Abstract
Randomized trials showing that high dose therapy with autologous stem cell transplant (ASCT) improved overall survival in multiple myeloma (MM) excluded patients over age 65. To compare outcomes of older adults with MM who underwent ASCT with non-transplant strategies, we identified 146 patients age 65 – 77 with newly diagnosed MM seen Washington University School of Medicine from 2000–2010. Survival among patients who did (N=62) versus did not (N=84) undergo ASCT was compared using Cox proportional hazards modeling, controlling for comorbidities, ECOG performance status (PS) and propensity to undergo ASCT. Median age was 68 (range 65–77). PS and comorbidities did not differ significantly between those who did vs. did not undergo ASCT. Median overall survival was significantly longer in patients who underwent ASCT than those who did not [median 56.0 months (95% confidence intervals 49.1–65.4) vs. 33.1 months (24.3–43.1), p=0.004]. Adjusting for PS, comorbidities, Durie-Salmon stage and propensity to undergo ASCT, ASCT was associated with superior overall survival [HR for mortality 0.52 (95% CI 0.30–0.91), p=0.02]. In a cohort of older adults with MM, undergoing ASCT was associated with a nearly 50% lower mortality, after controlling for PS, comorbidities, stage and propensity to undergo ASCT.
Introduction
Multiple myeloma (MM) is a disease of older adults, with almost two-thirds of cases occurring in patients over the age of 65 years. With the aging of the population, the number of older adults with MM is expected to nearly double by the year 2030.(1) While advances in treatment have improved survival rates substantially, the improvement among older adults have been modest compared to that among younger adults.(2,3) Advancing age is one of the most important negative prognostic factors in MM.(4) The factors underlying this age-related disparity in prognosis are likely related to a combination of factors, including comorbidity, performance status, decreased physiologic reserve, social support, referral bias and undertreatment.(5)
In randomized trials, high dose therapy with autologous stem cell transplantation (ASCT) prolongs survival in patients with MM, compared to conventional chemotherapy.(6,7) However, these studies specifically excluded patients over the age of 65, with a median age of enrolled patients of 55–57 years. Retrospective studies suggest that select older adults can tolerate ASCT and benefit to the same extent that younger individuals do(8–11), but the role of ASCT in older adults in the era of modern therapy with immunomodulatory agents and proteasome inhibitors is unclear.
Thus, we sought to examine relationships between baseline factors and initial MM treatment strategy with or without ASCT and to then compare survival between older adults who did versus did not undergo ASCT as part of their initial treatment.
Subjects and methods
Patients
With the approval of the Human Studies Committee, we identified all patients age 65 years and older with multiple myeloma diagnosed or treated at Washington University School of Medicine 2000–2010 from the Barnes-Jewish Hospital Oncology Data Services cancer registry. Patients with amyloid, smoldering MM or other concomitant malignancies were excluded. Patients who received no treatment/palliative care or corticosteroids only were excluded. Because in the United States, the Center for Medicare and Medicaid Services policy excluded coverage of ASCT for individuals over age 77 through 2003, we restricted the analyses to patients aged 77 years and younger. To mitigate immortal time bias, patients who survived fewer than 4 months and thus would not have survived to proceed to ASCT were excluded.
Clinical Data
The Barnes-Jewish Hospital Oncology Data Services cancer registry includes data on the age, gender, race, insurance status, and comorbidities. Comorbidities were graded using the ACE-27 Index.(12) Insurance status/payer was categorized as Medicare alone or with a supplemental plan, Medicare managed care plan, Medicare/Medicaid dual-eligible, and Other/Unknown. Medical records were reviewed for data not available within the Oncology Data Services Registry, including ECOG performance status(13), body mass index (BMI), baseline laboratory values, staging, details on pathology, cytogenetics, paraprotein isotype, and initial treatment, including whether the patient underwent high-dose therapy and autologous stem cell transplant as part of initial therapy. Creatinine clearance was calculated using the Cockcroft-Gault equation.(14) Staging was determined using both the Durie-Salmon Staging System(15) and the International Staging System (ISS).(16) Initial treatment was categorized as: conventional agents (such as melphalan or doxorubicin-based regimens) with corticosteroids, novel agents (thalidomide, lenalidomide or bortezomib) with corticosteroids, novel agents with a second agent and corticosteroids. As common in retrospective studies, missing data presented a potential challenge to this study though every effort has been made to retrieve patient and clinical data. Among the 10 clinicopathologic factors considered, 5 covariates (ASCT status, age, gender, race and insurance status) had complete data, while the other 5 covariates had some extent of missing values - ACE-27 comorbidity index (n=7, 5%), initial therapy (n=35, 24%), Durie-Salmon stage (n=41, 28%), ECOG performance status (n=42, 29%), and creatinine clearance (n=49, 34%). Rather than omit patients with missing values, multivariate regression models were used to impute the missing values.(17) Specifically, the 5 covariates with complete data were first used to impute the comorbidity index, which had only a few missing values, and then these covariates were used as predictors for the remaining covariates with more missing data. To better satisfy the assumption of "missing at random", we also included body mass index (BMI) and several other baseline laboratories (WBC, HGB, ANC, and platelets) in the regression models for multiple imputations.
Statistical Analyses
The primary endpoint was overall survival (OS) defined as time from diagnosis until death, censored at last follow-up. The distribution of demographic and clinical characteristics between patients with and without ASCT was compared using Fisher's exact test, Mann-Whitney rank-sum test, or two-sample t-test as appropriate. For significantly imbalanced factors, multivariate logistic regression was used to create propensity scores for undergoing ASCT.(18) Survival curves by ASCT status were estimated using the Kaplan-Meier product-limit method and compared by log-rank test. Univariate Cox proportional hazard models were fitted to identify factors significantly related to OS. To assess whether ASCT was an independent predictor of OS, a multivariate Cox model was constructed to adjust for other significant predictors as well as propensity scores. Multivariate regression models were used to impute the missing values in the predictors.(17) All analyses were two-sided and significance was set at a p-value of 0.05. Statistical analyses were performed using SAS 9.2 (SAS Institutes, Cary, NC).
Results
A total of 199 patients were initially identified. Fifty-three patients who were over age 77, who received only supportive care or corticosteroids, or survived fewer than 4 months were excluded. This resulted in an analysis cohort of 146 patients; baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics
| Entire cohort (n = 146) |
Subgroups | |||
|---|---|---|---|---|
| Nontransplant (n = 84) |
ASCT (n = 62) |
P | ||
| Age (median, range) | 68 (65 – 77) | 70 (65 – 77) | 67 (65 – 74) | <0.0001 |
| Male Gender(frequency, percent) | 77 (52.7%) | 50 (59.5%) | 27 (43.5%) | 0.06 |
| Race | 0.2 | |||
| White | 119 (81.5%) | 65 (77.4%) | 54 (87.1%) | |
| Other | 27 (18.5%) | 19 (22.6% | 8 (12.9%) | |
| Ace-27 comorbidity index (frequency*, percent) | 0.12 | |||
| None | 35/139 (25.2%) | 16/77 (20.8%) | 19 (30.6%) | |
| Mild | 61/139 (43.9%) | 35/77 (45.4%) | 26 (41.9%) | |
| Moderate | 30/139 (21.6%) | 15/77 (19.5%) | 15 (24.2%) | |
| Severe | 13/139 (9.4%) | 11/77 (14.3%) | 2 (3.2%) | |
| Durie-salmon stage (frequency*, percent) | 0.14 | |||
| 1 | 10/105 (9.5%) | 1/46 (2.2%) | 9/59 (15.2%) | |
| 2 | 19/105 (18.1%) | 9/46 (19.6%) | 10/59 (17.0%) | |
| 3 | 76/105 (72.4%) | 36/46 (78.3%) | 40/59 (67.8%) | |
| ECOG performance status (frequency*, percent) | 0.55 | |||
| 0 | 19/104 (18.3%)* | 9/45 (20.0%) | 10/59 (17.0%) | |
| 1 | 53/104 (51.0%) | 19/45 (42.2%) | 34/59 (57.6%) | |
| 2 | 22/104 (21.2%) | 13/45 (28.9%) | 9/59 (15.2%) | |
| 3 | 10/104 (9.6%) | 4/45 (8.9%) | 6/109 (10.2%) | |
| Insurance | 0.02 | |||
| Medicare +/− supplement | 120 (82.2%) | 72 (85.7%) | 48 (77.4%) | |
| Medicare managed care | 14(9.6%) | 5 (6.0%) | 9 (14.5%) | |
| Medicare/Medicaid dual-eligible | 5 (3.4%) | 5 (6.0%) | 0 | |
| Other/unknown | 7 (4.8%) | 2 (2.4%) | 5 (8.1%) | |
| Initial therapy | 0.3 | |||
| Novel combination therapy** | 18 (16.2%) | 7 (14.3%) | 11 (17.7%) | |
| Novel agent | 65 (58.6%) | 26 (53.1%) | 39 (62.9%) | |
| Alkylating agents | 28 (25.2%) | 16 (32.7%) | 12 (19.4%) | |
| Creatinine clearance (Median, range) | 60.9 ml/min (8.7–126.4) | 58.0 ml/min (8.7–126.3) | 64.1 ml/min (16.8 – 126.4) | 0.2 |
Denominator reflects missing data.
Indicates an immunomodulatory agent or proteosome inhibitor with a second agent plus corticosteroids
ASCT, high dose therapy with autologous stem cell transplant
Regarding staging and baseline prognostic factors, beta-2-microglobulin was not available for the majority of patients in the cohort; therefore, ISS stage was not included in the analyses. Cytogenetics were not performed for most of the patients in the cohort, and therefore this variable was excluded from analysis. Of those for whom data on cytogenetics and/ or fluorescence in situ hybridization for common chromosomal abnormalities were performed, 13.3% (4 of 30 tested) had translocation t(4;14), 32.5% (13 of 40 tested) had deletion 13, 9.7% (3 of 31 tested) had translocation t(11;14), 11.8% (4 of 34 tested) had deletion 17p, 10.5% (6 of 57 tested) had hypodiploidy and 21.2% (12 of 57 tested) had hyperdiploidy.
Induction regimens are listed in Table 1. Over half (57.5%, N=84) of the cohort did not undergo ASCT, while 42.5% (N=62) did. Of those who underwent ASCT, all but one received melphalan 200 mg/m2 for conditioning; one received melphalan 140 mg/m2. No patients underwent tandem ASCT. One patient who underwent ASCT died within 100 days due to toxicity, yielding a 100-day non-relapse mortality rate of 1.6%. Following ASCT, most (85.5%) did not receive maintenance therapy; 2 patients (3.2%) received thalidomide maintenance, 3 (4.8%) received bortezomib maintenance and 4 (6.4%) received lenalidomide maintenance. Data on response to initial therapy was available in only 44% (37/84) of patients who did not undergo ASCT; in the 37 in whom response was assessable, 78.4% achieved PR or better. In those who underwent ASCT, most (88.7%, 55/62) had data available allowing ascertainment of response, with 98% achieving PR or better. Chi-square analysis was not performed due to concern for ascertainment bias, given the significant imbalance in proportion of patients with data available for ascertainment of response in the two treatment groups.
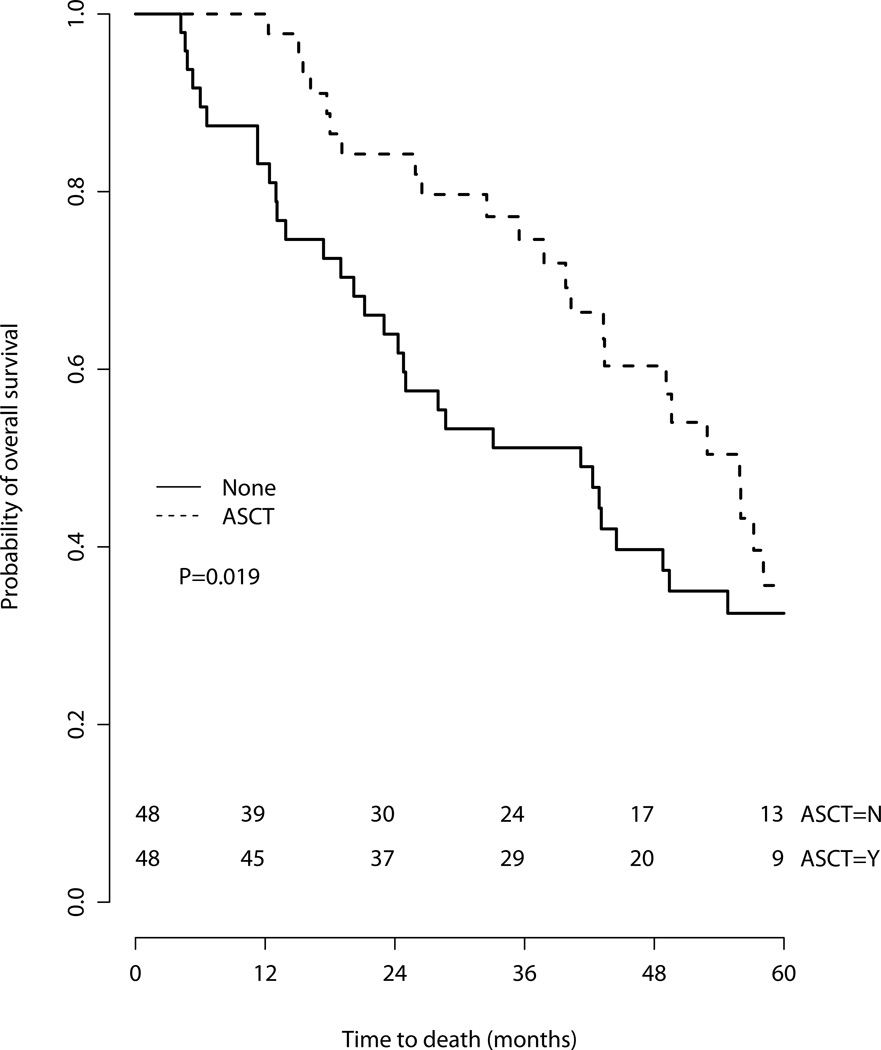
The median overall survival of the entire cohort was 43.4 months [95% Confidence Intervals (CI) 39.8 – 52.9 months]. The median follow-up time for censored patients was 48.4 months (range 5.5 – 141.7 months). As would be expected, there were imbalances in some baseline characteristics between patients who did versus did not undergo ASCT: Patients who did not undergo ASCT tended to be older and were more likely to be Medicare-Medicaid dual-eligible (Table 1). The median OS in the cohort that did not undergo ASCT was 33.1 months (95% CI 24.3–43.1) while the median OS in the cohort that did undergo ASCT was 56.0 months (95% CI 49.1–65.4)(Figure 1). The 3-year overall survival was 78.3% (95% CI 68.2–90.0%) among those who did undergo ASCT versus 49.5% (95% confidence intervals 39.8–61.5%) for those who did not.
Figure 1.
Overall survival of entire cohort by treatment
On univariate analysis, ASCT and performance status were associated with overall survival (Table 2). Race, gender, comorbidities, insurance, creatinine clearance and initial therapy were not associated with survival. The multivariate analysis was summarized over 10 imputed datasets. After controlling for performance status, comorbidity, stage and propensity to undergo ASCT, ASCT remained associated with superior overall survival [Hazard Ratio (HR) for death 0.52 (95% CI 0.30–0.91), p=0.02]. To visualize the results, Figure 2 also presents the Kaplan-Meier curves for 48 pairs of patients, matched by the propensity scores to undergo ASCT, from the first imputed dataset.
Table 2.
Factors associated with overall survival
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| Hazard Ratio (95% confidence intervals) |
P | Hazard Ratio (95% confidence intervals) |
P | |
| Transplant vs no transplant | 0.54 (0.35–0.82) | 0.004 | 0.52 (0.30–0.91) | 0.02 |
| Age | 1.03 (0.97–1.09) | 0.33 | ||
| Male gender | 1.30 (0.87–1.96) | 0.2 | ||
| Race (other relative to white) | 1.40 (0.84–2.33) | 0.2 | ||
| Performance status | ||||
| 0 | Ref | - | Ref | - |
| 1 | 2.2 (1.04–4.92) | 0.04 | 1.85 (0.74 – 4.63) | 0.18 |
| 2 | 3.35 (1.41–7.95) | 0.006 | 2.71(0.79 – 9.29) | 0.11 |
| 3 | 3.54 (1.34–9.31) | 0.01 | 2.79 (0.79 – 9.81) | 0.11 |
| Comorbidity | ||||
| None | Ref | - | ||
| Mild | 1.25 (0.73–2.12) | 0.42 | 1.33 (0.72–2.44) | 0.36 |
| Moderate | 1.24 (0.67–2.31) | 0.5 | 1.31 (0.64–2.70) | 0.46 |
| Severe | 1.73 (0.78–3.82) | 0.17 | 1.99 (0.73–5.44) | 0.18 |
| Insurance | ||||
| Medicare +/− supplemental | Ref | - | ||
| Medicare managed care | 1.0 (0.50–1.99) | 0.99 | ||
| Medicaid | 1.74 (0.63–1.79) | 0.28 | ||
| Other | 0.66 (0.21–2.09) | 0.48 | ||
| Creatinine clearance | 1.0 (0.99–1.01) | 0.44 | ||
| Initial therapy | ||||
| Novel combination therapy | Ref | - | ||
| Novel agent + steroids | 1.19 (0.58–2.48) | 0.64 | ||
| Alkylating agents | 1.21 (0.56–2.68) | 0.62 | ||
| Durie salmon stage | ||||
| 1 | Ref | - | Ref | - |
| 2 | 1.60 (0.50–5.14) | 0.43 | 1.06 (0.31–3.59) | 0.93 |
| 3 | 2.56 (0.93–7.16) | 0.069 | 0.93 (0.30–2.86) | 0.9 |
Adjusted for other variables in the model and propensity to undergo transplant.
Figure 2.
Overall survival of 48 propensity-score matched pairs
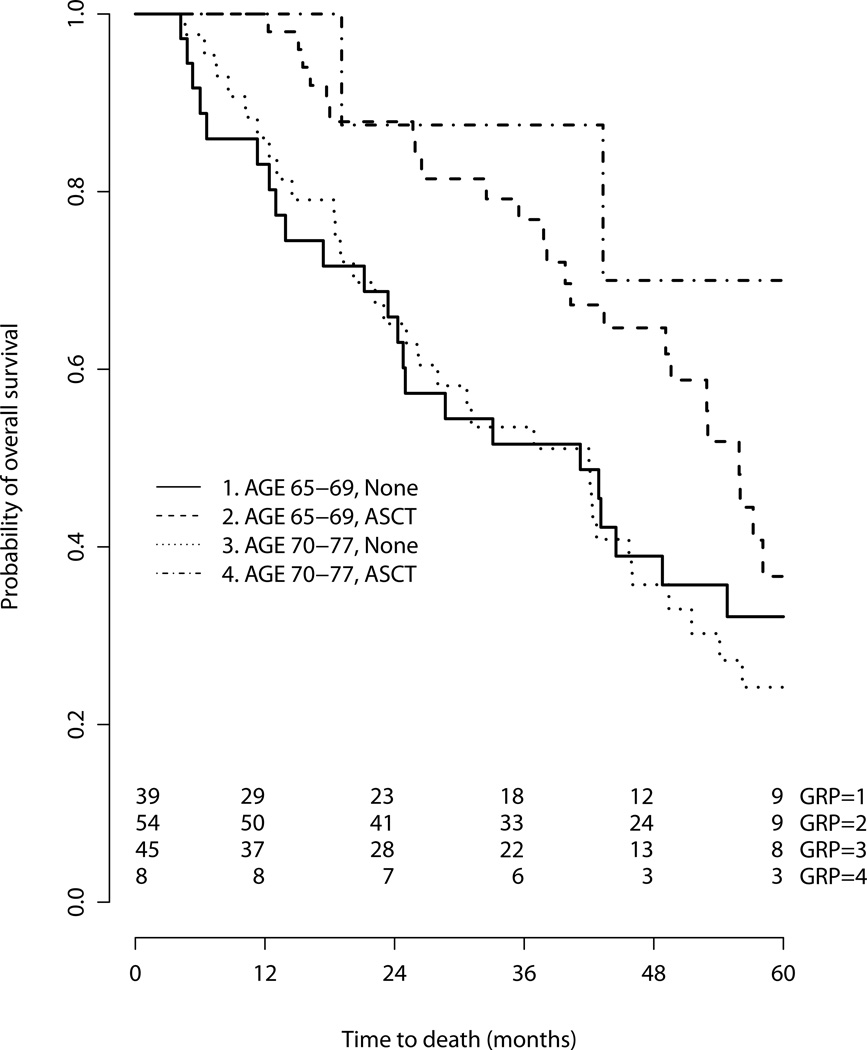
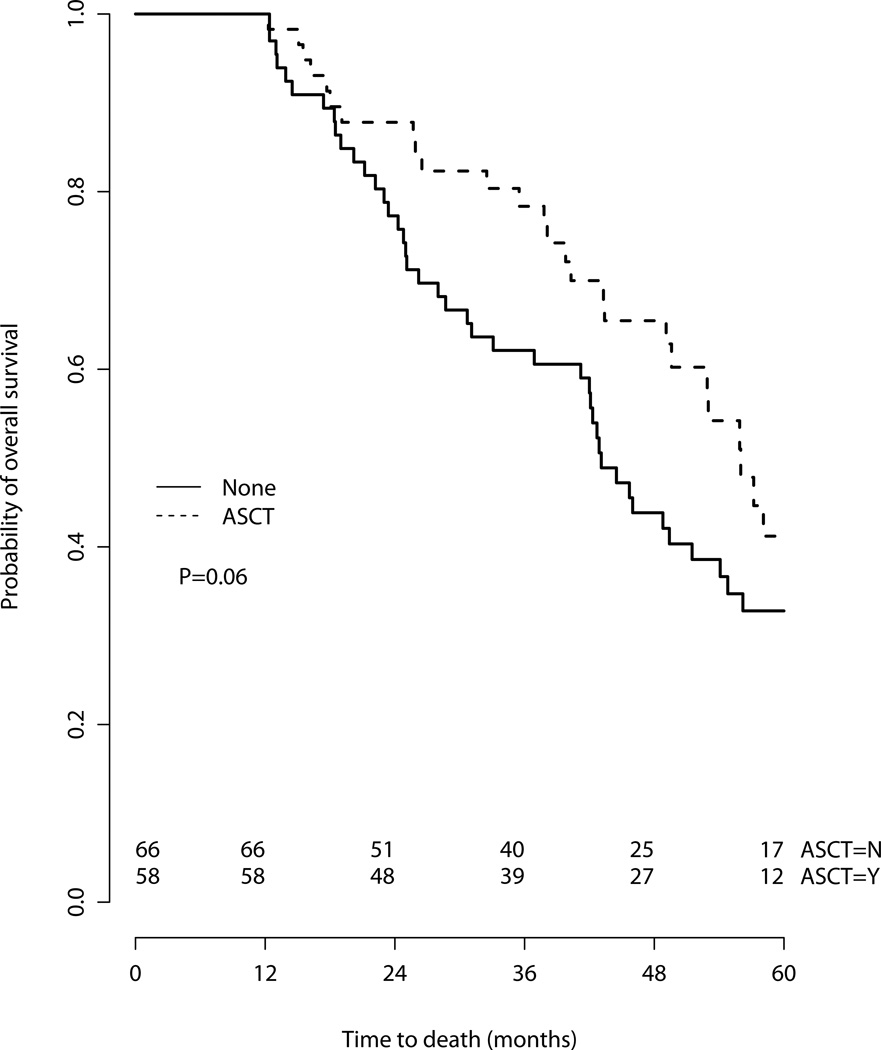
To interrogate our findings, we performed sensitivity analyses. First, we compared survival by treatment in the subgroups aged 65–69 and those aged over 70 (Figure 3), with similar results. ASCT was associated with superior overall survival in both subgroups, though the HR failed to meet significance in the strata of patients over age 70 [Age 65–69 HR for death 0.60 (95% CI 0.36–1.00), p=0.049); Age ≥70 HR 0.33 (95% CI 0.10–1.07), p=0.066]. Second, further attempt to mitigate immortal time bias and confounding by indication(19,20), we performed a 12-month landmark analysis, excluding patients with follow-up less than 1 year (Figure 4). On a multivariate analysis including performance status, comorbidity and age≥70, ASCT was still associated with a significantly superior survival [HR for death 0.49 (95% CI 0.25–0.94), p=0.03]. Finally, in multivariate analysis in which ASCT was treated as a time-dependent variable, ASCT maintained its association with superior survival [HR for death 0.44 (95% CI 0.25–0.78), p=0.0048].
Figure 3.
Overall survival, stratified by age group
Figure 4.
12-month landmark analysis, overall survival
Discussion
In this study, we show that, among adults over age 65 with MM, undergoing ASCT is associated with a nearly 50% reduction in the hazard ratio for mortality, after controlling for potential confounders including performance status, comorbidity, stage and propensity to undergo ASCT. This finding supports the potential utility of ASCT among older adults with MM who are deemed eligible for this treatment option.
Our findings add to the current literature regarding the benefit of ASCT among older adults, some of which is conflicting. While the superiority of ASCT over conventional therapy in younger adults with MM was established by randomized trials,(6,7) its utility among older adults is less well-established. A number of studies have compared outcomes of ASCT in older adults with MM to outcomes in younger patients and demonstrated similar response rates, progression-free survival and overall survival.(9,21–23) This line of evidence presupposes that if ASCT is superior to conventional therapy in younger adults, and the outcomes of ASCT are similar in younger and older patients, then ASCT is superior in older patients as well. However, other studies have suggested lower complete response rates and lower overall survival among older adults (Table 3).(11,24,25)
Table 3.
| Subgroups | Response rates | EFS/PFS/TTP | OS from transplant | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study design | Age group |
Sample size |
Melphalan dose | CR | P value |
Median(months) | P value | Median(months) | P value |
| Merz (21) | Retrospective cohort study |
60–64 | 83 | 100–200 mg/m2 | 38%(nCR+CR) | NS | EFS 27 | NS | Not reached | NS |
| 65–69 | 93 | 33%(nCR+CR) | EFS 23 | Not reached | ||||||
| 70–75 | 26 | 31%(nCR+CR) | EFS 23 | Not reached | ||||||
| Muta (11) | Retrospective cohort study |
51–64 | 63 | 100–200 mg/m2 | 24% | 0.06 | PFS 20.8 | 0.26 | 72.5 | 0.07 |
| 65–76 | 25 | 12% | PFS 17.1 | 40.8 | ||||||
| El Cheikh (9) | Retrospective cohort study |
60–65 | 104 | 100–200 mg/m2 | 48% CR or VGPR |
0.58 | PFS 45 | <0.0001 | 5 Yr OS 57% | NS |
| 65–77 | 82 | 41% CR or VGPR |
PFS 27 | 5Yr OS 54% | ||||||
| Kumar (22) | Matched pair analysis |
37–64 | 60 | 140–200 mg/m2 | 28% | NS | TTP 17.8 | 0.07 | 53.3 | NS |
| 70–75 | 33 | 42% | TTP 28.5 | Not reached | ||||||
| Gertz (23) | Retrospective cohort study |
≤65 | 541 | 140–200 mg/m2 | 30% | NS | TTP 17 | 0.09 | 44 | 0.28 |
| >65 | 137 | 40% | TTP 17 | 44 | ||||||
|
Jantunen (36) |
Retrospective cohort study |
39–64 | 79 | 200 mg/m2 | 36% | NR | PFS 21 | NS | 66 | NS |
| 65–73 | 22 | 44% | PFS 23 | 57 | ||||||
| Krejci (24) | Retrospective cohort study |
31–60 | 103 | 140–200 mg/m2 | NR | NR | 25.7 | 0.002 | ||
| 66–69 | 30 | NR | NR | 71 | ||||||
| Terpos (37) | Retrospective cohort study |
27–60 | 95 | 100–200 mg/m2; 140mg/m2+/−TBI |
NR | NR | 50.4 | 0.57 | ||
| 61–70 | 32 | NR | NR | 37.6 | ||||||
| Reece (38) | Registry | 30–59 | 382 | Several doses/regimens +/− TBI |
34% | NS | 3Yr PFS 44% |
NS | 3Yr OS 55% | NS |
| 60–73 | 110 | 33% | 3Yr PFS 35% |
3Yr OS 58% | ||||||
| O’Shea (39) | Retrospective cohort study |
26–60 | 151 | 100–200 mg/m2; 140mg/m2+/−TBI |
NR | NR | 50.9 | NS | ||
| 61–72 | 60 | NR | NR | 48.3 | ||||||
| Lenhoff (25) | Population- based registry |
<60 | 294 | 200mg/m2 | 36% | NS | EFS 36 | 0.005 | 67 | 0.004 |
| 60–64 | 120 | 37% | EFS 24 | 48 | ||||||
ASCT, autologous stem cell transplant; EFS, event-free survival; PFS, progression-free survival; OS, overall survival; CR, complete response; nCR, near complete response; VGPR, very good partial response; NS, nonsignificant; TTP, time to progresion; NR, not reported; TBI, total body irradiation.
Studies directly comparing outcomes among older adults who did undergo ASCT with older adults who did not are few, and similarly inconsistent (Table 4). In a randomized trial of melphalan and prednisone (MP) versus melphalan, prednisone and thalidomide (MPT) versus induction chemotherapy followed by intermediate-dose melphalan (MEL100) and ASCT, MPT produced similar complete response rates and superior overall survival compared to ASCT following MEL100.(26) However, this dose of melphalan is inferior to higher doses of melphalan(27), and does not refute the potential role of higher dose melphalan ASCT in older adults. In a population-based registry by the Nordic Myeloma Study Group, older adults (aged 60–64 in their cohort) who underwent ASCT had lower mortality than patients who did not, with a reduction in risk similar to that in our present study [Risk ratio 0.65 (95% confidence intervals 0.42–0.92), p=0.02].(25) In a recent cohort patients with MM diagnosed between 2001 and 2010, patients over age 65 who received ASCT experienced longer median overall survival compared to older patients who did not undergo ASCT [median OS not reached (95% confidence intervals 5.4 years – not reached) with ASCT compared with 3.1 years (95% CI 2.5, 3.7) for those who did not, P<0.01].(3) Finally, in a retrospective cohort study of 318 patients aged 65–70, including 38 who underwent ASCT, ASCT was associated with improved OS on univariate analysis but not on multivariate analysis.(28) Thus, further study is needed to clarify the role of ASCT in older adults in the era of modern therapy.
Table 4.
Studies of the efficacy and effectiveness of ASCT in older adults with multiple myeloma: Comparisons of autologous stem cell transplant and non-transplant treatment in older adults with multiple myeloma
| ASCT (N, age range) |
Non- ASCT (N, age range) |
Treatment | Median PFS (months) | Median OS (months) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCT group |
Non-ASCT group |
P value | ASCT group |
Non- ASCT group |
Hazard Ratio | P value | |||||
|
Present study |
Retrospective cohort study |
62 (65– 74) |
84 (65– 77) |
ASCT: Various induction, Mel 140– 200 mg/m2 Non-ASCT: Various |
NR | NR | 56 | 33.1 | 0.54 (95% CI 0.35–0.82) |
0.004 | |
|
Facon (IFM99- 06) (26) |
Randomized controlled trial |
N=126 | MPT arm N=125 MP arm N=196 |
ASCT: VAD × 2, chemomobilization, Melphalan 100mg/m2 , repeated 2 months later MPT: × 12 cycles MP: × 12 cycles |
19.4 | MPT: 27.5 MP: 17.8 |
P=0.0002 | 38.3 | MPT:51.6 MP: 33.2 |
NR | MPT vs MEL100 P=0.027 |
| Lenhoff(25) | Population- based registry |
120 (60– 64) |
97 (60– 64) |
ASCT: VAD then ASCT and IFN alfa- 2B maintenance Non-ASCT: MP +/− IFN alfa-2B |
EFS 24 |
NR | P=0.02 | 48 | 28 | Risk ratio 0.65, (95% CI 0.42–0.92) |
0.02 |
|
Offidani (40) |
Post-hoc analysis of Phase II trial |
26 (65– 75) |
62 (65– 91) |
Non-ASCT: ThaDD × 6 cycles, then maintenance thalidomide ASCT: ThaDD × 4 then ASCT |
32 | 29 | NS | 3Yr OS 82% 5Yr OS 49% |
3Yr OS 66% 5Yr OS 46% |
NR | NS |
|
Kumar (3) |
Retrospective cohort study |
1038 (52% over age 65) |
Various | NR | NR | Not reached (95%CI 5.4 years –not reached) |
3.1 years (95% CI 2.5–3.7) |
NR | <0.01 | ||
|
Ozaki (28) |
Retrospective cohort study |
N=17 ASCT + novel agents; N=21 conventional chemotherapy +ASCT (Age 65– 70) |
N=192 conventional chemotherapy; N=88 novel agents (Age 65– 70) |
Various | Not reached |
57.9 months |
<0.001 | ||||
ASCT, autologous stem cell transplant; PFS, progression-free survival; OS, overall survival; NS, nonsignificant; NR, not reported; MPT, melphalan, prednidone and thalidomide; MP, melphalan and prednisone; VAD, vincristine, doxorubicin and dexamethasone; IFN, interferon; ThaDD, thalidomide, pegylated liposomal doxorubicin and dexamethasone.
There are a number of limitations to our study. First, as an observational study, there are a number of potential confounders. Patients were selected for ASCT by clinicians who incorporate multiple facets of an older adult’s health into the decision, such as laboratory values, comorbidities, performance status and patient preference. We attempted to control for differences in the populations who did versus did not undergo ASCT by controlling for performance status, comorbidity, stage and propensity to undergo ASCT in the multivariate model of survival and still saw a benefit associated with ASCT. Further, we saw persistence of the improvement in OS in a 12-month landmark analysis and treating ASCT as a time-dependent variable. It is possible that the survival benefit seen among the patients who underwent ASCT is related to residual confounding by additional factors which are associated with treatment allocation and directly impact prognosis, but unmeasured in our study: for example, functional status, as measured by scales such as the Katz Activities of Daily Living(29) or the Lawton Instrumental Activities of Daily Living (IADL) Scale(30). Dependence in IADLs is predictive of chemotherapy toxicity(31,32); since toxicity of therapy is associated with poorer survival in older adults with myeloma,(33) imbalances in geriatric assessment parameters such as functional status between the groups may explain the differences in survival seen in our study, rather than ASCT. Future studies that comprehensively evaluate the health of older adults with MM, including common geriatric syndromes such as functional dependence, impaired cognition and social support, are needed to ensure analyses to control for differences in the populations who undergo different treatment strategies.
In our analysis cohort, which was restricted to older adults who survived 4 months after diagnosis (and thus would have potentially been eligible for ASCT) and those under age 78, comorbidities were not independently associated with survival. Kleber et al developed a comorbidity index which is prognostic in MM, independent of International Staging System (ISS) stage.(34,35) The comorbidity index developed by Kleber et al includes the Karnofsky performance status (KPS) ≤70% as an independent prognostic factor; ECOG performance status was associated with survival in our model on univariate, but not multivatiate analysis. In our model, we employed the ACE-27 comorbidity index, which does not include performance status. The lack of prognostic power of comorbidities in this cohort may simply reflect the relatively small samples size, or that the discriminatory power of the ACE-27 comorbidity index is limited when the cohort is restricted to patients who survived 4 months after diagnosis (i.e. there were only 13 patients categorized as having severe comorbidities).
Another limitation of our study is the lack of disease-specific prognostic data, including ISS stage, cytogenetics and response to initial therapy. Given that we included cases from 2000–2010, prior to the promulgation of the ISS stage, many patients did not have data on beta-2-microglobulin to allow calculation of the ISS stage.(16) In addition, data on cytogenetics and fluorescence in situ hybridization for specific chromosomal abnormalities were frequently not available and could not be included in the survival model. It is possible that there were differences in the biology of disease that could explain the observed differences in survival, such that those with more aggressive biology of disease were less likely to undergo ASCT.
In conclusion, in a cohort of patients with multiple myeloma over the age of 65, undergoing ASCT was associated with superior survival, with a nearly 50% lower risk of mortality after controlling for comorbidities, performance status, stage and propensity to undergo ASCT. We recognize the limitations of a retrospective cohort study in examining all of the factors associated with treatment selection, which may confound the association between treatment and outcomes. Future studies must focus on prospectively incorporating greater detail on disease characteristics, functional status and other geriatric assessment parameters in order to further perform comparative effectiveness research to clarify the role of ASCT in older adults with multiple myeloma.
Acknowledgements
This publication was made possible by Grant Numbers KM1CA156708 and K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH. The authors also wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Conflict of Interest
The authors have no relevant conflicts of interest.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. Journal of Clinical Oncology. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Improvement in Survival of Older Adults with Multiple Myeloma: Results of an Updated Period Analysis of SEER Data. Oncologist. 2011;16(11):1600–1603. doi: 10.1634/theoncologist.2011-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Klepin HD, Hurd DD. Autologous transplantation in elderly patients with multiple myeloma: are we asking the right questions? Bone Marrow Transplantation. 2006;38(9):585–592. doi: 10.1038/sj.bmt.1705486. [DOI] [PubMed] [Google Scholar]
- 6.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 7.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 8.Roncon S, Barbosa IL, Campilho F, Lopes SM, Campos A, Carvalhais A. Mobilization and collection of peripheral blood stem cells in multiple myeloma patients older than 65 years. Transplant Proc. 2011;43(1):244–246. doi: 10.1016/j.transproceed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Cheikh ElJ, Kfoury E, Calmels B, Lemarie C, Stoppa A-M, Bouabdallah R, et al. Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther. 2011;4(1):30–36. doi: 10.5144/1658-3876.2011.30. [DOI] [PubMed] [Google Scholar]
- 10.Bashir Q, Shah N, Parmar S, Wei W, Rondon G, Weber DM, et al. Leuk Lymphoma. 1. Vol. 53. London: Informa Healthcare; 2012. Feasibility of autologous hematopoietic stem cell transplant in patients aged≥ 70 years with multiple myeloma; pp. 118–122. [DOI] [PubMed] [Google Scholar]
- 11.Muta T, Miyamoto T, Fujisaki T, Ohno Y, Kamimura T, Kato K, et al. Evaluation of the feasibility and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Intern Med. 2013;52(1):63–70. doi: 10.2169/internalmedicine.52.8390. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo JF, Creech CM, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Registry Manage. 1999;26(2):66–70. [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 14.Launay-Vacher V, Chatelut E, Lichtman SM, Wildiers H, Steer C, Aapro M, et al. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Annals of oncology. 2007;18(8):1314–1321. doi: 10.1093/annonc/mdm011. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Greipp PR. International Staging System for Multiple Myeloma. Journal of Clinical Oncology. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 17.Little R, Rubin DB. Statistical Analysis with Missing Data. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 18.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 19.Liu J, Weinhandl ED, Gilbertson DT, Collins AJ, St Peter WL. Issues regarding “immortal time” in the analysis of the treatment effects in observational studies. Kidney Int. 2012;81(4):341–350. doi: 10.1038/ki.2011.388. [DOI] [PubMed] [Google Scholar]
- 20.Boyko EJ. Observational research — opportunities and limitations. Journal of Diabetes and its Complications. 2013;27(6):642–648. doi: 10.1016/j.jdiacomp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merz M, Neben K, Raab MS, Sauer S, Egerer G, Hundemer M, et al. Autologous stem cell transplantation for elderly patients with newly diagnosed multiple myeloma in the era of novel agents. Annals of Oncology. 2014;25(1):189–195. doi: 10.1093/annonc/mdt509. [DOI] [PubMed] [Google Scholar]
- 22.Kumar SK, Dingli D, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol. 2008;83(8):614–617. doi: 10.1002/ajh.21191. [DOI] [PubMed] [Google Scholar]
- 23.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S, Leung N, et al. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplantation. 2007;39(10):605–611. doi: 10.1038/sj.bmt.1705627. [DOI] [PubMed] [Google Scholar]
- 24.Krejci M, Buchler T, Hajek R, Svobodnik A, Krivanova A, Pour L, et al. Autologous bone marrow transplantation in multiple myeloma: a single centre experience of 23 patients. Bone Marrow Transplantation. 2005;35(2):159–164. doi: 10.1038/sj.bmt.1704728. [DOI] [PubMed] [Google Scholar]
- 25.Lenhoff S, Hjorth M, Westin J, Brinch L, Backstrom B, Carlson K, et al. Impact of age on survival after intensive therapy for multiple myeloma: a population-based study by the Nordic Myeloma Study Group. British Journal of Haematology. 2006;133(4):389–396. doi: 10.1111/j.1365-2141.2006.06042.x. [DOI] [PubMed] [Google Scholar]
- 26.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. The Lancet. 2007;370(9594):1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M, et al. Melphalan 200 mg/m(2) versus melphalan 100 mg/m(2) in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. 2010;115(10):1873–1879. doi: 10.1182/blood-2009-09-241737. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki S, Harada T, Saitoh T, Shimazaki C, Itagaki M, Asaoku H, et al. Survival of Multiple Myeloma Patients Aged 65–70 Years in the Era of Novel Agents and Autologous Stem Cell Transplantation. A Multicenter Retrospective Collaborative Study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014;132(2):211–219. doi: 10.1159/000357394. [DOI] [PubMed] [Google Scholar]
- 29.Katz S, Ford AB, Moskowitz R, Jackson B, Jaffe M. Studies of Illness in the Aged: The Index of ADL. JAMA: The Journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 30.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–986. [PubMed] [Google Scholar]
- 31.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of Clinical Oncology. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 33.Bringhen S, Mateos M-V, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–987. doi: 10.3324/haematol.2012.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleber M, Ihorst G, Terhorst M, Koch B, Deschler B, Wäsch R, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM–comorbidity score. Blood Cancer Journal. 2011;1(9):e35–e38. doi: 10.1038/bcj.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleber M, Ihorst G, Groß B, Koch B, Reinhardt H, Wäsch R, et al. Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk. 2013;13(5):541–551. doi: 10.1016/j.clml.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Jantunen E, Kuittinen T, Penttilä K, Lehtonen P, Mahlamäki E, Nousiainen T. High-dose melphalan (200 mg/m2) supported by autologous stem cell transplantation is safe and effective in elderly (> 65 years) myeloma patients: comparison with younger patients treated on the same protocol. Bone Marrow Transplantation. 2006;37(10):917–922. doi: 10.1038/sj.bmt.1705360. [DOI] [PubMed] [Google Scholar]
- 37.Terpos E, Apperley JF, Samson D, Giles C, Crawley C, Kanfer E, et al. Autologous stem cell transplantation in multiple myeloma: improved survival in nonsecretory multiple myeloma but lack of influence of age, status at transplant, previous treatment and conditioning regimen. A single-centre experience in 127 patients. Bone Marrow Transplantation. 2003;31(3):163–170. doi: 10.1038/sj.bmt.1703818. [DOI] [PubMed] [Google Scholar]
- 38.Reece DE, Bredeson C, Pérez WS, Jagannath S, Zhang M-J, Ballen KK, et al. Autologous stem cell transplantation in multiple myeloma patients /=60 years of age. Bone Marrow Transplantation. 2003;32(12):1135–1143. doi: 10.1038/sj.bmt.1704288. [DOI] [PubMed] [Google Scholar]
- 39.O'Shea D, Giles C, Terpos E, Perz J, Politou M, Sana V, et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transplantation. 2006;37(8):731–737. doi: 10.1038/sj.bmt.1705307. [DOI] [PubMed] [Google Scholar]
- 40.Offidani M, Leoni P, Corvatta L, Polloni C, Gentili S, Savini A, et al. ThaDD plus high dose therapy and autologous stem cell transplantation does not appear superior to ThaDD plus maintenance in elderly patients with de novo multiple myeloma. European Journal of Haematology. 2010;84(6):474–483. doi: 10.1111/j.1600-0609.2010.01418.x. [DOI] [PubMed] [Google Scholar]