome of primary combined immunodeficiency with microcephaly, cerebellar hypoplasia, growth failure and progressive pancytopenia. Eur J Pediatr. 1994;153:333–338. doi: 10.1007/BF01956413. [DOI] [PubMed] [Google Scholar]
- Berthet F, Tuchschmid P, Boltshauser E, Seger RA. The Hoyeraal-Hreidarsson syndrome: don’t forget the associated immunodeficiency. Eur J Pediatr. 1995;154:998. doi: 10.1007/BF01958649. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and Signaling at the Telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Borggraefe I, Koletzko S, Arenz T, Fuehrer M, Hoffmann F, Dokal I, Vulliamy T, Weiler V, Griese M, Belohradsky BH, Lang T. Severe Variant of X-linked Dyskeratosis Congenita (Hoyeraal-Hreidarsson Syndrome) Causes Significant Enterocolitis in Early Infancy. Journal of Pediatric Gastroenterology and Nutrition. 2009;49:359–363. doi: 10.1097/MPG.0b013e3181a15b94. [DOI] [PubMed] [Google Scholar]
- Brault ME, Lauzon C, Autexier C. Dyskeratosis congenita mutations in dyskerin SUMOylation consensus sites lead to impaired telomerase RNA accumulation and telomere defects. Human Molecular Genetics. 2013;22:3498–3507. doi: 10.1093/hmg/ddt204. [DOI] [PubMed] [Google Scholar]
- Canudas S, Houghtaling BR, Bhanot M, Sasa G, Savage SA, Bertuch AA, Smith S. A role for heterochromatin protein 1gamma at human telomeres. Genes & Development. 2011;25:1807–1819. doi: 10.1101/gad.17325211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, González A, Manguán-García C, Pintado-Berninches L, Perona R. p53 pathway activation by telomere attrition in X-DC primary fibroblasts occurs in the absence of ribosome biogenesis failure and as a consequence of DNA damage. Clinical and Translational Oncology. 2013;16:529–538. doi: 10.1007/s12094-013-1112-3. [DOI] [PubMed] [Google Scholar]
- Chen LY, Majerska J, Lingner J. Molecular basis of telomere syndrome caused by CTC1 mutations. Genes & Development. 2013;27:2099–2108. doi: 10.1101/gad.222893.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu F, Vulliamy T, Marrone A, Badiali M, Cao A, Dokal I. A novel DKC1 mutation, severe combined immunodeficiency (T+B–NK– SCID) and bone marrow transplantation in an infant with Hoyeraal–Hreidarsson syndrome. British Journal of Haematology. 2002;119:765–768. doi: 10.1046/j.1365-2141.2002.03822.x. [DOI] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & Development. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Molecular and Cellular Biology. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Glousker G, Molczan A, Fox AJ, Lamm N, Dheekollu J, Weizman OE, Schertzer M, Wang Z, Vladimirova O, Schug J, Aker M, Londono-Vallejo A, Kaestner KH, Lieberman PM, Tzfati Y. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proceedings of the National Academy of Sciences. 2013;110:E3408–E3416. doi: 10.1073/pnas.1300600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, Tam PPL, Nagy A, Lansdorp PM. Regulation of Murine Telomere Length by Rtel: An Essential Gene Encoding a Helicase-like Protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Donehower LA. Using mice to examine p53 functions in cancer, aging, and longevity. Cold Spring Harb Perspect Biol. 2009;1:a001081. doi: 10.1101/cshperspect.a001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du HY, Pumbo E, Ivanovich J, An P, Maziarz RT, Reiss UM, Chirnomas D, Shimamura A, Vlachos A, Lipton JM, Goyal RK, Goldman F, Wilson DB, Mason PJ, Bessler M. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MSH. Zscan4: A novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Developmental Biology. 2007;307:539–550. doi: 10.1016/j.ydbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedick AM, Shi L, Jalas C, Treff NR, Ekstein J, Kornreich R, Edelmann L, Mehta L, Savage SA. Carrier screening of RTEL1 mutations in the Ashkenazi Jewish population. Clinical Genetics. 2014 doi: 10.1111/cge.12459. [DOI] [PubMed] [Google Scholar]
- Frescas D, de Lange T. A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice. Genes & Development. 2014;28:153–166. doi: 10.1101/gad.233395.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell A, Nguyen Jennifer HG, Petalcorin Mark IR, Turner Katherine D, Boulton Simon J, Freudenreich Catherine H, Lahue Robert S. RTEL1 Inhibits Trinucleotide Repeat Expansions and Fragility. Cell Reports. 2014;6:827–835. doi: 10.1016/j.celrep.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Horikawa I, Mondal AM, Jenkins LM, Appella E, Vojtesek B, Bourdon JC, Lane DP, Harris CC. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat Cell Biol. 2010;12:1205–1212. doi: 10.1038/ncb2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramatges MM, Qi X, Sasa GS, Chen J, Bertuch AA. A homozygous telomerase T-motif variant resulting in markedly reduced repeat addition processivity in siblings with Hoyeraal Hreidarsson syndrome. Blood. 2013;121:3586–3593. doi: 10.1182/blood-2012-08-447755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian Telomeres End in a Large Duplex Loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Grozdanov PN, Fernandez-Fuentes N, Fiser A, Meier UT. Pathogenic NAP57 mutations decrease ribonucleoprotein assembly in dyskeratosis congenita. Human Molecular Genetics. 2009;18:4546–4551. doi: 10.1093/hmg/ddp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kartawinata M, Li J, Pickett HA, Teo J, Kilo T, Barbaro PM, Keating B, Chen Y, Tian L, Al-Odaib A, Reddel RR, Christodoulou J, Xu X, Hakonarson H, Bryan TM. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood. 2014;124:2767–2774. doi: 10.1182/blood-2014-08-596445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy T, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature Genetics. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Hodes RJ, Hathcock KS, Weng N-p. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- Hoyeraal HM, Lamvik J, Moe PJ. Congenital hypoplastic thrombocytopenia and cerebral malformations in two brothers. Acta Paediatr Scand. 1970;59:185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Hreidarsson S, Kristjansson K, Johannesson G, Johannsson JH. A syndrome of progressive pancytopenia with microcephaly, cerebellar hypoplasia and growth failure. Acta Paediatr Scand. 1988;77:773–775. doi: 10.1111/j.1651-2227.1988.tb10751.x. [DOI] [PubMed] [Google Scholar]
- Jack K, Bellodi C, Landry Dori M, Niederer Rachel O, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean Alison M, Thompson Sunnie R, Ruggero D, Dinman Jonathan D. rRNA Pseudouridylation Defects Affect Ribosomal Ligand Binding and Translational Fidelity from Yeast to Human Cells. Molecular Cell. 2011;44:660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi S, Forbes L, Ruchelli E, Sullivan KE. Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum – a single-center pediatric experience. Pediatric Allergy and Immunology. 2011;22:313–319. doi: 10.1111/j.1399-3038.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- Keller RB, Gagne KE, Usmani GN, Asdourian GK, Williams DA, Hofmann I, Agarwal S. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatric Blood & Cancer. 2012;59:311–314. doi: 10.1002/pbc.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NK, Theimer CA, Mitchell JR, Collins K, Feigon J. Effect of pseudouridylation on the structure and activity of the catalytically essential P6.1 hairpin in human telomerase RNA. Nucleic Acids Research. 2010;38:6746–6756. doi: 10.1093/nar/gkq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Fayet-Lebaron E, Jády BE. Box H/ACA Small Ribonucleoproteins. Molecular Cell. 2010;37:597–606. doi: 10.1016/j.molcel.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Knight S, Heiss NS, Vulliamy T, Aalfs CM, McMahon C, Richmond P, Jones A, Hennekam RC, Poustka A, Mason PJ, Dokal I. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. British Journal of Haematology. 1999a;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- Knight S, Heiss NS, Vulliamy T, Grescner S, Stavrides G, Pai GS, Lestringant G, Varma N, Mason PJ, Dokal I, Poustka A. X-Linked Dyskeratosis Congenita Is Predominantly Caused by Missense Mutations in the DKC1 Gene. American Journal of Medical Genetics. 1999b;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S, Vulliamy T, Morgan B, Devriendt K, Mason P, Dokal I. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Hum Genet. 2001;108:299–303. doi: 10.1007/s004390100494. [DOI] [PubMed] [Google Scholar]
- Knight SW, Vulliamy TJ, Heiss NS, Matthijs G, Devriendt K, Connor JM, D’Urso M, Poustka A, Mason PJ, Dokal I. 1.4 Mb candidate gene region for X linked dyskeratosis congenita defined by combined haplotype and X chromosome inactivation analysis. J Med Genet. 1998;35:993–996. doi: 10.1136/jmg.35.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch H, Tennstedt C, Brueck W, Hammer H, Vulliamy T, Dokal I, Lehmann Rd, Hanefeld F, Tinschert S. Two brothers with findings resembling congenital intrauterine infection-like syndrome (pseudo-TORCH syndrome) American Journal of Medical Genetics. 2003;120A:261–265. doi: 10.1002/ajmg.a.20138. [DOI] [PubMed] [Google Scholar]
- Kocak H, Ballew BJ, Bisht K, Eggebeen R, Hicks BD, Suman S, O’Neil A, Giri N, Maillard I, Alter BP, Keegan CE, Nandakumar J, Savage SA. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes & Development. 2014;28:2090–2102. doi: 10.1101/gad.248567.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwashima S. Hoyeraal-Hreidarsson syndrome: magnetic resonance imaging findings. Japanese Journal of Radiology. 2009;27:324–327. doi: 10.1007/s11604-009-0344-1. [DOI] [PubMed] [Google Scholar]
- Lai W, Deng WP, Liu X, Chen HM, Dai SX. A recurrent p. A353V mutation in DKC1 responsible for different phenotypes of dyskeratosis congenita in a Chinese family. 2011;63:122–124. doi: 10.1016/j.jdermsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Lamm N, Ordan E, Shponkin R, Richler C, Aker M, Tzfati Y. Diminished Telomeric 3′ Overhangs Are Associated with Telomere Dysfunction in Hoyeraal-Hreidarsson Syndrome. PLoS ONE. 2009;4:e5666. doi: 10.1371/journal.pone.0005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, Carpentier W, Nitschke P, Picard C, Couillault G, Soulier J, Fischer A, Callebaut I, Jabado N, Londono-Vallejo A, de Villartay JP, Revy P. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Human Molecular Genetics. 2013;22:3239–3249. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- Liu D, O’Connor MS, Qin J, Songyang Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. The Journal of biological chemistry. 2004;279:51338–51342. doi: 10.1074/jbc.M409293200. [DOI] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, Seyfang A, Blasco MA, Keefe DL. Telomere lengthening early in development. Nature Cell Biology. 2007;9:1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- Machado-Pinilla R, Sanchez-Perez I, Ramon Murguia J, Sastre L, Perona R. A dyskerin motif reactivates telomerase activity in X-linked dyskeratosis congenita and in telomerase-deficient human cells. Blood. 2007;111:2606–2614. doi: 10.1182/blood-2007-04-083261. [DOI] [PubMed] [Google Scholar]
- Malbora B, Avci Z, Ozbek N. Aplastic anemia and Hoyeraal-Hreidarsson syndrome. Skinmed. 2014;12:117–118. [PubMed] [Google Scholar]
- Marrone A, Walne A, Tamary H, Masunari Y, Kirwan M, Beswick R, Vulliamy TJ, Dokal I. Telomerase reverse-transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999a;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Cheng J, Collins K. A Box H/ACA Small Nucleolar RNA-Like Domain at the Human Telomerase RNA 3′ End. Molecular and Cellular Biology. 1999b;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proceedings of the National Academy of Sciences. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492:285–289. doi: 10.1038/nature11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Ozdemir MA, Karakukcu M, Kose M, Kumandas S, Gumus H. The longest surviving child with Hoyeraal-Hreidarsson Syndrome. Haematologica. 2004;89:ECR38. [PubMed] [Google Scholar]
- Parry EM, Alder JK, Lee SS, Phillips JA, Loyd JE, Duggal P, Armanios M. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. Journal of Medical Genetics. 2011;48:327–333. doi: 10.1136/jmg.2010.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Curtis F, Al-Eyadhy A, Al-Tamemi S, Mazer B, Dror Y, Abish S, Bale S, Compton J, Ray R, Scott P, Der Kaloustian VM. An intronic mutation in DKC1 in an infant with Hoyeraal-Hreidarsson syndrome. Am J Med Genet A. 2008;146A:2159–2161. doi: 10.1002/ajmg.a.32412. [DOI] [PubMed] [Google Scholar]
- Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic Acids Res. 2008;36:D339–343. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvi A, Linnankivi T, Kivela T, Herva R, Keating JP, Makitie O, Pareyson D, Vainionpaa L, Lahtinen J, Hovatta I, Pihko H, Lehesjoki AE. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. American Journal of Human Genetics. 2012;90:540–549. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Molecular Cell. 2006;21:249–260. doi: 10.1016/j.molcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. DOG 1.0: illustrator of protein domain structures. Cell Res. 2009;19:271–273. doi: 10.1038/cr.2009.6. [DOI] [PubMed] [Google Scholar]
- Revesz T, Fletcher S, al-Gazali LI, DeBuse P. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? J Med Genet. 1992;29:673–675. doi: 10.1136/jmg.29.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revy P, Busslinger M, Tashiro K, Arenzana F, Pillet P, Fischer A, Durandy A. A syndrome involving intrauterine growth retardation, microcephaly, cerebellar hypoplasia, B lymphocyte deficiency, and progressive pancytopenia. Pediatrics. 2000;105:E39. doi: 10.1542/peds.105.3.e39. [DOI] [PubMed] [Google Scholar]
- Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- Sarek G, Vannier JB, Panier S, Petrini JH, Boulton SJ. TRF2 Recruits RTEL1 to Telomeres in S Phase to Promote T-Loop Unwinding. Mol Cell. 2015 doi: 10.1016/j.molcel.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasa GS, Ribes-Zamora A, Nelson ND, Bertuch AA. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clinical Genetics. 2012;81:470–478. doi: 10.1111/j.1399-0004.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA. Connecting complex disorders through biology. Nature Genetics. 2012;44:238–240. doi: 10.1038/ng.2206. [DOI] [PubMed] [Google Scholar]
- Savage SA, Alter BP. Dyskeratosis Congenita. Hematology/Oncology Clinics of North America. 2009;23:215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a Component of the Shelterin Telomere Protection Complex, Is Mutated in Dyskeratosis Congenita. The American Journal of Human Genetics. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer M, Jouravleva K, Perderiset M, Dingli F, Loew D, Le Guen T, Bardoni B, de Villartay JP, Revy P, Londono-Vallejo A. Human regulator of telomere elongation helicase 1 (RTEL1) is required for the nuclear and cytoplasmic trafficking of pre-U2 RNA. Nucleic Acids Res. 2015 doi: 10.1093/nar/gku1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Bernstein Douglas A, Mumbach Maxwell R, Jovanovic M, Herbst Rebecca H, León-Ricardo Brian X, Engreitz Jesse M, Guttman M, Satija R, Lander Eric S, Fink G, Regev A. Transcriptome-wide Mapping Reveals Widespread Dynamic-Regulated Pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JL, Zyner KG, Pickett HA, Cohen SB, Bryan TM. Telomerase recruitment requires both TCAB1 and Cajal bodies independently. Molecular and Cellular Biology. 2012;32:2384–2395. doi: 10.1128/MCB.00379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznajer Y, Baumann C, David A, Journel H, Lacombe D, Perel Y, Blouin P, Segura JF, Cezard JP, Peuchmaur M, Vulliamy T, Dokal I, Verloes A. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal-Hreidarsson syndrome) Eur J Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Molecular Cell. 2011;44:647–659. doi: 10.1016/j.molcel.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumati NR, Zeng XL, Au HHT, Jang CJ, Jan E, Wong JMY. Severity of X-linked Dyskeratosis Congenita (DKCX) Cellular Defects Is not Directly Related to Dyskerin (DKC1) Activity in Ribosomal RNA Biogenesis or mRNA Translation. Human Mutation. 2013;34:1698–1707. doi: 10.1002/humu.22447. [DOI] [PubMed] [Google Scholar]
- Touzot F, Callebaut I, Soulier J, Gaillard L, Azerrad C, Durandy A, Fischer A, de Villartay JP, Revy P. Function of Apollo (SNM1B) at telomere highlighted by a splice variant identified in a patient with Hoyeraal-Hreidarsson syndrome. Proceedings of the National Academy of Sciences. 2010;107:10097–10102. doi: 10.1073/pnas.0914918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot F, Gaillard L, Vasquez N, Le Guen T, Bertrand Y, Bourhis J, Leblanc T, Fischer A, Soulier J, de Villartay JP, Revy P. Heterogeneous telomere defects in patients with severe forms of dyskeratosis congenita. Journal of Allergy and Clinical Immunology. 2012;129:473–482. doi: 10.1016/j.jaci.2011.09.043. [DOI] [PubMed] [Google Scholar]
- Trahan C, Martel C, Dragon F. Effects of dyskeratosis congenita mutations in dyskerin, NHP2 and NOP10 on assembly of H/ACA pre-RNPs. Human Molecular Genetics. 2009;19:825–836. doi: 10.1093/hmg/ddp551. [DOI] [PubMed] [Google Scholar]
- Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski KT, Shu MD, Kukoyi A, Steitz JA. A Conserved WD40 Protein Binds the Cajal Body Localization Signal of scaRNP Particles. Molecular Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uringa EJ, Lisaingo K, Pickett HA, Brind’Amour J, Rohde JH, Zelensky A, Essers J, Lansdorp PM. RTEL1 contributes to DNA replication and repair and telomere maintenance. Molecular biology of the cell. 2012;23:2782–2792. doi: 10.1091/mbc.E12-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelij P, Chrzanowska KH, Godthelp BC, Rooimans MA, Oostra AB, Stumm M, Zdzienicka MÇZ, Joenje H, de Winter JP. Warsaw Breakage Syndrome, a Cohesinopathy Associated with Mutations in the XPD Helicase Family Member DDX11/ChlR1. The American Journal of Human Genetics. 2010;86:262–266. doi: 10.1016/j.ajhg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier JB, Pavicic-Kaltenbrunner V, Petalcorin MIR, Ding H, Boulton SJ. RTEL1 Dismantles T Loops and Counteracts Telomeric G4-DNA to Maintain Telomere Integrity. Cell. 2012;149:795–806. doi: 10.1016/j.cell.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Vannier JB, Sandhu S, Petalcorin MI, Wu X, Nabi Z, Ding H, Boulton SJ. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science. 2013;342:239–242. doi: 10.1126/science.1241779. [DOI] [PubMed] [Google Scholar]
- Venteicher AS, Artandi SE. TCAB1: Driving telomerase to Cajal bodies. Cell Cycle. 2009;8:1329–1331. doi: 10.4161/cc.8.9.8288. [DOI] [PubMed] [Google Scholar]
- Vogiatzi P, Perdigones N, Mason PJ, Wilson DB, Bessler M. A family with Hoyeraal-Hreidarsson syndrome and four variants in two genes of the telomerase core complex. Pediatric Blood & Cancer. 2013;60:E4–E6. doi: 10.1002/pbc.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy T, Knight SW, Heiss NS, Smith OP, Poustka A, Dokal I, Mason PJ. Dyskeratosis Congenita Caused by a 3′ Deletion: Germline and Somatic Mosaicism in a Female Carrier. Blood. 1999;94:1254–1260. [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genetics. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood cells, molecules & diseases. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy TJ, Kirwan MJ, Beswick R, Hossain U, Baqai C, Ratcliffe A, Marsh J, Walne A, Dokal I. Differences in Disease Severity but Similar Telomere Lengths in Genetic Subgroups of Patients with Telomerase and Shelterin Mutations. PLoS ONE. 2011;6:e24383. doi: 10.1371/journal.pone.0024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliamy T, Beswick R, Kirwan MJ, Hossain U, Walne AJ, Dokal I. Telomere length measurement can distinguish pathogenic from non-pathogenic variants in the shelterin component, TIN2. Clinical Genetics. 2012;81:76–81. doi: 10.1111/j.1399-0004.2010.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbott H, Machado-Pinilla R, Liger D, Blaud M, Rety S, Grozdanov PN, Godin K, van Tilbeurgh H, Varani G, Meier UT, Leulliot N. The H/ACA RNP assembly factor SHQ1 functions as an RNA mimic. Genes & Development. 2011;25:2398–2408. doi: 10.1101/gad.176834.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Dokal I. Dyskeratosis Congenita: A historical perspective. Mechanisms of ageing and development. 2008;129:48–59. doi: 10.1016/j.mad.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Human Molecular Genetics. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am J Hum Genet. 2013a;92:448–453. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne AJ, Bhagat T, Kirwan M, Gitiaux C, Desguerre I, Leonard N, Nogales E, Vulliamy T, Dokal IS. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica. 2013b;98:334–338. doi: 10.3324/haematol.2012.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- White Malcolm F. Structure, function and evolution of the XPD family of iron–sulfur-containing 5′ →3′ DNA helicases. Biochemical Society Transactions. 2009;37:547–551. doi: 10.1042/BST0370547. [DOI] [PubMed] [Google Scholar]
- Wong JMY, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes & Development. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes & Development. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Takai H, de Lange T. Telomeric 3′ Overhangs Derive from Resection by Exo1 and Apollo and Fill-In by POT1b-Associated CST. Cell. 2012;150:39–52. doi: 10.1016/j.cell.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Suhasini AN, Brosh RM., Jr Welcome the Family of FANCJ-like Helicases to the Block of Genome Stability Maintenance Proteins. Cellular and Molecular Life Sciences. 2009;66:1209–1222. doi: 10.1007/s00018-008-8580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Beauchamp AD, Calado RT, Bradford JW, ARJ, Shenoy A, Liang Y, Lansdorp PM, Young NS, Ly H. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- Xin ZT, Ly H. Characterization of interactions between naturally mutated forms of the TIN2 protein and its known protein partners of the shelterin complex. Clinical Genetics. 2012;81:301–302. doi: 10.1111/j.1399-0004.2011.01784.x. [DOI] [PubMed] [Google Scholar]
- Yaghmai R, Kimyai-Asadi A, Rostamiani K, Heiss NS, Poustka A, Eyaid W, Bodurtha J, Nousari HC, Hamosh A, Metzenberg A. Overlap of dyskeratosis congenita with the Hoyeraal-Hreidarsson syndrome. The Journal of Pediatrics. 2000;136:390–393. doi: 10.1067/mpd.2000.104295. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the Gene for Telomerase Reverse Transcriptase, in Aplastic Anemia. New England Journal of Medicine. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Yang D, He Q, Kim H, Ma W, Songyang Z. TIN2 Protein Dyskeratosis Congenita Missense Mutants Are Defective in Association with Telomerase. Journal of Biological Chemistry. 2011;286:23022–23030. doi: 10.1074/jbc.M111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes & Development. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee SL, Stagg CA, Hoang HG, Yang HT, Indig FE, Wersto RP, Ko MSH. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–863. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug AJ, Crary SM, Jesse Fioravanti M, Campbell K, Cech TR. Many disease-associated variants of hTERT retain high telomerase enzymatic activity. Nucleic Acids Research. 2013;41:8969–8978. doi: 10.1093/nar/gkt653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XL, Thumati NR, Fleisig HB, Hukezalie KR, Savage SA, Giri N, Alter BP, Wong JMY. The accumulation and not the specific activity of telomerase ribonucleoprotein determines telomere maintenance deficiency in X-linked dyskeratosis congenita. Human Molecular Genetics. 2011;21:721–729. doi: 10.1093/hmg/ddr504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes & Development. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Franklin L, Batista Luis FZ, Freund A, Pech Matthew F, Venteicher Andrew S, Artandi Steven E. TPP1 OB-Fold Domain Controls Telomere Maintenance by Recruiting Telomerase to Chromosome Ends. Cell. 2012;150:481–494. doi: 10.1016/j.cell.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a Sentinel or a Subset of Short Telomeres Determine Replicative Senescence? Molecular biology of the cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]