Abstract
Background
Predictors of venous thromboembolism (VTE) recurrence are uncertain.
Objective
To identify predictors of VTE recurrence, adjusted for treatments and interim exposures.
Materials and Methods
Using Rochester Epidemiology Project resources, all Olmsted County, MN residents with objectively-diagnosed incident VTE over the 13-year period, 1988–2000, who survived ≥1 day were followed for first objectively-diagnosed VTE recurrence. For all patients with recurrence, and a random sample of all surviving incident VTE patients (n=415), we collected demographic and baseline characteristics, treatments and interim exposures. In a case-cohort study design, demographic, baseline, treatment and interim exposure characteristics were tested as potential predictors of VTE recurrence using time-dependent Cox proportional hazards modeling.
Results
Among 1262 incident VTE patients, 306 developed recurrence over 6,440 person-years. Five-year recurrence rates, overall and for cancer-associated, idiopathic and non-cancer secondary VTE, were 24.5%, 43.4%, 27.3% and 18.1%, respectively. In multivariable analysis, interim hospitalization, active cancer, pregnancy, central venous catheter and respiratory infection were associated with increased hazards of recurrence, and warfarin and aspirin were associated with reduced hazards. Adjusting for treatments and these interim risk factors, male sex, baseline active cancer and failure to achieve a therapeutic aPTT in the first 24 hours were independently associated with increased hazards of VTE recurrence over the entire follow-up period, while the hazards of recurrence for patient age, chronic lung disease, leg paresis, prior superficial vein thrombosis and idiopathic VTE varied over the follow-up period.
Conclusions
Baseline and interim exposures can stratify VTE recurrence risk and may be useful for directing secondary prophylaxis.
Keywords: epidemiology, pulmonary embolism, recurrence, thrombophlebitis, venous thrombosis
INTRODUCTION
Venous thromboembolism, consisting of deep vein thrombosis (DVT) and its complication, pulmonary embolism (PE), is a chronic disease with episodic recurrence; anticoagulation therapy treats the acute thrombotic episode but does not cure the underlying predisposition to VTE.[1–4] Current recommendations are to treat acute VTE for about three months;[3, 4] after three months, the aim of any continuing antithrombotic therapy is to prevent VTE recurrence (i.e., secondary prophylaxis).[2, 4] Secondary prophylaxis should be continued as long as the risk of recurrent VTE (particularly recurrent idiopathic fatal PE) exceeds the risk of anticoagulant-associated bleeding.[2–4] The 10-year overall VTE cumulative recurrence rate is about 30%.[1, 5] To aid in identifying those patients at increased risk for VTE recurrence, baseline characteristics that are independent predictors of recurrence (adjusting for time-dependent anticoagulation treatment) have been identified[1, 6, 7] and several recurrence risk prediction tools for idiopathic incident VTE have been derived.[8, 9, 10–12] However, none of these studies accounted for new (interim) exposures after the incident VTE event that may be risk factors for VTE recurrence. Whether previously identified baseline characteristics remain predictors of recurrence after adjusting for treatments and interim exposures is unknown. To address this important gap in knowledge, we performed a population-based case-cohort study to identify interim exposures that are independent risk factors for VTE recurrence, and to test baseline characteristics as potential predictors for recurrence after adjusting for treatments and interim exposures.
MATERIALS AND METHODS
Study Setting, Population and Design
Using the resources of the Rochester Epidemiology Project (REP; see APPENDIX),[13–15] we identified the inception cohort of all Olmsted County, Minnesota residents with incident deep vein thrombosis (DVT) or pulmonary embolism (PE) over the 35-year period, 1966–2000, as previously described.[16] For this study, we restricted our analyses to residents with objectively-diagnosed, incident VTE (see APPENDIX for definitions) over the 13-year period, 1988–2000, who lived at least one day after the incident VTE event. We followed each case from the onset of incident VTE symptoms or signs forward in time using their complete (inpatient and outpatient) medical records in the community for first objectively-diagnosed VTE recurrence, death or 12/31/05, whichever came first.[1, 6] Recurrent VTE was defined as thrombosis of a venous site that was either previously uninvolved or had documentation of incident thrombus resolution. For deceased patients, all death certificates and autopsy reports were reviewed regardless of the location at death. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Using REP resources, we performed a case-cohort study.[17] The case-cohort design incorporates an efficient sampling technique to provide the research capabilities of a large cohort study without the expense of data collection for the entire cohort.[18] This is accomplished by selecting all recurrent cases in the incident VTE cohort and by randomly sampling the entire incident VTE cohort who lived at least one day, regardless of recurrence status or follow-up time, to create a sub-cohort. The sub-cohort is a representative sample of the original cohort and thus is useful for describing characteristics of incident VTE subjects and for analyzing the association of exposures with recurrence since this sample also serves as the source of comparison for the full set of recurrent cases.
Measurements
Using explicit criteria, trained and experienced nurse abstractors reviewed all medical records in the community for consenting cases from date first seen by a REP healthcare provider until the earliest of death, date of VTE recurrence, date of last medical record follow-up, or 12/31/2005 as previously performed.[1, 6] Data were collected on date and type of incident VTE, baseline characteristics, dates of heparin and warfarin initiation and completion, IVC filter placement, date and type of first VTE recurrence, vital status at last clinical contact, major bleeding on anticoagulation,[6] and baseline characteristics (see APPENDIX), as described in detail elsewhere.[1, 6, 19]
For all patients with VTE recurrence and a random sample of all patients with incident VTE (including those with recurrence), 1988–2000 (subcohort n=415; incident VTE cases without recurrence n=307),[20] we collected treatments (regardless of indication, including standard heparin, low molecular weight heparin and warfarin [including proportion of time in therapeutic range],[6] IVC filter, aspirin [≥100 mg per day], lipid-lowering therapy), primary VTE prophylaxis, and new exposures occurring after the incident VTE event date and dates of those exposures, including hospitalization for surgery (general, cardiac, orthopedic or gynecological surgery; neurosurgery); hospitalization for acute medical illness; trauma and/or fracture requiring hospital admission; new active cancer (excluding non-melanoma skin cancer), including cancer stage, stage progression, cancer metastasis and multiple cancers; cancer therapy (including type and dates of therapy); serious neurologic disease with leg paresis; infection and infection location; a new diagnosis of hyperlipidemia or autoimmune disease; and for women, pregnancy, oral contraceptive, estrogen therapy and progestin therapy. The sub cohort sample size was chosen to maximize the asymptotic relative efficiency (ARE) while minimizing the size of the cohort so as to minimize the cost of data collection. With 306 recurrent VTE events, a sub cohort of n=415 provides an ARE=0.64.
Statistical Analyses
Descriptive statistics were reported as percentage (count), mean (standard deviation [SD] or median (interquartile range [IQR]) for baseline data as appropriate, or as rate of cumulative incidence (via the Kaplan-Meier estimator) for VTE recurrence. The association of candidate predictor variables with VTE recurrence was analyzed with multivariable Cox proportional hazards (PH) regression using the counting process style of input, which utilizes a time-dependent data structure with follow-up of an individual partitioned into a number of intervals with non-overlapping start and stop days.[21] Baseline exposures were specified as fixed covariates in the regression modeling, whereas interim exposures were expressed as a function of follow-up time and analyzed as time-dependent variables. To assign an appropriate risk interval after onset of an interim cancer (any new diagnosis or recurrence of cancer occurring >92 days after the incident VTE event date), we derived an algorithm based on cancer type, stage, treatment and progression that predicted length of time over follow-up a cancer remained active (see Appendix). Patients were classified as rapidly achieving a therapeutic APTT if the first APTT within 24±4 hours of starting heparin was therapeutic (≥58 seconds), or as failing to achieve this if the value was sub-therapeutic; an additional category indicating incomplete data (those who never received standard heparin or only received LMW heparin, or those who received standard heparin but had no APTT values within 24±4 hours of starting heparin) in order to retain all subjects in the regression analysis (see Appendix). Although all levels of this variable were tested simultaneously (i.e., 2 d.f. test) for an association with recurrence, the final interpretation was based on the individual pairwise contrast of those who attained vs. failed to rapidly achieve a therapeutic APTT.
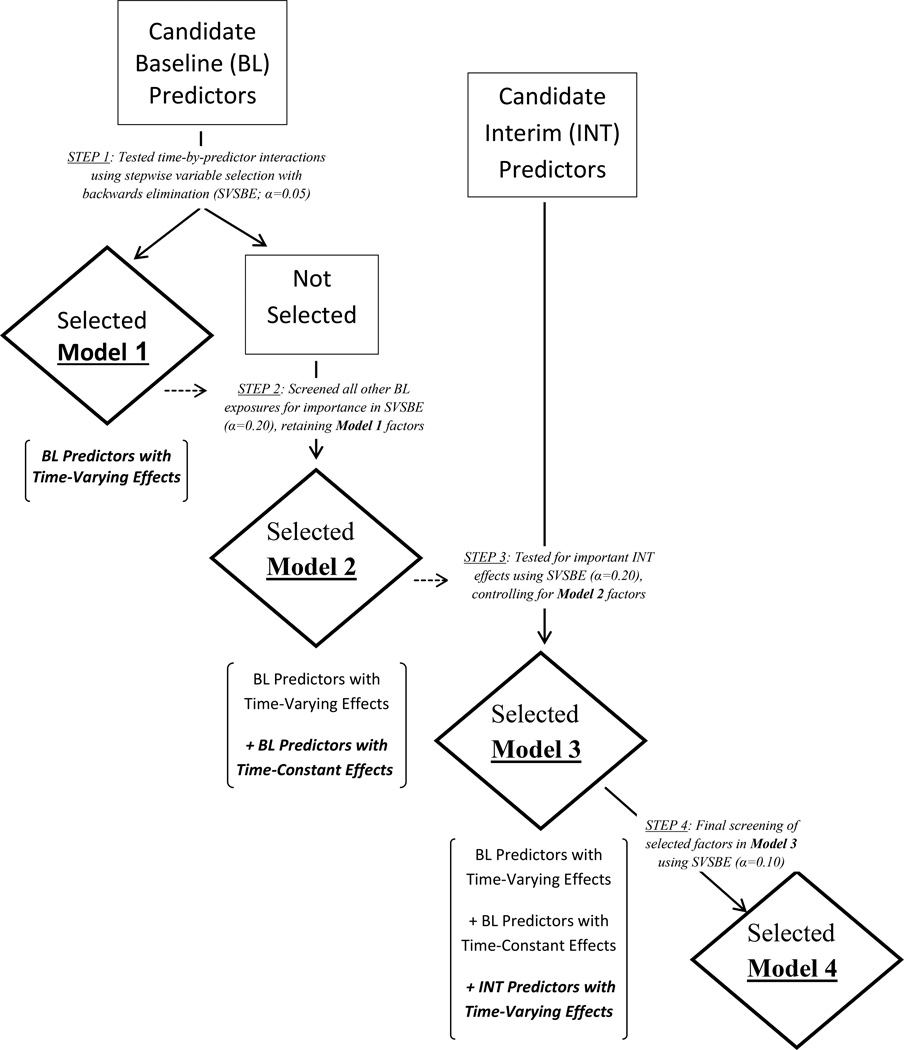
Although baseline characteristics were coded with fixed values that were constant over time, the model assumption of a constant proportional effect over time (i.e., PH assumption) was tested for validity for each characteristic using both graphical and analytical methods. In particular, the log hazard ratio for each baseline predictor was plotted over follow-up time based on weighted Schoenfeld residuals from a fitted Cox model. Visual inspection of these plots suggested certain baseline predictors had changing effects on the hazard function, with changes most often evident roughly three months and/or one year after the index VTE event date (see Appendix Figure). To further assess the importance of baseline predictors as a function of time, a time-dependent variable distinguishing three follow-up intervals (as guided by the Appendix Figure plots: 0–92 days, 93–365 days, and >365 days) was used to construct time-by-predictor interactions in Cox regression. Formal tests for PH were then derived by testing the interaction coefficients for significance in multivariable modeling using stepwise variable selection with backwards elimination (alpha level of 0.05). The non-PH baseline predictors retained in this model thus express the relative hazard of VTE recurrence as a step function of time, constant only within each interval. However, since the time-varying effects might be reasonably summarized as a contrast of two rather than all three time intervals, a post hoc analysis testing the adequacy of a simpler two-interval form of time in the interaction was performed for each non-PH variable using likelihood ratio (LR) tests with the two-interval version preferred if the LR result was non-significant. The selected model of non-PH baseline predictors was re-fit using the final choices of time interval by predictor interactions (Figure 1, “Model 1”).
Figure 1.
Study analyses flow diagram.
Controlling for the non-PH predictors from Model 1, the remaining set of baseline exposures were then entered as additive terms in a multivariable model and screened for an association with VTE recurrence using backwards elimination at an alpha level of 0.20 (note a less stringent selection criteria was used here to allow the possibility that a baseline effect with a marginal signal might become significant while adjusting for interim exposures in the succeeding models). The resulting model (Figure 1, “Model 2”) was the union of baseline exposures with a potentially marginal to significant proportional effect, either as a function or independent of follow-up time intervals, on the hazard function of VTE recurrence. To expand beyond baseline predictors, a new stepwise model (Figure 1, “Model 3”) was derived based on screening all time-dependent interim exposures and discarding the insignificant effects (again using backwards variable selection and an alpha level of 0.20), under the constraint that all baseline terms in the preceding model were retained unconditionally at each step of the stepwise process. Lastly, for the sake of reporting a more parsimonious final model, all baseline and interim predictors in the preceding model were subjected to further screening via stepwise variable selection with backwards elimination but using a more stringent alpha level of 0.10 (Figure 1, “Model 4”).
For descriptive and univariable analyses, incident VTE was classified as non-idiopathic, either due to cancer or secondary (non-cancer) causes, or idiopathic (see Appendix);[19] this baseline characteristic was, however, not included in the initial multivariable analyses since the individual exposures that define non-idiopathic VTE were each assessed as prognostic factors in their own right. Note that if the entire set of baseline exposures that classify a non-idiopathic VTE are included as predictor variables in a full multivariable model, the reference group becomes those with idiopathic VTE, thereby preventing estimation of an idiopathic effect itself. However, since these baseline effects were screened for importance using backwards variable selection, it was possible to define the reference group as those whose incident VTE was explained by the insignificant factors removed in the stepwise procedure, thus permitting the estimation of an idiopathic VTE effect. The baseline variable selection described above (corresponding to Models 1 and 2) was performed initially on all baseline variables except idiopathic VTE, and then repeated with the indicator variable for idiopathic VTE also allowed to compete as a potential predictor of recurrence. We emphasized the latter analysis which showed that idiopathic VTE contained prognostic value concerning recurrence, and since non-idiopathic results from the modeling with and without the inclusion of idiopathic VTE did not differ appreciably. All analyses were performed using the statistical programming language SAS, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Over the 13-year period, 1988–2000, 1402 Olmsted County residents developed an incident DVT and/or PE, 1262 of whom survived ≥ one day after their incident VTE onset and were included in the analyses; the demographic and baseline characteristics of these patients are shown in Table 1. Of the 1262 VTE cases, the distribution by VTE event type was 490 PE with or without (±) DVT and 772 DVT alone (662 leg DVT [9.3% isolated distal leg DVT], 80 arm DVT, 27 abdominal vein thrombosis, and 3 inferior vena cava [IVC] thrombosis); 1030 (82%), 270 (21%) and 1076 (85%) received standard heparin (subcutaneous heparin, n=93 or 7%), low molecular weight heparin and warfarin therapy, respectively. The 128 patients not receiving heparin were more likely to have neurological disease, stage 4 cancer, liver disease or recent neurosurgery, and less likely to have an infection or chronic lung disease or receive warfarin. Of these 128 patients, 33 (29%) received an IVC filter. Among the 1181 patients who received anticoagulation therapy, 22 developed major bleeding[6] (20 within the first six months of anticoagulation); 30-, 90- and 180-day, one- and five-year major bleeding rates were 1.5%, 1.6%, 1.9%, 2.5% and 2.5%, respectively.
Table 1.
Baseline Demographic, and Baseline and Interim* Clinical Characteristics, of Olmsted County, MN Residents with Incident Deep Vein Thrombosis or Pulmonary Embolism, 1988–2000, Surviving ≥One Day after the Incident Venous Thromboembolism Event
| Characteristic | VTE Incidence Cohort Surviving ≥One Day (n=1262) |
Subcohort (n=415; includes 108 with VTE recurrence) |
|
|---|---|---|---|
| Baseline | Baseline | Interim | |
| Patient age at incident deep vein thrombosis or pulmonary embolism, years; mean ± SD (range) | 63.9±19.0 | 63.9±19.5 | – |
| Male sex, n (%) | 567 (45%) | 178 (43%) | – |
| Body mass index (BMI; kg/m2); mean ± SD | 28.2±7.0 | 28.0±7.5 | – |
| Incident PE±DVT; n (%) | 486 (39%) | 153 (37%) | – |
| Classification of Incident VTE, n (%) | |||
| Idiopathic | 295 (23%) | 85 (20%) | – |
| Active cancer | 284 (23%) | 107 (26%) | – |
| Other secondary | 683 (54%) | 223 (54%) | – |
| Major VTE Risk Factors | |||
| Hospitalization for surgery | 336 (27%) | 115 (28%) | 126 (30%) |
| Hospitalization for acute medical illness | 420 (33%) | 140 (34%) | 347 (84%) |
| Nursing home confinement | 120 (10%) | 48 (12%) | |
| Active cancer | 291 (23%) | 107 (26%) | 33 (8%) |
| Trauma/fracture | 164 (13%) | 63 (15%) | 61 (15%) |
| Neurological disease with leg paresis | 86 (7%) | 31 (7%) | 17 (4%) |
| Superficial vein thrombosis | 186 (15%) | 62 (15%) | |
| Central venous catheter | 137 (11%) | 35 (8%) | 60 (14%) |
| Transvenous pacemaker | 32 (3%) | 11 (3%) | 13 (3%) |
| Pregnancy or postpartum | 27 (2%) | 7 (2%) | 6 (1%) |
| Oral contraceptive | 61 (5%) | 20 (5%) | 1 (<1%) |
| Estrogen therapy | 128 (10%) | 43 (10%) | 20 (5%) |
| Progestin therapy | 96 (8%) | 33 (8%) | 16 (4%) |
| Congestive heart failure‡; n (%) | 205 (16%) | 67 (16%) | |
| Chronic lung disease; n (%) | 237 (19%) | 78 (19%) | |
| Chronic liver disease; n (%) | 13 (1%) | 2 (<1%) | |
| Chronic renal disease; n (%) | 40 (3%) | 18 (4%) | |
| Diabetes mellitus; n (%) | 144 (11%) | 44 (11%) | |
| Any infection | 456 (36%) | 161 (39%) | 294 (71%) |
| Respiratory infection | 179 (14%) | 67 (16%) | 176 (42%) |
| Urinary tract infection | 165 (13%) | 57 (14%) | 149 (36%) |
| IVC filter | 3 (<1%) | 1 (<1%) | 38 (9%) |
Interim characteristic is a continuing or new exposure occurring from ≥one day after the incident VTE event date to the VTE recurrence date, death or immigration, or 12/31/2005, whichever came first.
Hospitalization for surgery or for acute medical illness, nursing home confinement, trauma/fracture, pregnancy/postpartum and oral contraceptive/hormone therapy had to be documented as present in the three months prior to the incident venous thromboembolism date. Active cancer had to be documented in the three months before or after the incident VTE event.
Neurological disease with leg paresis, congestive heart failure and chronic lung, liver or renal disease had to be documented as present at any time prior to the incident venous thromboembolism date.
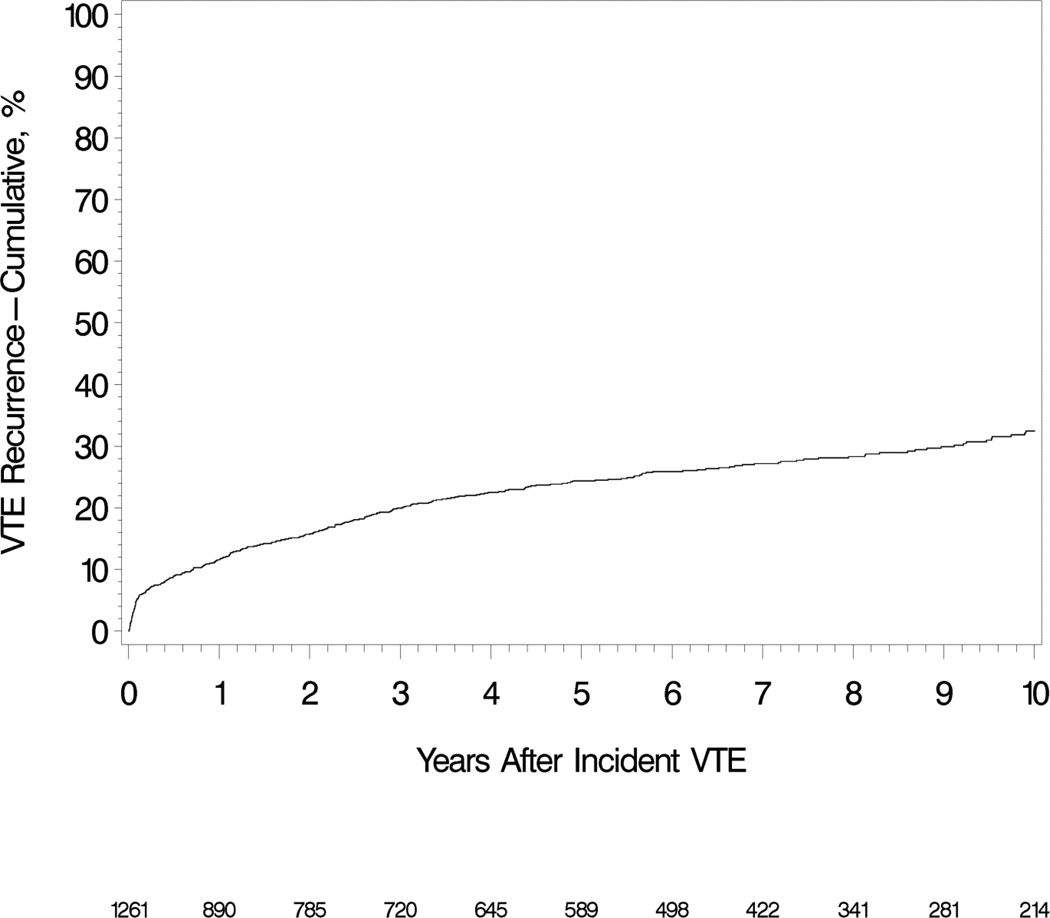
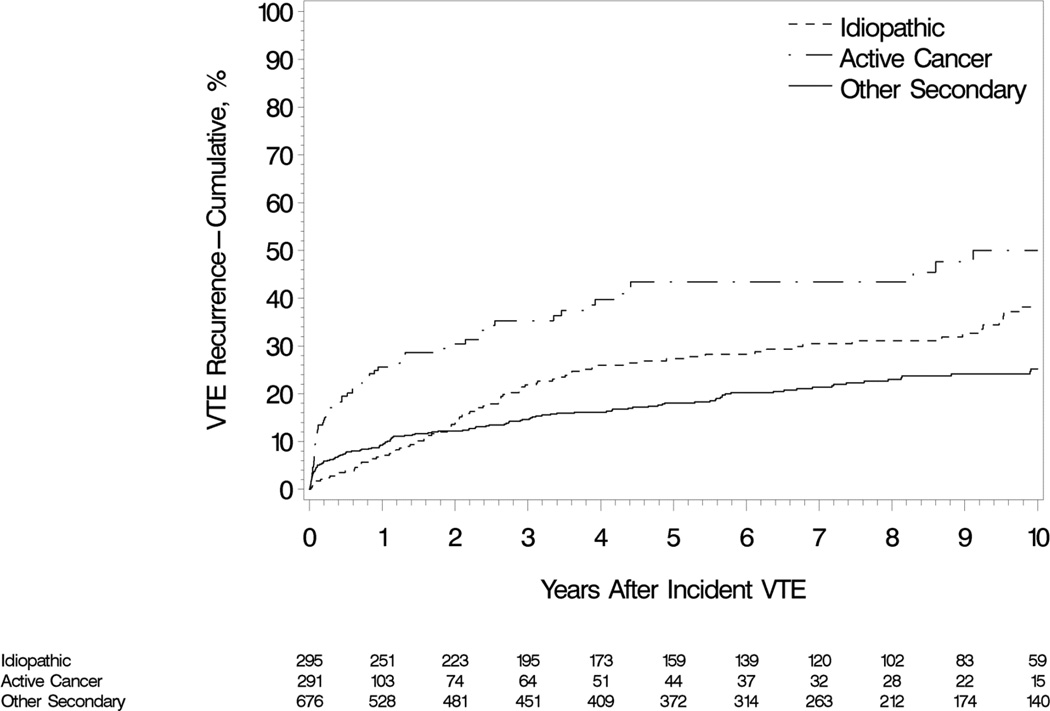
Over 6,440 person-years of follow-up, 306 patients developed recurrent VTE (i.e., a rate of 4.8 per 100 person-years). The 30-day, 180-day, one and five year cumulative recurrence rates were 4.8%, 8.9%, 11.6% and 24.5%, respectively (Figure 1). Of the VTE recurrences, 122 (40%) were PE ± DVT, and 184 (60%) were DVT alone. The 5-year cumulative recurrence rates among patients with incident cancer-associated,[7] idiopathic and secondary non-cancer VTE were 43.4%, 27.3% and 18.1%, respectively (Figure 2). There were 185 deaths over the 6,440 person-years of follow-up; 30- and 60-day, one- and five-year survival were 94.3%, 91.3%, 90.2% and 85.2%, respectively (mortality =2.87 [95%CI: 2.47, 3.32] per 100 person-years). Allowing for the competing risk of death, the five year cumulative recurrence rates, both overall and for cancer-associated, idiopathic and secondary non-cancer incident VTE were 22.2%, 33.8%, 26.2% and 16.8%, respectively.
Figure 2.
Cumulative incidence of venous thromboembolism recurrence among Olmsted County, Minnesota residents who survived ≥1 day after an incident deep vein thrombosis or pulmonary embolism over the 13-year period, 1988–2000, followed through 12/31/2005.
Among the random subcohort of 415 incident VTE cases, 383 (92%) and 362 (87%) received heparin and warfarin therapy for a median (inter quartile range [IQR]) 6 (5–12) and 128 (69–225) days, respectively. Among those in the subcohort receiving heparin with at least one APTT measured (n=338), the median (IQR) duration of heparin therapy with therapeutic APTT was 4 (2–5) days. The median (IQR) time from VTE onset to start of heparin therapy was 2 (0–4) days, and of overlapping heparin and warfarin was 5 (3–6) days. The time interval from start of heparin to start of warfarin decreased in later calendar years (Spearman correlation coefficient ρ=−0.18; p<.001), while the duration of overlapping heparin and warfarin increased (p=0.12 and p=0.028, respectively).
Among the random subcohort of 415 incident VTE cases, 183 (44%) received VTE prophylaxis at least once during follow-up (median [IQR] number of instances was 1 [1–2]); 103 (25%) and 78 (19%) received prophylaxis for an interim hospitalization or surgery, respectively, while 86 (21%) received prophylaxis for another reason.
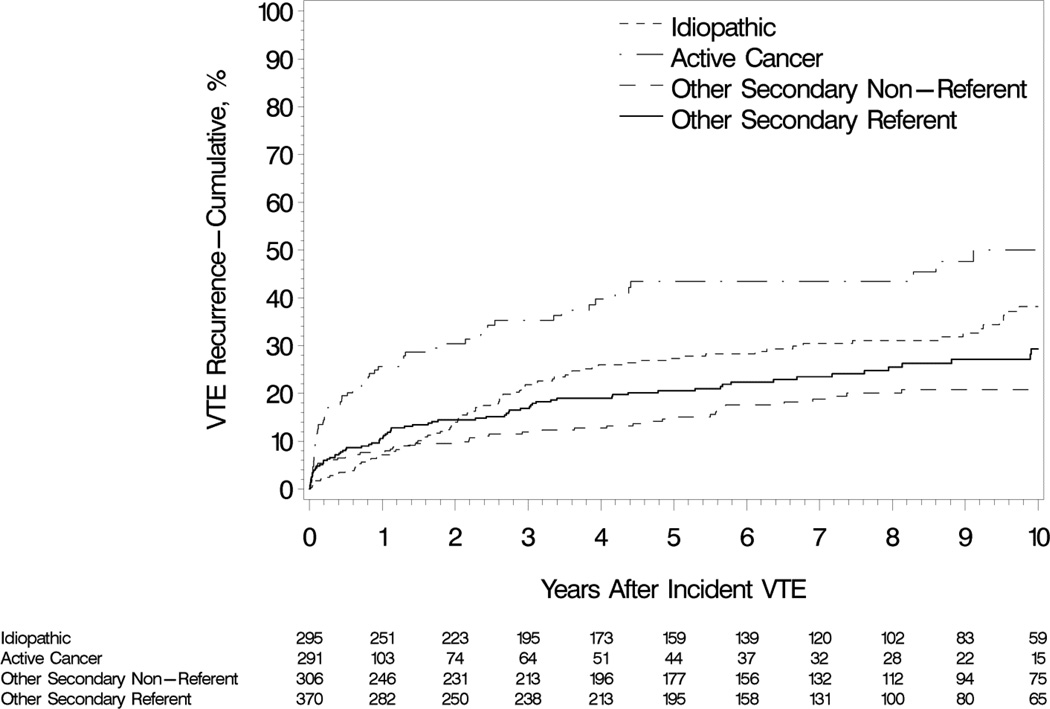
The univariate analyses of demographic and baseline characteristics and new or continuing (interim) exposures occurring after the incident VTE event date as potential predictors of VTE recurrence are presented in Table 2. In multivariable analyses, several baseline characteristics did not satisfy the criteria for proportional hazards (PH), including patient age, chronic lung disease, prior superficial vein thrombosis, neurological disease with leg paresis and idiopathic incident VTE (Table 3, model 1). When analyzed as a function of follow-up intervals, the effects of increasing age, chronic lung disease, superficial vein thrombosis and idiopathic VTE generally were associated with an equal or reduced hazard for recurrence in the early time period (either the first 92 days or <1 year of follow-up), with a comparatively worse hazard afterward (though the late-period effects of chronic lung disease and superficial vein thrombosis were not significant). Conversely, neurological disease with leg paresis was associated with an increased hazard of recurrence in the first follow-up year and a relatively reduced hazard after one year. In a second multivariable model with these non-PH variables carried forward and the remaining baseline characteristics screened for importance, active cancer was associated with increasing hazards of recurrence for succeeding cancer stages (Table 3, model 2). Several secondary non-cancer baseline characteristics (i.e., hospitalization for acute medical illness, nursing home confinement, trauma/fracture and pregnancy) were not predictors of recurrence; incident VTE cases with these characteristics were therefore included in the model’s reference group (illustrated by “other secondary referent” group in Figure 3).
Table 2.
Univariate Association of Baseline, Treatment and Interim Exposure Characteristics with Recurrent Venous Thromboembolism among a Random Subcohort of Olmsted County, MN Residents with Incident Deep Vein Thrombosis or Pulmonary Embolism, 1988–2000.
| Characteristic | Baseline and Interim Exposure Effect (Adjusted) Hazard Ratio (95% CI) [P-value] |
|
|---|---|---|
| Baseline | Interim Exposure | |
| Male sex | 1.19 (0.95, 1.49) [0.128] | – |
| Age (years; per 10 year increase in age) | 1.06 (1.00, 1.13) [0.054] | – |
| Body mass index (BMI, kg/m2; per 10 kg/m2 increase in BMI) | 1.11 (0.96, 1.29) [0.162] | – |
| Incident PE ± DVT | 0.77 (0.60, 0.99) [0.039] | – |
| Classification of incident VTE | ||
| Active cancer | 2.40 (1.81, 3.20) | – |
| Idiopathic | 1.43 (1.10, 1.87) | – |
| Other secondary (referent) | 1.0 (referent) [F test p<.001] | – |
| Hospitalization for surgery | 0.73 (0.56, 0.96) [0.022] | – |
| Hospitalization for surgery§ | [F test p<.001] | |
| No | – | 1.0 (referent) |
| Yes, hazard ratio for in-hospital | – | 10.73 (6.48, 17.76) |
| Yes, hazard ratio for 92 days post dismissal | – | 3.15 (1.97, 5.04) |
| Hospitalization for acute medical illness | 1.15 (0.90, 1.47) [0.257] | – |
| Hospitalization for acute medical illness§ | [F test p<.001] | |
| No | – | 1.0 (referent) |
| Yes, hazard ratio for in-hospital | – | 5.82 (3.73, 9.09) |
| Yes, hazard ratio for 92 days post dismissal | – | 2.62 (1.91, 3.60) |
| Nursing home confinement | 0.74 (0.45, 1.24) [0.254] | – |
| Active cancer | 2.11 (1.62, 2.75) [<.001] | 3.11 (1.95, 4.97) [<.001] |
| Multiple active cancer types | [F test p<.001] | [F test p<.001] |
| No cancer | 1.0 (referent) | 1.0 (referent) |
| Single cancer type | 2.08 (1.59, 2.72) | 2.58 (1.97, 3.37) |
| Multiple cancer types | 3.09 (1.14, 8.41) | 5.08 (2.23, 11.54) |
| Active cancer metastases | [F test p<.001] | [F test p<.001] |
| No cancer | 1.0 (referent) | 1.0 (referent) |
| Cancer without metastases | 1.59 (1.11, 2.28) | 1.37 (0.60, 3.09) |
| Cancer with metastases | 2.94 (2.09, 4.15) | 5.73 (3.52, 9.31) |
| Active cancer stage | [F test p<.001] | [F test p<.001] |
| No cancer | 1.0 (referent) | 1.0 (referent) |
| Stage 1–3 cancer | 1.54 (1.10, 2.16) | 2.24 (1.18, 4.25) |
| Stage 4 cancer | 3.84 (2.63, 5.61) | 5.49 (3.11, 9.68) |
| Active cancer stage progression | [F test p<.001] | [F test p<.001] |
| No cancer | 1.0 (referent) | 1.0 (referent) |
| Cancer without stage progression | 2.09 (1.59, 2.74) | 2.82 (1.63, 4.89) |
| Cancer with stage progression | 2.37 (1.16, 4.86) | 5.16 (2.71, 9.80) |
| Any lifetime cancer | 1.70 (1.33, 2.16) [<.001] | 1.92 (1.53, 2.43) [<.001] |
| Trauma/fracture | 0.72 (0.49, 1.05) [0.084] | 4.24 (2.34, 7.65) [<.001] |
| Neurological disease with leg paresis | 1.11 (0.69, 1.76) [0.669] | – |
| Neurological disease with leg paresis§ | [F test p<.001] | |
| No | – | 1.0 (referent) |
| Yes, 0–92 days | – | 8.36 (3.85, 18.15) |
| Yes, >92 days | – | 0.24 (0.03, 1.70) |
| Chronic Lung Disease | 0.97 (0.71, 1.32) [0.856] | – |
| Diabetes | 1.43 (0.99, 2.06) [0.058] | – |
| Superficial vein thrombosis | 0.95 (0.71, 1.27) [0.727] | – |
| Central venous catheter | 1.28 (0.88, 1.85) [0.198] | 7.91 (5.36, 11.67) [<.001] |
| Venous Transfemoral Catheter | 0.94 (0.30, 2.93) [0.918] | 3.60 (2.01, 6.44) [<.001] |
| Pacemaker | 0.57 (0.18, 1.77) [0.330] | 2.15 (1.05, 4.37) [0.035] |
| Any infection | 1.05 (0.82, 1.34) [0.678] | 2.29 (1.78, 2.94) [<.001] |
| Respiratory infection | 1.20 (0.85, 1.68) [0.304] | 2.29 (1.61, 3.26) [<.001] |
| Urinary tract infection | 1.20 (0.81, 1.77) [0.364] | 1.93 (1.31, 2.86) [<.001] |
| Pregnancy/ Postpartum† | 0.93 (0.41, 2.10) [0.858] | - |
| Pregnancy/ Postpartum§ † | [F test p=0.135] | |
| No | – | 1.0 (referent) |
| Yes, hazard ratio for pregnancy | – | 4.20 (1.03, 17.13) |
| Yes, hazard ratio for 92 days after post-partum | – | - |
| Oral contraceptive§ | – | 1.12 (0.41, 3.06) [0.826] |
| Estrogen therapy§ | – | 0.89 (0.52, 1.53) [0.683] |
| Progestin therapy§ | – | 1.69 (0.99, 2.89) [0.053] |
| Lipid-lowering drugs | ||
| Statinठ| Р| 0.99 (0.65, 1.51) [0.978] |
| Non-statinठ| Р| 0.44 (0.19, 0.99) [0.048] |
| Unfractionated heparin therapy§ | – | 3.36 (1.95, 5.79) [<.001] |
| Intravenous heparin therapy§ | – | 2.44 (1.27, 4.70) [0.008] |
| Subcutaneous heparin therapy§ | – | 4.72 (2.21, 10.12) [<.001] |
| Therapeutic APTT within first 24 hours of heparin therapy§ | [F test p=0.45] | |
| Yes | – | 1.0 (referent) |
| No | – | 1.24 (0.87, 1.77) |
| No APTT available | – | 1.19 (0.88, 1.61) |
| Cumulative proportion of time on heparin with therapeutic APTT^§ | – | 0.95 (0.92, 0.99) [0.01] |
| Heparin status/cumulative time with therapeutic APTT§ | [F test p<.001] | |
| ▪ ≥50% of time-spent on heparin with therapeutic APTT | 2.17 (1.09, 4.31) | |
| ▪ <50% of time-spent on heparin with therapeutic APTT | 4.53 (2.54, 8.10) | |
| ▪ No APTT measured while on heparin | 1.72 (0.39, 7.56) | |
| ▪ Not on heparin | 1.0 (referent) | |
| Low molecular weight heparin therapy§ | – | 1.47 (0.64, 3.38) [0.370] |
| Warfarin therapy§ | – | 0.40 (0.28, 0.56) [<.001] |
| Cumulative proportion of time on warfarin with INR≥1.5^§ | – | 0.94 (0.89, 0.99) [0.013] |
| Warfarin status/cumulative time with INR≥1.5 | – | [F test p<.001] |
| ▪ ≥50% of time-spent on warfarin with INR≥1.5 | 0.38 (0.26, 0.55) | |
| ▪ <50% of time-spent on warfarin with INR≥1.5 | 0.69 (0.37, 1.28) | |
| ▪ No INR measured while on warfarin | 0.18 (0.04, 0.73) | |
| ▪ Not on warfarin | 1.0 (referent) | |
| Cumulative proportion of time on warfarin with INR≥2.0^§ | – | 0.98 (0.94, 1.02) [0.343] |
| Warfarin status/cumulative time with INR≥2.0 | – | [F test p<.001] |
| ▪ ≥50% of time-spent on warfarin with INR≥2.0 | 0.37 (0.25, 0.55) | |
| ▪ <50% of time-spent on warfarin with INR≥2.0 | 0.53 (0.33, 0.87) | |
| ▪ No INR measured while on warfarin | 0.18 (0.04, 0.72) | |
| ▪ Not on warfarin | 1.0 (referent) | |
| IVC filter§ | – | 1.95 (1.33, 2.84) [<.001] |
| Aspirin therapy§ | – | 0.79 (0.57, 1.10) [0.168] |
N=613 subjects were analyzed for each univariate model except for proportion of time on heparin (n=496) and on warfarin (n=484) variables
Effects for female-specific exposures were adjusted for sex.
Missing interim (binary) data were imputed with a value of “yes” if the coefficient for a category assigned to unknown was similar to that of the yes level.
Time-dependent variable constructed for interim exposures only.
Table 3.
Multivariable Association of Baseline, Treatment and Interim Exposure Characteristics with Recurrent Venous Thromboembolism among a Random Subcohort of Olmsted County, MN Residents with Incident Deep Vein Thrombosis or Pulmonary Embolism, 1988–2000.
| Baseline Terms | Risk Interval | Model 1† (Non-PH Baseline Terms) |
Model 2 (Model 1 + PH Baseline Terms) |
Model 3 (Model 2 + Interim Exposure Terms) |
Model 4 (Model 3 Pruned Using α = 0.1) |
|---|---|---|---|---|---|
| Non-Proportional Hazards Terms | |||||
| Age (per 10-year increase) | [P<.001] | [P<.001] | [P<.001] | [P<.001] | |
| 0–365 days | 0.92 (0.85, 1.01) | 0.91 (0.83, 1.00) | 0.94 (0.86, 1.04) | 0.94 (0.86, 1.03) | |
| >365 days | 1.21 (1.10, 1.33) | 1.22 (1.11, 1.34) | 1.28 (1.15, 1.41) | 1.28 (1.16, 1.41) | |
| Chronic lung disease | [P=0.060] | [P=0.075] | [P=0.016] | [P=0.019] | |
| 0–92 days | 0.52 (0.25, 1.10) | 0.52 (0.24, 1.09) | 0.35 (0.16, 0.76) | 0.35 (0.16, 0.76) | |
| >92 days | 1.15 (0.80, 1.63) | 1.09 (0.76, 1.56) | 0.99 (0.68, 1.44) | 0.96 (0.67, 1.39) | |
| Prior superficial vein thrombosis | [P=0.005] | [P=0.007] | [P<.001] | [P<.001] | |
| 0–365 days | 0.47 (0.25, 0.87) | 0.47 (0.25, 0.88) | 0.31 (0.16, 0.60) | 0.33 (0.17, 0.63) | |
| >365 days | 1.28 (0.91, 1.81) | 1.25 (0.88, 1.78) | 1.30 (0.90, 1.89) | 1.30 (0.90, 1.88) | |
| Neurological disease with leg paresis | [P=0.021] | [P=0.004] | [P=0.006] | [P=0.004] | |
| 0–365 days | 1.80 (1.04, 3.12) | 2.33 (1.34, 4.07) | 1.81 (1.02, 3.20) | 1.77 (1.00, 3.13) | |
| >365 days | 0.47 (0.17, 1.28) | 0.44 (0.16, 1.20) | 0.34 (0.12, 0.98) | 0.31 (0.11, 0.89) | |
| Idiopathic incident VTE | [P<.001] | [P<.001] | [P<.001] | [P<.001] | |
| 0–92 days | 0.23 (0.10, 0.53) | 0.31 (0.13, 0.73) | 0.29 (0.12, 0.70) | 0.30 (0.13, 0.73) | |
| >92 days | 1.54 (1.16, 2.03) | 1.46 (1.05, 2.04) | 1.81 (1.29, 2.55) | 1.81 (1.30, 2.53) | |
| Proportional Hazards Terms | |||||
| BMI (per 10 kg/m2 increase) | 1.14 (0.97, 1.33) [0.111] | 1.08 (0.93, 1.27) [0.315] | |||
| Male sex | 1.23 (0.97, 1.57) [0.085] | 1.31 (1.02, 1.68) [0.037] | 1.29 (1.01, 1.65) [0.041] | ||
| Incident PE (±DVT) | 0.83 (0.65, 1.07) [0.160] | 0.88 (0.68, 1.15) [0.366] | |||
| Diabetes mellitus | 1.41 (0.95, 2.09) [0.086] | 1.43 (0.96, 2.13) [0.079] | 1.46 (0.99, 2.15) [0.057] | ||
| Active cancer | No | 1.0 (referent) [P<.001] | 1.0 (referent) [P<.001] | 1.0 (referent) [P<.001] | |
| Stage 1–3 active cancer | 1.73 (1.20, 2.48) | 1.49 (1.02, 2.19) | 1.53 (1.05, 2.25) | ||
| Stage 4 active cancer | 3.93 (2.61, 5.93) | 2.71 (1.77, 4.14) | 2.62 (1.72, 4.00) | ||
| Central Vein Catheter | 1.42 (0.94, 2.13) [0.093] | 0.87 (0.56, 1.34) [0.517] | |||
| Hospitalization for Surgery | 0.75 (0.56, 1.02) [0.071] | 0.82 (0.60, 1.12) [0.219] | 0.77 (0.57, 1.03) [0.080] | ||
| Interim Exposure Terms | |||||
| Therapeutic APTT within 24 hrs of heparin | Yes | 1.0 (referent) [P=0.038] | 1.0 (referent) [P=0.054] | ||
| No | 1.63 (1.12, 2.39) | 1.58 (1.09, 2.30) | |||
| No APTT available | 1.36 (0.99, 1.87) | 1.34 (0.97, 1.84) | |||
| Trauma/Fracture | 1.66 (0.87, 3.18) [0.127] | ||||
| Pregnancy | 12.0 (2.80, 51.4) [<.001] | 12.9 (3.01, 54.8) [<.001] | |||
| Active cancer | No | 1.0 (referent) [P<.001] | 1.0 (referent) [P<.001] | ||
| Stage 1–3 active cancer | 1.31 (0.67, 2.58) | 1.27 (0.65, 2.48) | |||
| Stage 4 active cancer | 3.52 (1.86, 6.66) | 3.44 (1.83, 6.48) | |||
| Hospitalization for medical illness | Not hospitalized | 1.0 (referent) [P<.001] | 1.0 (referent) [P<.001] | ||
| Yes, HR for in-hospital | 4.94 (3.14, 7.75) | 5.21 (3.33, 8.16) | |||
| Yes, HR for next 92 days | 2.88 (2.12, 3.93) | 3.02 (2.23, 4.10) | |||
| Hospitalization for surgery | Not hospitalized | 1.0 (referent) [P<.001] | 1.0 (referent) [P<.001] | ||
| Yes, HR for in-hospital | 5.87 (3.32, 10.37) | 6.61 (3.79, 11.52) | |||
| Yes, HR for next 92 days | 1.89 (1.13, 3.15) | 2.04 (1.23, 3.36) | |||
| Central vein catheter | 4.33 (2.76, 6.79) [<.001] | 4.31 (2.81, 6.60) [<.001] | |||
| Venous transfemoral catheter | 1.91 (1.03, 3.55) [0.041] | 1.83 (0.99, 3.38) [0.053] | |||
| Pacemaker | 1.74 (0.82, 3.69) [0.150] | ||||
| Respiratory infection | 1.51 (1.02, 2.24) [0.039] | 1.53 (1.04, 2.25) [0.029] | |||
| Aspirin therapy | 0.62 (0.44, 0.88) [0.007] | 0.60 (0.43, 0.86) [0.005] | |||
| Lipid lowering non-statin drugs | 0.52 (0.23, 1.21) [0.131] | ||||
| Low molecular weight heparin | 1.79 (0.75, 4.32) [0.192] | ||||
| Warfarin therapy | 0.38 (0.27, 0.53) [<.001]‡ | 0.37 (0.26, 0.51) [<.001] | |||
Effects estimated from multivariable Cox PH regression are expressed as hazard ratio (95% confidence interval) [p-value]; Models 1–4 derived using stepwise variable selection with backwards elimination with varying alpha levels defining the selection criteria (refer to Methods section for more details); based on the inclusion of an idiopathic term and the removal of insignificant baseline terms in Model 2, the reference level defined in Models 2–4 would include incident VTE cases with the following secondary non-idiopathic factors: medical-related hospitalization, nursing home confinement, trauma/fracture and pregnancy
Note that the original fit of Model 1 specified time as 3 intervals (0–92, 93–365 and >365 days) for the time-by-predictor interactions; the reported results are from a re-fitted model in which the choice of a simpler 2 interval form of time was decided for each predictor using post-hoc likelihood ratio (LR) tests
LR tests comparing Model 3 with a re-fitted model that replaced the binary warfarin term with a categorical measure of time in therapeutic range (1. INR>1.5, 2. INR>2.0) while on warfarin (vs. not on warfarin), holding all other covariates fixed, showed that the therapeutic range on warfarin measures, although statistically significant, were not more informative as predictors than the simple indication of warfarin (1. P=0.194; 2. P=0.190)
Figure 3.
Cumulative incidence of venous thromboembolism recurrence among Olmsted County, Minnesota residents who survived ≥1 day after an incident deep vein thrombosis or pulmonary embolism over the 13-year period, 1988–2000, followed through 12/31/2005 by idiopathic, active cancer-associated and secondary non-active cancer-associated incident venous thromboembolism event.
In a third multivariable model that accounted for the baseline variables in the preceeding model while testing all interim exposures, male sex was independently associated with an increased hazard of VTE recurrence, as were the interim exposures of: 1) failure to rapidly achieve a therapeutic APTT after starting standard heparin (specifically, the contrast of those who attained vs. failed to rapidly achieve a therapeutic APTT); 2) hospitalization for medical illness or for surgery, 3) active cancer, 4) pregnancy, and 5) respiratory infection. Conversely, the interim exposures of warfarin therapy and aspirin therapy were independently associated with reduced hazards of recurrence (Table 3, model 3). Similar findings were obtained in the final model, which removed baseline or interim exposures not significant with alpha=0.10 (Table 3, model 4). Baseline characteristics that were not independent predictors (p<0.10) of recurrence were any lifetime history of cancer, central vein catheter, BMI and type of incident VTE (DVT alone vs. PE with or without DVT; isolated calf vein DVT vs. PE or proximal leg vein DVT) and were removed, while both hospitalization for surgery and diabetes mellitus were retained but were not significant with alpha=0.05.
DISCUSSION
After completing about three months of anticoagulation treatment for acute VTE, a decision must be made regarding whether to recommend secondary prophylaxis against recurrent VTE. This decision is complex and requires assessment of the individual patient’s risk of idiopathic recurrent VTE (particularly idiopathic recurrent fatal PE), anticoagulant-related bleeding (particularly intracranial hemorrhage) and patient preference. Current guidelines differ regarding recommendations for secondary prophylaxis.[3, 22] Moreover, while multiple studies have tested baseline characteristics as potential predictors of VTE recurrence,[1, 6, 7] none have adjusted for both treatment effects and new exposures that may be risk factors for recurrent VTE. Whether previously identified baseline characteristics remain predictors of recurrence after adjusting for treatments and interim exposures is unknown. Interim exposure to lipid-lowering drugs[23] and among women, to oral contraceptives[24] and hormone therapy,[25] have been identified as predictors of VTE recurrence, but no study has comprehensively studied interim exposures; we do not know which exposures are independent predictors of recurrence nor the magnitude of recurrence risk associated with each. These are important issues because the 5-year VTE recurrence rate has changed little over the last 35 years.[1, 5] To address these gaps in knowledge, we performed a population-based case-cohort study in which we identified those interim exposures that are independent predictors of VTE recurrence, and then identified baseline characteristics that are independent predictors of recurrence after adjusting for treatments and interim exposure to these VTE risk factors.
We identified new or continuing (interim) exposure to active cancer, pregnancy, central vein catheter and respiratory infection after the incident VTE as independent predictors of VTE recurrence (Table 3, models 3 and 4). We also identified new hospitalization, including for surgery and for acute medical illness, as an independent predictor of recurrence; compared to no hospitalization, the magnitude of risk was highest while in hospital although the increased risk persisted to a lesser magnitude for 92 days after hospital discharge. We confirmed that secondary prophylaxis with warfarin[6, 7] and aspirin[26, 27] therapy independently reduce the hazard of recurrence. In secondary analyses, aspirin was associated with a significantly reduced hazard of recurrence (HR=0.59; p=0.028) among patients with non-idiopathic incident VTE. We corroborated previous findings that failure to rapidly achieve a therapeutic APTT after starting standard heparin was an independent predictor of recurrence.[6, 28] We previously showed that this reflects heparin resistance rather than under-dosing,[6] possibly due to high factor VIII activity as an acute phase reactant.[29–31] This variable was independently associated with an increased hazard of recurrence for the entire follow-up period, suggesting that this is an inherent individual characteristic. We could not confirm interim exposure to oral contraceptives[24] or hormone therapy[25] as independent predictors of VTE recurrence. Non-statin lipid-lowering therapy was marginally associated with a reduced hazard of recurrence (HR=0.52 [0.23, 1.21; p=0.13]; Table 3).[23] Sixty-six (16%) patients in the sub cohort received lipid-lowering therapy for at least one day during follow-up; 59 received a statin and 26 received a non-statin (20 of these 26 also received a statin). An association of lipid-lowering therapy with a reduced hazard of recurrence may have been missed due to inadequate power.
After adjusting for treatments and interim exposures that were independent predictors of recurrence, we confirmed that among demographic and baseline characteristics, patient age,[1, 5, 32, 33] male sex,[1, 6, 33, 34] active cancer,[1, 7, 11] neurologic disease with leg paresis,[1] prior superficial vein thrombosis[1, 35] and idiopathic incident VTE were independently associated with VTE recurrence (Table 3, models 3 and 4). However, the effects of several baseline characteristics on the hazard of VTE recurrence varied across early to late follow-up and require careful interpretation. While baseline patient age was not associated with recurrence in the first follow-up year, there was a significant association between increasing age and a higher hazard of recurrence after one year (among those still at risk). Conversely, prior superficial vein thrombosis and chronic lung disease were associated with a reduced harzard of recurrence in the first 365 and 92 days of followup, respectively, with no association with recurrence thereafter. Neurological disease with leg paresis was characterized by a decreasing hazard of recurrence over time, with borderline increased risk over the first year and significantly lower risk thereafter. On the other hand, idiopathic incident VTE was associated with a reduced hazard of recurrence over the first 92 days but an increased hazard thereafter (among those still at risk; also see Appendix Figure). Contrary to previous reports, patient BMI[1, 6, 36–38] and the type of incident VTE (DVT alone vs. PE with or without DVT) were not independent predictors of recurrence.[1, 6, 32, 34, 39]
Our study has several important strengths. The population-based study design avoided referral bias and insured that the entire spectrum of VTE disease occurring in the community was included. Thus, our results are generalizeable to populations of similar demography and baseline characteristics. We accurately separated incident from recurrent VTE events, used an unambiguous definition of VTE recurrence and our cohort follow up was virtually complete. Our sample size was relatively large and the observed VTE recurrence rate was comparable to contemporary studies. The APTT and PT/INR assays and assay instruments were standardized across the entire study timeframe, and we used a “carry-forward” method for calculation of proportion of time in therapeutic range that avoids the use of future information to bias present data. We used evidence-based criteria for categorizing baseline cancer as “active”, and for predicting the active duration of an interim cancer to analyze this as a time-dependent variable. However, our study also has important limitations. Because of our observational cohort study design, we could not insure equal and random patient allocation to differing durations and intensities of heparin and warfarin anticoagulation. However, the frequency distribution of these characteristics in the cohort was sufficiently broad that we had adequate power to assess their potential effects on VTE recurrence.
Our study has several important implications. First, VTE patients with new exposure to hospitalization for surgery or acute medical illness, respiratory infection, placement of a central venous catheter, and among women, pregnancy, are at increased risk for VTE recurrence and are candidates for brief secondary prophylaxis around the time of the new exposure. VTE patients with a new cancer diagnosis or cancer recurrence (particularly stage IV cancer) also are at increased risk for recurrence but prophylaxis in ambulatory cancer patients remains controversial.[40] Secondly, the risk of recurrence and duration of recurrence risk among those with non-cancer secondary VTE can now be further stratified by patient age, male sex, and the baseline characteristics of chronic lung disease, prior superficial vein thrombosis, and neurological disease with leg paresis. We have previously identified those cancer types and cancer characteristics that are predictors of recurrence among patients with active cancer-associated incident VTE,[7] and others have identified characteristics that are predictors of recurrence among patients with idiopathic incident VTE.[8–10, 12, 41] Thus, we are developing the tools to better assess the risk of recurrence and direct secondary prophylaxis recommendations for the individual. Finally, we have shown that secondary prophylaxis with warfarin and aspirin reduce the hazard of VTE recurrence by about 60% and 40%, respectively, independent of whether the incident event was idiopathic or secondary. Thus, among patients with incident non-cancer secondary VTE who are judged to be at relatively high risk for recurrence but have unacceptable risk for bleeding from secondary prophylaxis with warfarin or an oral direct thrombin or factor Xa inhibitor, secondary prophylaxis with aspirin is an alternative.
In conclusion, heparin resistance and interim major surgery, hospitalization for acute medical illness, active cancer (particularly stage 4), respiratory infection, central venous catheter and pregnancy after the incident VTE are associated with increased hazards of recurrence, while warfarin and aspirin are associated with reduced hazards. Adjusting for these treatments and interim exposures, the baseline characteristics of male sex, active cancer, increasing patient age (after 1 year follow-up, among those still at-risk), and neurologic disease with leg paresis (within first year of follow-up) are independently associated with increased hazards of VTE recurrence.
Supplementary Material
Figure 4.
Cumulative incidence of venous thromboembolism recurrence among Olmsted County, Minnesota residents who survived ≥1 day after an incident deep vein thrombosis or pulmonary embolism over the 13-year period, 1988–2000, followed through 12/31/2005 by idiopathic and non-idiopathic-associated incident venous thromboembolism events, with referent and non-referent patients from the multivariable modeling distinguished within the secondary non-active cancer group.
Highlights.
We tested baseline and interim characteristics as predictors of VTE recurrence
5-year % recurrence for cancer, idiopathic and secondary VTE were 43, 27 and 18
New hospitalization, cancer, pregnancy, catheter and infection predicted recurrence
Warfarin and aspirin were associated with reduced hazards of recurrence
Male sex, baseline cancer and heparin resistance were predictors of recurrence
ACKNOWLEDGEMENTS
We gratefully acknowledge Catherine L. Brandel, R.N., Diadra H. Else, R.N., Jane A. Emerson, R.N., and Cynthia L. Nosek, R.N. for excellent data collection and Cynthia E. Regnier, R.N., as research project manager. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Research reported in this publication was supported in part by grants from the National Heart Lung and Blood Institute under Award Numbers R01HL066216 to JAH, and was made possible by the Rochester Epidemiology Project (Award Number R01AG034676 of the National Institute on Aging, National Institutes of Health). Research support also was provided by Mayo Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflict of interest.
J.A. Heit, M.D. designed the study, collected the data, participated in the analyses and wrote the manuscript.
B.D. Lahr, M.S. performed the statistical analyses and participated in writing the manuscript.
A.A. Ashrani, M.D. participated in the study design, data collection, interpretation of the analyses and manuscript preparation.
T.M. Petterson, M.S. participated in the study design, data collection, performance and interpretation of the analyses and manuscript preparation.
K.R. Bailey, Ph.D. participated in the study design and data collection, directed the statistical analyses, and participated in manuscript preparation.
References
- 1.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ., 3rd Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Archives of Internal Medicine. 2000;160:761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA. Estimating the risk of recurrent venous thromboembolism. American Journal of Hematology. 2012;87(Supppl 1):S63–S67. doi: 10.1002/ajh.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e494S. doi: 10.1378/chest.11-2301. PMCID: PMC 3278049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123:1794–1801. doi: 10.1182/blood-2013-12-512681. [DOI] [PubMed] [Google Scholar]
- 5.Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, Iotti M, Tormene D, Simioni P, Pagnan A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 6.Heit JA, Lahr BD, Petterson TM, Bailey KR, Ashrani AA, Melton LJ., 3rd Heparin and warfarin anticoagulation intensity as predictors of recurrence after deep vein thrombosis or pulmonary embolism: a population-based cohort study. Blood. 2011;118:4992–4999. doi: 10.1182/blood-2011-05-357343. PMCID: 3208304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ, 3rd, Heit JA. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood. 2014;123:3972–3978. doi: 10.1182/blood-2014-01-549733. PMCID: 4064333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, Solymoss S, Crowther M, Perrier A, White R, Vickars L, Ramsay T, Betancourt MT, Kovacs MJ. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. Canadian Medical Association Journal. 2008;179:417–426. doi: 10.1503/cmaj.080493. PMCID: 2518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121:1630–1636. 1. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 10.Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC, Douketis J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) Journal of Thrombosis and Haemostasis. 2012;10:1019–1025. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 11.Louzada ML, Carrier M, Lazo-Langner A, Dao V, Kovacs MJ, Ramsay TO, Rodger MA, Zhang J, Lee AY, Meyer G, Wells PS. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. 2012;126:448–454. doi: 10.1161/CIRCULATIONAHA.111.051920. [DOI] [PubMed] [Google Scholar]
- 12.Kyrle PA, Eischer L. Predicting the risk of recurrent venous thromboembolism. The Austrian study on recurrent venous thromboembolism (AUREC) Hamostaseologie. 2013;33:201–209. doi: 10.5482/HAMO-13-03-0018. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American Journal of Epidemiology. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. PMCID: 3105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic Proceedings. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. PMCID: 3538404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Archives of Internal Medicine. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 17.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 18.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 19.Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ., 3rd Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1399–1405. doi: 10.1161/ATVBAHA.109.189290. PMCID: 2735343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Self SG, Prentice RL. Asymptotic distribution theory and efficiency results for case-cohort studies. Ann Stat. 1988;16:64–81. [Google Scholar]
- 21.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of Clinical Epidemiology. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 22.2014 www.nice.org.uk/guidance/CG144/chapter/1-Guidance. [Google Scholar]
- 23.Delluc A, Tromeur C, Le Moigne E, Nowak E, Mottier D, Le Gal G, Lacut K. Lipid lowering drugs and the risk of recurrent venous thromboembolism. Thrombosis Research. 2012;130:859–863. doi: 10.1016/j.thromres.2012.08.296. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352–2361. doi: 10.1001/jama.293.19.2352. [DOI] [PubMed] [Google Scholar]
- 25.Hoibraaten E, Qvigstad E, Arnesen H, Larsen S, Wickstrom E, Sandset PM. Increased risk of recurrent venous thromboembolism during hormone replacement therapy--results of the randomized, double-blind, placebo-controlled estrogen in venous thromboembolism trial (EVTET) Thrombosis and Haemostasis. 2000;84:961–967. [PubMed] [Google Scholar]
- 26.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, Grandone E, Prandoni P. Aspirin for preventing the recurrence of venous thromboembolism. The New England Journal of Medicine. 2012;366:1959–1967. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 27.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, Kirby A, Simes J. Low-dose aspirin for preventing recurrent venous thromboembolism. The New England Journal of Medicine. 2012;367:1979–1987. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 28.Hull RD, Raskob GE, Hirsh J, Jay RM, Leclerc JR, Geerts WH, Rosenbloom D, Sackett DL, Anderson C, Harrison L, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. The New England Journal of Medicine. 1986;315:1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 29.Edson JR, Krivit W, White JG. Kaolin partial thromboplastin time: high levels of procoagulants producing short clotting times or masking deficiencies of other procoagulants or low concentrations of anticoagulants. The Journal of laboratory and clinical medicine. 1967;70:463–470. [PubMed] [Google Scholar]
- 30.Cirisano FD, Lee S, Greenspoon JS. Apparent heparin resistance form elevated factor VIII in a patient with postoperative deep venous thrombosis. A case report. The Journal of reproductive medicine. 1996;41:191–194. [PubMed] [Google Scholar]
- 31.Legnani C, Cosmi B, Cini M, Frascaro M, Guazzaloca G, Palareti G. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. British Journal of Haematology. 2004;124:504–510. doi: 10.1046/j.1365-2141.2003.04795.x. [DOI] [PubMed] [Google Scholar]
- 32.Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thrombosis and Haemostasis. 2002;88 [PubMed] [Google Scholar]
- 33.Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The risk of recurrent venous thromboembolism in men and women. The New England Journal of Medicine. 2004;350:2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 34.Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, Kyrle P, Poli D, Tait RC, Iorio A. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813. doi: 10.1136/bmj.d813. PMCID: 3044449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galanaud JP, Genty C, Sevestre MA, Brisot D, Lausecker M, Gillet JL, Rolland C, Righini M, Leftheriotis G, Bosson JL, Quere I. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thrombosis and Haemostasis. 2011;105:31–39. doi: 10.1160/TH10-06-0406. [DOI] [PubMed] [Google Scholar]
- 36.Romualdi E, Squizzato A, Ageno W. Abdominal obesity and the risk of recurrent deep vein thrombosis. Thrombosis Research. 2007;119:687–690. doi: 10.1016/j.thromres.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Eichinger S, Pecheniuk NM, Hron G, Deguchi H, Schemper M, Kyrle PA, Griffin JH. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. 2007;115:1609–1614. doi: 10.1161/CIRCULATIONAHA.106.649954. [DOI] [PubMed] [Google Scholar]
- 38.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, Heinze G, Kyrle PA. Overweight, obesity, and the risk of recurrent venous thromboembolism. Archives of Internal Medicine. 2008;168:1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 39.Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, Hirsh J, Kearon C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ. 2011;342:d3036. doi: 10.1136/bmj.d3036. PMCID: 3100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connors JM. Prophylaxis against venous thromboembolism in patients with cancer. The New England Journal of Medicine. 2014;371:1263–1264. doi: 10.1056/NEJMc1408866. [DOI] [PubMed] [Google Scholar]
- 41.Palareti G, Cosmi B, Legnani C, Antonucci E, De Micheli V, Ghirarduzzi A, Poli D, Testa S, Tosetto A, Pengo V, Prandoni P. D-dimer to guide the duration of anticoagulation in patients with venous thromboembolism: a management study. Blood. 2014;124:196–203. doi: 10.1182/blood-2014-01-548065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.