Abstract
BACKGROUND
Maternal obesity is a major problem in obstetrics, and the placenta is involved in obesity-related complications via its roles at the maternal–fetal interface. We have recently shown a causative role for micro(mi)RNA-210, a so called ‘hypoxamir’ regulated by HIF-1α, in mitochondrial dysfunction in placentas from women with preeclampsia. We also reported mitochondrial dysfunction in placentas with maternal obesity. Here we hypothesized that expression of miR-210 is dysregulated in the placentas with obesity.
METHODS
Placentas from uncomplicated pregnancies were collected at term from healthy weight or control (CTRL, pre-pregnancy body mass index (BMI)<25), overweight (OW, BMI = 25–24.9) and obese (OB, BMI>30) women following C-section with no labor. Expression of miRNA-210 and its target genes was measured by reverse transcription–PCR and Western Blot, respectively. Mitochondrial respiration was assessed by Seahorse Analyzer in syncytiotrophoblast (ST) 72 h after cytotrophoblast isolation.
RESULTS
Expression of miR-210 was significantly increased in placentas of OB and OW women with female but not male fetuses compared with CTRL placentas of females. However, expression of HIF-1α in these placentas remained unchanged. Levels of tumor-necrosis factor-alpha (TNFα) were increased in OW and OB placentas of females but not males, and in silico analysis suggested that activation of miR-210 expression in these placentas might be activated by NFκB1 (p50) signaling. Indeed, chromatin Immunoprecipitation assay showed that NFkB1 binds to placental miR-210 promoter in a fetal sex-dependent manner. Female but not male STs treated with TNFα showed overexpression of miR-210, reduction of mitochondrial target genes and decreased mitochondrial respiration. Pre-treatment of these STs with small interfering RNA to NFkB1 or antagomiR-210 prevented the TNFα-mediated inhibition of mitochondrial respiration.
CONCLUSIONS
Our data suggest that the inflammatory intrauterine environment associated with maternal obesity induces an NFκB1-mediated increase in miR-210 in a fetal sex-dependent manner, leading to inhibition of mitochondrial respiration and placental dysfunction in the placentas of female fetuses.
INTRODUCTION
Obesity is a major challenge to the overall health of the population and to the economics of the health care system. Pregnancy in obese mothers generates an adverse intrauterine environment via the inflammatory milieu1 and metabolic derangements.2,3 Obesity impacts the outcome of the pregnancy per se, being associated with hypertensive disorders, gestational diabetes and thromboembolic events.4–6 In addition, maternal obesity affects the fetus and newborn causing congenital malformations, large-for-gestational-age and intrauterine growth-restricted infants, stillbirth, shoulder dystocia, and leads to subsequent complications in later life, including obesity, cardiovascular disease and diabetes as a result of fetal programming.4,5,7–9
Placental function is recognized as a critical regulator of fetal growth and development, as the organ that communicates the maternal and uterine environment to the fetus, and as a mediator of fetal programming.10–12 We and others have demonstrated the presence of hypoxia, inflammation and oxidative stress in the placenta in various pathologic situations13,14 and their relationship to altered placental function and fetal programming.15 Additional evidence for altered placental function in these pathologies is provided by proteomic studies, where differences in the placental proteome between normal pregnancies and those complicated by maternal obesity16 are seen. Obesity was associated with marked downregulation of genes involved in the anti-inflammatory response, cell integrity and structure.16
MicroRNAs (miRNAs) are highly conserved, regulatory molecules that have an important role in the post-transcriptional regulation of target gene expression by promoting mRNA instability or translational inhibition.17 MiRNAs are expressed in placenta and alterations in their expression have been described in association with exposure to xenobiotics,18 cigarette smoking19 or with adverse pregnancy outcomes including preeclampsia20,21 and growth restriction.22 MiR-210, a hypoxia-related miRNA (hypoxamir), was found to be involved in the hypoxic state in several tissues23 under direct control of HIF-1α.24 In addition, miR-210 is associated with mitochondrial dysfunction in various types of cancer25 by shifting tumor metabolism from oxidative phosphorylation to glycolysis (Warburg effect).26 We have previously shown an increase in the expression of miR-210 in placentas from women with preeclampsia and a causative role of miR-210 in placental mitochondrial dysfunction.27 We have also recently reported inhibition of mitochondrial respiration, a decrease in ATP content and in mitochondrial biogenesis in placentas from women with increased adiposity.28 Based on these findings, we hypothesized that miR-210 expression is dysregulated in the placenta with maternal obesity.
PATIENTS AND METHODS
Ethical approval and study participants
Placentas were collected with informed consent from patients from the Labor and Delivery unit at University Hospital San Antonio under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center San Antonio.
Materials
Oligomycin, FCCP (4-(trifluoromethoxy) phenylhydrazone), rotenone and antimycin A were obtained from Sigma (St Louis, MO, USA) and dissolved in dimethylsulfoxide as 2.5mm stock solutions. TNFα was purchased from Sigma. NFkB1 (p50), NFkB (p65) and Histone 3 antibodies were obtained from Cell Signaling (Danvers, MA, USA), and HIF-1α antibody was obtained from BD Biosciences (San Jose, CA, USA).
Collection of placental tissue
Collection of placental tissue was performed immediately following delivery by cesarean section at term in the absence of labor from uncomplicated pregnancies from women with a range of pre-pregnancy BMI, grouped as healthy or control (CTRL, body mass index (BMI) 18.0–24.9), overweight (OW, BMI 25–29.9) and obese (OB, BMI > 30).
Tissue processing and sampling
A random sampling technique was used to collect placental tissue from five sites. Villous tissue was dissected out from beneath the chorionic plate, avoiding the basal plate, flash frozen and stored at −80 °C. Tissue was subsequently thawed, the five samples from each placenta were combined and homogenized by mini bead beater (Biospec Products, Bartlesville, OK, USA) in lysis buffer as described earlier.14 Total protein in the homogenates was estimated using Bradford’s reagent (Bio-Rad, Hercules, CA, USA).
Placental TNFα content
Placental TNFα content was determined by Quantikine ELISA kit from R&D Systems (Minneapolis, MN, USA) according to manufacturer's instructions. The range of the standard curve was from 0 to 1000 pg ml−1. For the assay, 20 µg of placental total protein was used. The samples were read at 450 nm in 96-well plates. The within-assay variation was 7.3% (n = 36). The TNFα content was calculated as pg per mg placental protein.
Reverse transcription–PCR
Total RNA was isolated using miRNAeasy kit from Qiagen (Valencia, CA, USA). To determine the expression of miR-210, 5–10 ng of total RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Foster City, CA, USA). TaqMan reactions were conducted using commercially available validated primer/probe sets (Applied Biosystems, Foster City, CA, USA) and normalized to U18 as an internal control. Expression of Iron-Sulfur Cluster Assembly Enzyme (ISCU) and NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4 (NDUFA4) messenger RNAs (mRNAs) was measured by reverse transcription–PCR (RT–PCR) as described before.27
Western blotting
Proteins were separated on 4–20% gradient precast gels (Bio-Rad), transferred onto nitrocellulose membranes and blocked with 5% milk in 0.1% Tween, 20mm Tris (pH7.5)-buffered saline (TTBS) (w/v) for 1 h. Blots were probed with primary antibody in 1% nonfat milk powder/TTBS overnight at 4 °C and were detected using peroxidase-conjugated secondary antibody in 5% milk/TTBS for 1 h. Products were visualized by chemiluminescence (Millipore, Billerica, MA, USA). Band intensity was measured in a G:Box using Gene Snap and Gene Tools software (Syngene, Frederick, MD, USA).
Chromatin Immunoprecipitation (ChIP)
ChIP assay was conducted using a kit from Millipore. Trophoblast cells were fixed with 1% formaldehyde on ice to cross-link the proteins bound to the chromatin DNA. After washing, the cells were homogenized and the chromatin DNA was sheared by sonication to produce DNA fragments of ~ 500–1000 bp. The same amounts of sheared DNA were used for immunoprecipitation with antibodies against NFkB1 or an equal amount of pre-immune rabbit IgG (Millipore). The immunoprecipitate was then incubated with protein A agarose/salmon sperm DNA (Millipore), and the antibody/protein/DNA/agarose complex was collected for subsequent reverse cross-linking. The same amount of sheared DNA without antibody precipitation was processed for reverse cross-linking and served as input control. DNA recovered from reverse cross-linking was used for quantitative RT–PCR. The primers for quantitative RT–PCR are as follows: proximal promoter 1 Forward 5′-TCAGCCGCTGTCACACGCAC-3′, Reverse 5′-CGCGCGAGGGATCCCAGGTT-3′; distal promoter Forward 5′-GGGTGGGA GTCAGGAGGTAT-3′, Reverse 5′-ATGTCAGACAGCACTCAGGC-3′. DNA precipitated with antibody against acetylated histone 3 lysine 9 was used as a positive control. The amount of DNA precipitated was calculated from the threshold cycle number of the amplification curve of quantitative RT–PCR. The percentage of antibody-precipitated and pre-immune IgG-precipitated DNA to input DNA was compared.
Isolation and culture of primary trophoblasts
Villous cytotrophoblasts (CT) were cultured as described29 from placentas obtained from term uncomplicated pregnancies in lean women delivered by cesarean section in the absence of labor. CTs were plated at a density of ~3.5 × 106 cells on 6-well plates or ~8 × 105 cells in Seahorse XF24 plates (Seahorse Biosciences, North Billerica, MA, USA) and incubated for 72 h to allow syncytialization (syncytiotrophoblasts). Cell culture media (DMEM/ Hams F-12, supplemented with l-glutamine, penicillin, streptomycin, gentamicin and 10% fetal bovine serum) was changed daily. For NFκB1 manipulation, CTs were transfected after 24 h of plating with either 5 nm of siRNA NFκB1 or control siRNA using the Dharmafect transfection reagent (all from Thermo Scientific, Waltham. MA, USA). Transfection with antagomiR-210 was performed as described before.27 Forty-eight hours after transfection, cells were either remained untreated or were treated with TNFα (10ng ml−1) for 2 h. Twenty-four hours after the treatment, trophoblast oxygen consumption was measured or cells were harvested for future RNA and protein analysis.
Assessment of oxygen consumption rates
Of the cultured syncytiotrophoblasts (ST) was performed using a XF24 analyzer (Seahorse Biosciences) as described previously.27,30 oxygen consumption rate were normalized to total cellular protein (Bradford method).
Statistical analysis
Data are reported as mean ± s.e.m. Comparisons between two groups were performed using a Student’s t-test. One or two-way analysis of variance and Tukey’s post-hoc test was used to compare data sets with more than two groups. P < 0.05 was considered significant. The TNFα data were analyzed by regression and correlation analysis against maternal BMI using Excel (Redmond, WA, USA) and GraphPad (version 5.0, La Jolla, CA, USA).
RESULTS
Demographic and clinical characteristics of study subjects
Demographic and clinical characteristics of study subjects are presented in Table 1. Data from 36 women (n = 6 in each group and each gender) were analyzed according to the original group assignment. The patient groups did not differ by maternal and gestational ages, or by placental and fetal weights. As expected, the maternal BMI was significantly higher in the overweight and obese groups as compared with controls (P < 0.05).
Table 1.
Demographic and clinical characteristics of study subjects
| BMI range | Pre-gravid BMI | Maternal age (years) | Gestational age (weeks) | Placental weight (g) | Birth weight (g) |
|---|---|---|---|---|---|
| Female | |||||
| Healthy (n=6) | 22.3 ± 0.2 | 26.8 ± 2.1 | 38.7 ± 0.5 | 754 ± 38 | 3203 ± 354 |
| Overweight (n=6) | 27.0 ± 0.3* | 29.5 ± 1.5 | 39.3 ± 0.4 | 724 ± 40 | 3401 ± 176 |
| Obese (n=6) | 33.1 ± 2.5* | 25.0 ± 2.3 | 39.3 ± 0.9 | 792 ± 61 | 3344 ± 243 |
| Male | |||||
| Healthy (n=6) | 22.5 ± 0.2 | 29.0 ± 2.1 | 38.9 ± 0.5 | 702 ± 14 | 3307 ± 354 |
| Overweight (n=6) | 28.3 ± 0.3* | 28.6 ± 1.5 | 38.6 ± 0.4 | 785 ± 43 | 3576 ± 174 |
| Obese (n=6) | 34.1 ± 2.5* | 26.1 ± 2.3 | 39.0 ± 0.9 | 718 ± 47 | 3454 ± 243 |
Abbreviation: BMI, body mass index.
P < 0.05 vs healthy group. Values are mean ± s.e.m.
Effect of maternal obesity on expression of miR-210 and target genes
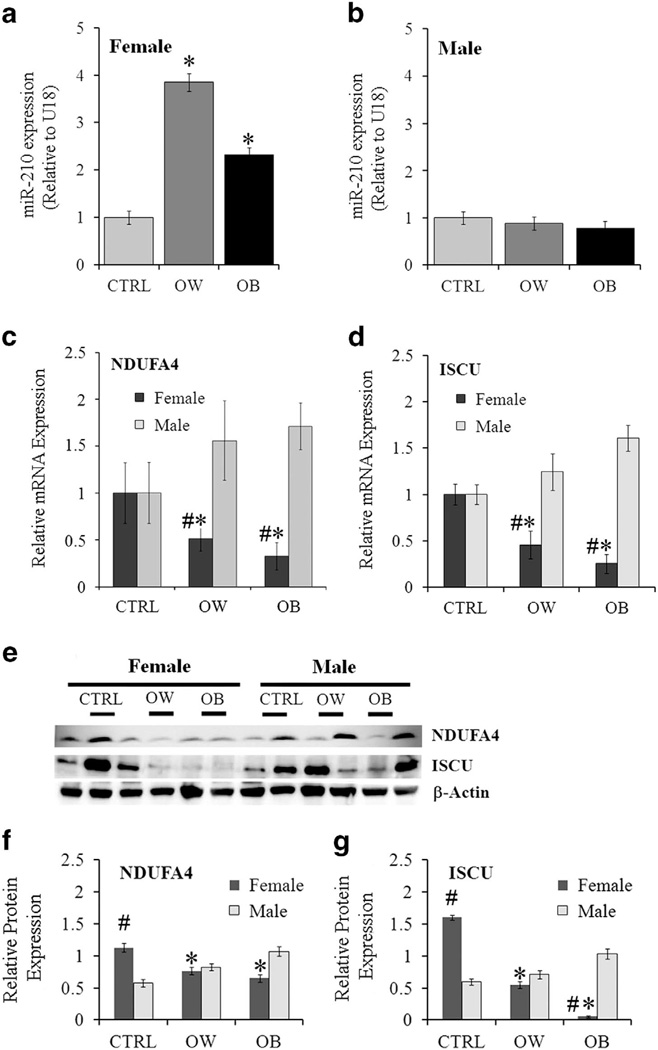
Expression of miR-210 was significantly increased in placentas from OW and OB women with female fetuses compared with controls (Figures 1a, P < 0.05) but remained unchanged across groups in placentas of males (Figure 1b). This increased miR-210 expression was associated with a significant reduction in the mRNA and protein levels of two mitochondria-related miR-210 target genes, NDUFA4 and ISCU, in the OW and OB placentas of females compared with corresponding CTRL (P < 0.05, Figures 1c–g). In placentas of males, no significant changes in the mRNA and protein levels of target genes with maternal adiposity were observed. Notable, protein levels of ISCU and NDUFA4 were significantly higher in CTRL placentas of females (P < 0.05) compared with males.
Figure 1.
Expression of miR-210 and its target genes in placentas of healthy weight, overweight and obese women. (a and b) Quantification of miR-210 by RT–PCR in CTRL, OW and OB placentas of females (a) and males (b). U18 was used as internal control. (c and d) mRNA levels of miR-210 targets NFUFA4 (c) and ISCU (d). (e–g) Protein levels of target genes normalized to β-actin measured by Western blot. (e) Representative images and quantification for NDUFA4 (f) and ISCU (g). Values are represented as mean ± s.e.m. *P < 0.05 vs CTRL group; #P < 0.05 vs males within the same group of adiposity; n=6 per gender per group.
The transcription factor NFκB1 functions upstream of miR-210 in placentas with maternal obesity
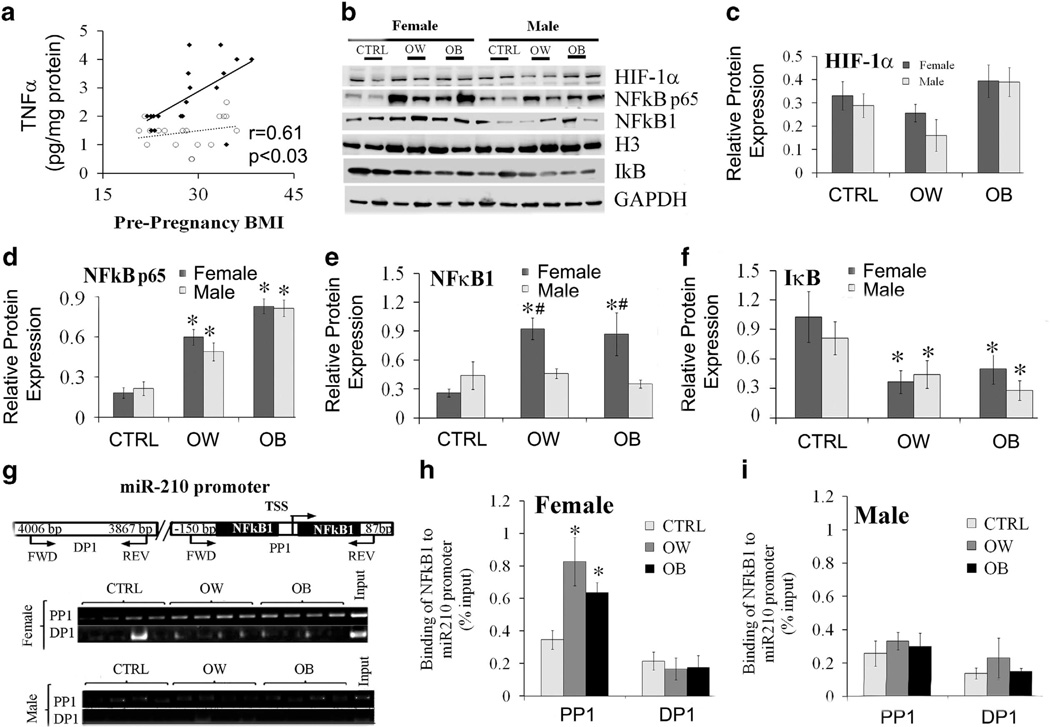
We next sought to determine the mechanisms, whereby miR-210 was upregulated in placentas with obesity. It was previously reported that miR-210 is induced by oxidative stress31 in several tissues23 under direct control of HIF-1α.24 However, we did not find any differences in expression of HIF-1α with maternal obesity in male or female placentas (Figures 2b and c). Obesity in pregnancy creates a physiologic state of chronic, low-grade inflammation, and inflammatory immune responses have been shown to act via degradation of IκB and activation of NFκB signaling.32–34 Using enzyme-linked immunosorbent assay (ELISA), we have measured the concentration of pro-inflammatory TNFα in placentas of males and females over the range of adiposity. We found that placental TNFα content correlates positively with maternal pre-gravid BMI (r = 0.61, P < 0.03) but only in placentas of females (Figure 2a). To examine the potential involvement of NFκB pathways in placentas with maternal obesity, we examined protein expression of NFκB p65, NFκB1 and IκB by Western Blot (Figures 2b and d–f). When compared with CTRL group, protein expression of NFκB p65 was increased three to fourfold in OW and OB placentas of both males and females. Placental IκB was significantly reduced, potentially due to proteomic degradation, in OW and OB women with either male or female fetuses, indicating activation of NFκB signaling. In contrast, NFκB1 (p50) was significantly increased only in OW and OB placentas of females vs corresponding CTRL with no change in placentas of males. In addition, protein expression of NFκB1 was significantly greater in the female OW and OB placentas vs corresponding male groups.
Figure 2.
Changes in expression of placental TNFα and hypoxia- and inflammation-related transcription factors with maternal obesity and fetal sex. (a) Placental TNFα expression measured by ELISA. (b–f) Protein expression of transcription factors in response to maternal obesity and fetal sex. Representative Western blots (b) and quantification of HIF-1α (c), NFκB p65 (d), NFκB1 (e) and IκB (f). GAPDH was used as loading control. G-I, DNA-binding activity of NFkB1 to miR-210 promoter. Chromatin Immunoprecipitation (CHIP) assay (g) and quantification data showing binding of NFκB1 to miR-210 promoter in female (h) and male (i) placentas. TSS, transcription start site, PP1, proximal promoter, positive binding site and DP1, distal promoter, negative binding site. Histone 3 (H3) was used as loading control. Values are represented as mean ± s.e.m. *P < 0.05 vs. CTRL, #P < 0.05 vs males within the same group of adiposity, n=6 per gender per group.
Since a scan of 1.5 kb of genomic sequence located upstream of the predicted pri-miR-210 start site using Transcription Element Search System (TESS, University of Pennsylvania, PA, USA) and the CHIPBase software (Sun Yat-sen University, Guangdong, China) identified a putative NFkB1 consensus binding site, CHIP assay was performed using anti-NFκB1 antibody (Figures 2g–i). NFκB1–coprecipitating DNA was analyzed by RT–PCR using miR-210 proximal promoter, positive binding site and distal promoter, negative binding site. We found significantly increased binding of NFκB1 to the miR-210 promoter in placentas of females from both OW and OB women (Figures 2g and h). No changes in NFκB1 binding were detected in placentas of males over the range of maternal adiposity (Figures 2g and i).
Inflammation increases the expression of miR-210 in a fetal sex-specific manner
To dissect the effect of NFκB1 on placental miR-210 expression, we mimicked an inflammatory response in vitro using primary trophoblasts isolated from placentas of women with a healthy BMI (< 25) and with uncomplicated pregnancies (Figure 3). TNFα, a primary regulator of inflammatory pathways, was used to activate NFκB signaling. We confirmed the induction of NFκB p65 and NFκB1 in TNFα–treated trophoblasts by Western Blot (Figures 3a–c). As expected, NFκB p65 expression was equally increased in the trophoblasts of males and females treated with TNFα (P < 0.05, n = 3 independent experiments in each group). However, NFκB1 was induced only in TNFα–treated trophoblasts of females. Correspondingly, the expression of miR-210 was increased by 3.5-fold (P < 0.05) in TNFα-treated trophoblasts of females only (Figure 3f). Pretreatment of trophoblasts with siRNA to NFκB1 (Figure 3e) and with antagomir-210 (Figure 3f) was sufficient to prevent the increase in miR-210 expression in TNFα-treated cells (Figure 3f). While mRNA levels were similar (Figure 3g), protein levels of the miR-210 mitochondria-related target genes, NDUFA4 and ISCU, were significantly decreased in TNFα-treated cells as compared with controls (Figure 3h). CHIP analysis confirmed an almost twofold increase in binding of NFκB1 to miR-210 promoter in TNFα-treated trophoblasts of female but not male fetuses, suggesting sexual dimorphism in trophoblast inflammatory responses (Figures 4a–c).
Figure 3.
Sex differences in expression of NFκB1, miR-210 and target genes in cultured trophoblasts on TNFα-mediated inflammatory stress. Representative Western Blots (a) and quantification (b and c) of protein levels of transcription factors NFκB p65 and NFκB1 in primary trophoblasts isolated from healthy weight women and treated with TNFα. NFκB1 was induced only in female trophoblasts treated with TNFα. (d and e) Infection of trophoblasts with siRNA to NFκB1 caused a 90% reduction in the protein level of NFκB1. (f) Activation of miR-210 expression in TNFα-treated trophoblasts of females. Pre-treatment with siRNA NFκB1 or antagomir-210 abolished the activation of miR-210 expression. (g and h) Quantification of mRNA expression (g) protein levels (h) of NDUFA4 and ISCU in trophoblasts of females treated with TNFα. Values are represented as mean ± s.e.m. *P < 0.05 vs CTRL and #P < 0.05 vs female trophoblasts, n =3 independent experiments/gender.
Figure 4.
CHIP analysis (a) and quantification data showing binding of NFκB1 to miR-210 promoter in cultured trophoblasts of female (b) and male (c) fetuses on exposure to TNFα. Values are represented as mean ± s.e.m. *P < 0.05 vs CTRL, n=3 independent experiments per gender.
Inflammation affects mitochondrial function in primary trophoblasts
To assess the effect of inflammation on mitochondrial respiration, we measured oxygen consumption rates using mito stress test27,28,30 in TNFα-treated trophoblasts of males and females. In the trophoblasts of females, TNFα significantly decreased basal oxygen consumption as well as ATP-coupled respiration measured by adding the ATP synthase inhibitor oligomycin (Figure 5). FCCP-stimulated oxygen consumption, a measure of maximal mitochondrial respiration, was decreased by 40% (P < 0.05, n = 3 separate experiments) with TNFα treatment (Figure 5). Reduction in ATP-coupled and maximal respiration but not basal respiration was prevented when trophoblasts were pre-treated with siRNA to NFκB1 and antagomir-210 (Figure 5). No changes in mitochondrial respiration were detected in TNFα-treated trophoblasts of males (Figure 5).
Figure 5.
Mitochondrial function is inhibited in TNFa-treated syncytiotrophoblasts in a fetal sex-dependent manner. Mitochondrial respiration was measured in the syncytiotrophoblasts of females and males from healthy weight women. The syncytiotrophoblasts were either untreated or treated with TNFα in the absence or presence of siRNA to NFκB1 or antagomiR-210. Individual oxygen consumption rate (OCR) parameters (basal, ATP-coupled and maximal respiration) were calculated based on total cellular protein and expressed as percentage levels in untreated cells. Values are represented as mean ± s.e.m. *P < 0.05 vs untreated cells,#P < 0.05 in cells treated with either siRNA NFκB1+TNFα or antagomir-210+TNFα vs cells treated with TNFα, n =3 independent experiments/gender.
DISCUSSION
More than 60% of women entering pregnancy in the US are OW or OB.35 Data from animal models and human studies suggest that increased maternal adiposity, reflected by greater maternal pre-pregnancy BMI, may affect adiposity in the offspring later in life.36 Pregnancy per se is a state of inflammation and oxidative stress, which can be clearly demonstrated in the placenta, where production of reactive oxygen species by mitochondria and by various enzymes occurs.37 The inflammation and oxidative stress are heightened in pregnancies with maternal obesity.13 OB women are more likely to enter pregnancy in a subclinical inflammatory state since increased accumulation of body fat is associated with elevated cytokine levels.38 Alternatively, maternal adiposity could produce a hypoxic state if glycosylated hemoglobin levels are increased and affinity for oxygen is reduced, decreasing oxygen transfer to the uterus and impairing normal placental development.35
MiR-210 was classically described as a HIF-1α-regulated miRNA, and has been implicated in various pathophysiological pathways, such as cancer, cardiovascular diseases, oxidative stress and apoptosis.39–43 In the placenta, miR-210 is significantly upregulated during preeclampsia,20,44 and we have shown that increased placental expression of miR-210 is involved in mitochondrial abnormalities during preeclampsia.27 Using gain- and loss-of-function approaches, we found that miR-210 is necessary and sufficient for the placental mitochondrial dysfunction observed in preeclampsia. MiR-210 notably targets several mitochondria-related proteins including ISCU and NDUFA4, which are critical for mitochondrial oxidation–reduction reactions and the assembly of electron transport system complexes, illustrating how miR-210 may strongly impact mitochondrial function.
Anton et al.45 have shown that an increase in miR-210 expression causes repression of extravillous trophoblast invasion. These results supported a study performed by Zhang et al.,44 which found that miR-210 represses CT invasion and migration by inhibition of its other targets EphrinA3 and HOXA9, both involved in migration and vascular remodeling. In addition, Anton et al.45 suggested that miR-210 has the potential to be a predictive and diagnostic biomarker for preeclampsia because serum miR-210 is elevated months before the onset of clinical symptoms.
In our recently published report, we showed a mitochondrial dysfunction, inhibition in mitochondrial biogenesis and metabolic inflexibility in male and female placentas with increased maternal adiposity.46 Surprisingly, however, the increase in expression of miR-210 was observed only in the female placentas of OW and OB women but not males, suggesting that other obesity-related mechanisms might trigger the mitochondrial dysfunction in males. For example, our metabolomics analysis has recently shown a decrease in mitochondria-derived lipid metabolites in OB placentas of male but not female fetuses. This has been shown to be associated with a decreased β-oxidation47 and might potentially underlie placental mitochondrial dysfunction in males. Presence of defective autophagy in OB placentas of males is another potential explanation. Defective autophagy in obesity has been previously linked to progressive increase in mitochondrial dysfunction.48
Overexpression of miR-210 has already been shown in the settings of obesity. For example, pancreatic islets isolated from a mouse model of obesity-related diabetes had increased levels of miR-210 compared with lean mice,49 as did gingival biopsy samples collected from OB vs lean patients.50 Bye et al.51 found an increase in circulating miR-210 in patients with reduced aerobic fitness, however, no correlation with patients’ BMI was observed. We have studied a specific mechanism of activation of miR-210 expression in placenta in the obesogenic environment: the NFκB1 pathway (see schematic in Figure 6). We focused on this pathway because maternal obesity is associated with increased inflammation and oxidative stress in the placenta.52 We show increased expression of miR-210 in placentas of females born to OB and OW mothers. Despite the fact that pregnancies with maternal obesity are characterized by increased oxidative/nitrative stress,14 we did not find any changes in levels of the transcription factor HIF-1α. In fact, a recently published study reports a reduction in HIF-1α in placentas with maternal obesity.52 This led us to a search for other transcription factors involved in regulation of miR-210 expression, and we show here that NFκB1 (p50) is able to bind the miR-210 promoter in a fetal sex- and maternal adiposity-specific manner. Our results are supported by the study of Zhang et al.,44 wherein JAR cells are exposed to TNFα, and the expression of miR-210 was shown to be regulated by NFκB1.
Figure 6.
Proposed model for role of maternal inflammation in fetal sex-dependent activation of NFκB1 and miR-210. Chronic maternal inflammation leads to proteasomal degradation of IκB and activation of NFκB1, and in turn, induces expression of miR-210 and mitochondrial dysfunction in trophoblasts of female fetuses, potentially programming female offspring of obese mothers for metabolic diseases in later life.
The NFκB family of inducible dimeric transcription factors are involved in activation of an exceptionally large number of genes in response to infections, inflammation and other stressful situations requiring rapid reprogramming of gene expression.53 This study suggests that fetal sex has a major influence on regulation of NFκB1 activity in the placenta. Whether it is the presence of a Y chromosome, an extra X chromosome, epigenetic changes or a difference in the hormonal milieu, the sex differences in the placenta are remarkably reflected in the placental adaptation to adverse intrauterine environment and its programming effect on later life disease. While no changes in total DNA methylation of miR-210 promoter were observed (data not shown) in the placentas of males and females from the healthy, OW and OB women, other epigenetic mechanisms might be responsible for the sexual dimorphism seen in the settings of maternal obesity.
Estrogens, for example, have been shown to activate NFκB signaling. Vina et al.54 report that estradiol activates mitogen-activated protein kinases, specifically ERK1 and ERK2, which, in turn, activate signaling pathways involving the transcription factor NFκB1 as indicated by an increase in abundance of the p50 subunit of NFκB. It remains to be clarified what triggers the sexually dimorphic effect of maternal obesity on placental function but comprehensive proteomics and metabolomics studies are underway to understand this important phenomenon.
Hormonal and genetic differences between males and females have considerable impact on regulation of miRNA function as well, however, there are very few studies highlighting the role of miRNAs in sex-biased diseases. Sex differences in expression of miRNAs have been observed in embryonic stem cell differentiation and germ line determination, where miR-302 is enriched in the male embryonic stem cell but not in female cells.55 Another example is the sex-associated differential expression of miRNAs during lung development, which ultimately leads to differences in the structure and function of lungs between males and females.56
In a murine model of fetal programming, male embryos were found to be most susceptible to maternal stress compared with female embryos. As adults, these male mice showed demasculinization in their response to stress, as well as a ‘female’ pattern of gene expression associated with neurodevelopment. The miRNA expression profile in these male brains showed a similarity to control female brains.57
Sex differences have a major role in non-communicable diseases, such as obesity and metabolic syndrome.58,59 Wang et al.60 have discovered that miR-221 and let-7 g were expressed more prominently in the plasma of women than men manifesting metabolic syndrome. This may have significant implications in the susceptibility of women to cardiovascular risk factors. Thus, it is likely that miRNAs are able to influence sex-specific responses to fetal programming, its pathogenesis and outcome.
In summary, our data provide evidence that the placenta responds to the maternal obesogenic environment in a fetal sex-specific manner. In placentas of female fetuses, an activation of signaling from inflammation via NFκB1 and miR-210 leading to mitochondrial dysfunction has been observed; all these features not being seen in placentas of males. Importantly, we demonstrate that primary trophoblasts derived from placentas of female fetuses showed higher sensitivity to inflammatory stress compared with placentas of males. Our findings reveal an essential role of maternal inflammatory status in regulation of placental mitochondrial metabolism and identify miR-210 as a central component of this fetal sex-biased metabolic regulatory mechanism.
ACKNOWLEDGEMENTS
We are thankful to the funding sources HD076259A (LM and AM), HL075297 (LM), and CTSA grant (UL1RR025767) from the Institute for Integration of Medicine and Science (IIMS) at UTHSCSA (AM).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Zhu MJ, Du M, Nathanielsz PW, Ford SP. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta. 2010;31:387–391. doi: 10.1016/j.placenta.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287:E472–E479. doi: 10.1152/ajpendo.00589.2003. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 4.Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am. 2009;36:285–300. doi: 10.1016/j.ogc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Reece EA. Obesity, diabetes, and links to congenital defects: a review of the evidence and recommendations for intervention. J Matern Fetal Neonatal Med. 2008;21:173–180. doi: 10.1080/14767050801929885. [DOI] [PubMed] [Google Scholar]
- 6.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed. 2011;96:F378–F382. doi: 10.1136/adc.2009.170928. [DOI] [PubMed] [Google Scholar]
- 7.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–2464. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417–421. doi: 10.1016/j.lfs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 11.Burton GJ, Fowden AL. Review: the placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33:S23–S27. doi: 10.1016/j.placenta.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 14.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30:169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myatt L, Roberts VH. Placental mechanisms and developmental origins of health and disease. In: Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. Cambridge, UK: Cambridge University Press; 2006. pp. 130–142. [Google Scholar]
- 16.Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, et al. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48:139–149. doi: 10.1530/JME-11-0123. [DOI] [PubMed] [Google Scholar]
- 17.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 18.Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF, Marsit CJ. Maternal cigarette smoking during pregnancy is associated with down regulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5:583–589. doi: 10.4161/epi.5.7.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196:261 e1–261 e6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011;204:178 e12–178 e21. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One. 2011;6:e21210. doi: 10.1371/journal.pone.0021210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2011;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakada C, Tsukamoto Y, Matsuura K, Nguyen TL, Hijiya N, Uchida T, et al. Overexpression of miR-210, a downstream target of HIF1alpha, causes centrosome amplification in renal carcinoma cells. J Pathol. 2011;224:280–288. doi: 10.1002/path.2860. [DOI] [PubMed] [Google Scholar]
- 25.Devlin C, Greco S, Martelli F, Ivan M. miR-210: more than a silent player in hypoxia. IUBMB Life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxiainducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307:E419–E425. doi: 10.1152/ajpendo.00025.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bax CM, Ryder TA, Mobberley MA, Tyms AS, Taylor DL, Bloxam DL. Ultrastructural changes and immunocytochemical analysis of human placental trophoblast during short-term culture. Placenta. 1989;10:179–194. doi: 10.1016/0143-4004(89)90039-8. [DOI] [PubMed] [Google Scholar]
- 30.Maloyan A, Mele J, Muralimanohara B, Myatt L. Measurement of mitochondrial respiration in trophoblast culture. Placenta. 2012;33:456–458. doi: 10.1016/j.placenta.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magenta A, Greco S, Gaetano C, Martelli F. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci. 2013;14:17319–17346. doi: 10.3390/ijms140917319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naeye RL. Maternal body weight and pregnancy outcome. Am J Clin Nutr. 1990;52:273–279. doi: 10.1093/ajcn/52.2.273. [DOI] [PubMed] [Google Scholar]
- 33.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–446. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- 34.Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstet Gynecol. 2007;110:743–744. doi: 10.1097/01.AOG.0000284990.84982.ba. [DOI] [PubMed] [Google Scholar]
- 35.King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- 36.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 37.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 39.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 41.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 42.Gee HE, Camps C, Buffa FM, Patiar S, Winter SC, Betts G, et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer. 2010;116:2148–2158. doi: 10.1002/cncr.25009. [DOI] [PubMed] [Google Scholar]
- 43.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One. 2010;5:e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anton L, Olarerin-George AO, Schwartz N, Srinivas S, Bastek J, Hogenesch JB, et al. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol. 2013;183:1437–1445. doi: 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307:E419–E425. doi: 10.1152/ajpendo.00025.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O'Connell TM, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One. 2012;7:e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, et al. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56:2203–2212. doi: 10.1007/s00125-013-2993-y. [DOI] [PubMed] [Google Scholar]
- 50.Perri R, Nares S, Zhang S, Barros SP, Offenbacher S. MicroRNA modulation in obesity and periodontitis. J Dent Res. 2012;91:33–38. doi: 10.1177/0022034511425045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bye A, Rosjo H, Aspenes ST, Condorelli G, Omland T, Wisloff U. Circulating microRNAs and aerobic fitness--the HUNT-Study. PLoS One. 2013;8:e57496. doi: 10.1371/journal.pone.0057496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35:171–177. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 54.Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males: control of longevity by sex hormones. Sci Aging Knowledge Environ. 2005;2005:pe17. doi: 10.1126/sageke.2005.23.pe17. [DOI] [PubMed] [Google Scholar]
- 55.Ciaudo C, Servant N, Cognat V, Sarazin A, Kieffer E, Viville S, et al. Highly dynamic and sex-specific expression of microRNAs during early ES cell differentiation. PLoS Genet. 2009;5:e1000620. doi: 10.1371/journal.pgen.1000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mujahid S, Logvinenko T, Volpe MV, Nielsen HC. miRNA regulated pathways in late stage murine lung development. BMC. Dev Biol. 2013;13:13. doi: 10.1186/1471-213X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambon A, Pauletto P, Crepaldi G. Review article: the metabolic syndrome--a chronic cardiovascular inflammatory condition. Aliment Pharmacol Ther. 2005;22:20–23. doi: 10.1111/j.1365-2036.2005.02589.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang YT, Tsai PC, Liao YC, Hsu CY, Juo SH. Circulating microRNAs have a sex-specific association with metabolic syndrome. J Biomed Sci. 2013;20:72. doi: 10.1186/1423-0127-20-72. [DOI] [PMC free article] [PubMed] [Google Scholar]