Abstract
Background
C-reactive protein has been evaluated as a risk factor for breast cancer in epidemiologic studies. However, results from prospective studies are inconsistent.
Methods
We evaluated the association using pre-diagnostic blood samples in a case-control study nested within the Nurses' Health Study (NHS) and the full cohort of the Women's Health Study (WHS). 943 cases in the NHS and 1919 cases in the WHS contributed to the analysis. Conditional logistic regression and Cox proportional hazards model were used in the NHS and WHS, respectively. We pooled our results with prior prospective studies using random effect meta-analysis.
Results
In the NHS, higher CRP levels were associated with a suggestively increased risk of breast cancer (quintile 5 vs. 1: relative risk [RR] = 1.27, 95% confidence interval [CI] = 0.93, 1.73; Ptrend = 0.02); results did not vary significantly by tumor invasiveness or hormone receptor status. However, no association was observed in the WHS for overall risk (quintile 5 vs. 1: RR = 0.89, 95%CI = 0.76, 1.06; Ptrend = 0.38) or by tumor invasiveness or hormone receptor status. The meta-analysis (including 5371 cases from 11 studies) showed a modestly increased risk among women in the highest vs. lowest categories of CRP (RR = 1.26, 95%CI = 1.07, 1.49).
Conclusions
Existing data from prospective studies suggest that CRP, a non-specific marker of inflammation, is modestly positively associated with breast cancer risk.
Impact
Our findings provide support to the concept that inflammation can influence breast cancer development.
Keywords: C-reactive Protein, Breast Cancer, Prospective, Epidemiology, Meta-analysis
Introduction
C-reactive protein (CRP), a non-specific acute phase protein produced primarily by the liver, has been widely used as a marker of systemic inflammation (1). A meta-analysis assessing pre-diagnostic circulating CRP and overall cancer risk in prospective studies published before 2008 reported a modest positive association (relative risk [RR] per natural log increase: 1.10; 95% confidence interval [CI]: 1.02, 1.18) (2) and these results have been supported by subsequent prospective studies (3-5). In terms of prospective assessments for specific cancers, a consistent positive association for lung cancer (3, 5-10), ovarian cancer (11) and a suggestive positive association for colon cancer (8, 12-14) have been reported.
Results of the association between circulating CRP and breast cancer risk in prospective studies are inconsistent. The meta-analysis mentioned above reported a non-significant increased risk (RR per log unit increase: 1.10; 95%CI: 0.97, 1.26) with moderate between study heterogeneity (I2 = 51%) (2). Of eight subsequent prospective studies (85 to 706 cases), no significant association was observed in four (3, 4, 15, 16), while a significant or borderline significant, increased risk (with extreme contrast RR∼1.3-2.0), was seen in the remaining studies (17-20). Further, elevated circulating CRP levels after breast cancer have been reported to be associated with a worse prognosis in some studies (21-23) but not others (24, 25). Despite the inconsistent association in epidemiologic studies, molecular studies suggest that CRP may be involved in breast tumorigenesis (26). Activation of Nuclear Factor Kappa B (NFkβ) leads to increased transcription of genes involved in inflammation, including cyclooxygenase 2 (COX-2). Overexpression of COX-2 results in elevated expression of prostaglandin E2 which can act as an aromatase promoter (27) and thus increase local estrogen production.
Given prior inconsistent results and the modest statistical power in most previous prospective studies, we evaluated pre-diagnostic circulating CRP levels and risk of incident breast cancer in two large prospective studies, the Nurses' Health Study (NHS) and the Women's Health Study (WHS). The current analysis in the WHS extends findings from an earlier report (28) by adding seven years of follow-up and more than doubling the number of breast cancer cases (892 to 1919). Finally, to summarize all available data on this association, we conducted a meta-analysis of prior prospective studies and our studies.
Materials and Methods
Study Population
Details of the study design of the NHS (29) and the WHS (30) have been reported elsewhere. Briefly, the NHS was established in 1976 when 121,700 U.S. female registered nurses, aged 30-55 years, completed an initial mailed questionnaire. The cohort has been followed biennially to update exposure status and ascertain newly diagnosed disease. During 1989-1990, 32,826 participants, aged 43-69 years, provided a blood sample; each participant arranged to have her blood drawn and shipped via overnight courier and with an ice pack to our laboratory, where it was processed and separated into plasma, red blood cell, and white blood cell components. Breast cancer cases had no previously reported cancer diagnosis before blood collection and were diagnosed after blood collection but before June 1998. A total of 981 cases were identified, with mean time from blood draw to diagnosis of 53.4 months (range 1-106 months). Cases were confirmed by medical record review (99%) or confirmation by the nurse (1%). One to two controls were matched to each case by age (±2 years), month of blood collection (±1 month), time of day of blood draw (±2 hours), fasting status (>8 or ≤8 hours), menopausal status at blood collection and diagnosis, and postmenopausal hormone (PMH) use at blood collection (yes or no). Covariate data for the NHS were obtained from the biennial questionnaires and the short questionnaire completed at the time of the blood draw.
The WHS is a completed randomized controlled clinical trial initiated in 1992, when 39,876 U.S. female health professionals, aged 45 years and older, were enrolled. The objective of the trial was to evaluate the benefits and risks of low-dose aspirin and vitamin E in the primary prevention of cancer and cardiovascular disease (30); results suggest that neither of the interventions decrease risk of cancer or major cardiovascular diseases (31, 32). Before random assignment, 28,345 participants provided whole blood samples that were sent to the laboratory via overnight courier with an ice pack; samples were separated into plasma, red blood cell, and white blood cell components. When the trial was completed in 2004, 88% of the women agreed to participate in a follow-up observational study. Breast cancer cases were self-reported on mailed questionnaires, sent every 6-months during the first year after enrollment and then annually. Medical records were then obtained to confirm self-reports and only confirmed cases (98% of reported) were included in the analysis. A total of 1,919 cases diagnosed after blood draw and before March 2011 were identified. Covariate data for WHS were obtained from the baseline questionnaire.
In both NHS and WHS, participant deaths were identified by reports from next of kin, postal authorities or by searching the National Death Index. Cause of death was ascertained from death certificates and physician review of medical records and National Death Index reports. Both the NHS and the WHS were approved by the Committee on the Use of Human Subjects in Research at the Brigham and Women's Hospital.
Laboratory Assays
Plasmas samples were assayed for high-sensitivity CRP in both studies and CRP levels were measured using a validated immunoturbidometric method (Denka Seiken, Tokyo, Japan) in the same laboratory (Dr. Nader Rifai's laboratory at the Children's Hospital Medical Center, Boston, MA). Assays were run in two batches in the NHS and one large continuous batch in the WHS. Case-control pairs in the NHS were assayed together and laboratory personnel were blinded to case, control, or quality control status. The coefficient of variation from blinded, replicate quality control samples were 7.7% and 15.0% in the two batches in the NHS, respectively and less than 7% in the WHS (33).
Statistical Analysis
Statistical outliers were identified using the generalized extreme Studentized deviate many-outlier detection approach (34) and no outliers were found in either of the studies. In the NHS, mean CRP levels of the quality control samples differed by batch (P-value = 0.002); thus, CRP levels were recalibrated to represent the average CRP distribution across batches as previously described (35). CRP levels were modeled as quintiles based on the distribution among controls (NHS) or the entire cohort (WHS) as well as in three categories (i.e. <1, 1-3, 3-10 mg/L) based on the cutoff points proposed for cardiovascular disease prevention (36); CRP levels > 10mg/L were excluded in the three-category analysis considering possible acute inflammation. All analyses were first conducted separately by cohort; results were then combined using meta-analysis (37). In the NHS, for the primary analysis, conditional logistic regression was used to estimate RR and 95% CI. In the WHS, follow-up time accrued from the date of randomization until the date of diagnosis of any type of cancer, death, or March 2011, whichever came first and Cox proportional hazards models were used to calculate hazard ratio (HR) and 95%CI. No evidence of violation of proportionality assumption was observed. Both analyses were adjusted for first degree family history of breast cancer, history of benign breast disease, age at menarche, parity and age at first birth, smoking status, alcohol consumption, BMI at blood draw, and physical activity. The WHS was further adjusted for age, randomized treatment assignment, menopausal status and PMH use. Results from age-adjusted analysis were similar to those from multivariable adjusted, and thus only the latter are shown. Given that the BMI and breast cancer association varies by menopausal status, we tried adding a product term of BMI and menopausal status to better control for BMI. However, adding this interaction term did not alter the association and thus only BMI was included in the multivariable model. Trend tests were performed by modeling the median values of CRP categories as a continuous variable in multivariable models.
We further evaluated the association between circulating CRP and breast cancer risk by tumor estrogen receptor [ER] and progesterone receptor [PR] expression status. Polytomous logistic regression (NHS) or a competing-risk model (WHS) was used to test if the association differed by tumor subtype. We also examined the association stratified by menopausal status, BMI at blood draw and aspirin use (non-current vs. current users at blood collection in the NHS and the randomized aspirin assignment groups in the WHS). Tests of interaction were performed using a likelihood ratio test by including an interaction term of the median values of the CRP categories and the stratified variable. For the stratified analyses, unconditional logistic regression models with adjustment for matching factors and other covariates were used in the NHS to maximize statistical power. In addition, to assess if CRP is merely a marker of occult cancer or inflammation, we performed sensitivity analysis by restricting to participants free of any cancer with at least 4 years of follow-up (median of the follow-up time) after blood sample collection in the NHS, while in the WHS, given the relatively long follow-up period, the sensitivity analysis was conducted in three strata (≤5, 6 -10, 10+ years). Similar analytical approaches for risk of incident breast cancer were applied when assessing risk of fatal breast cancer except that the outcome was fatal breast cancer and non-fatal breast cancer cases were excluded in the NHS or follow-up time was censored at diagnosis in the WHS. For this analysis, death follow-up ended in June 2012 for the NHS and March 2011 for the WHS.
Finally, we performed a meta-analysis. We searched for ‘c-reactive protein’ or ‘CRP’ and ‘breast cancer’ and ‘prospective’, and included prospective studies published online until December 2014. We excluded one eligible study in which CRP levels below 10mg/L were not quantified (38). In addition, an earlier paper based on data from the WHS (28) was not included since the present WHS includes all prior cases. The relative risk estimates were most frequently reported by quantile or category of CRP; we excluded four studies which did not evaluate CRP as a categorical variable (2, 8, 9, 16). In total, nine prior prospective studies were included in the meta-analysis, of which five studies included only invasive cases (3, 4, 6, 17, 39), and four studies included both invasive and in situ cases (15, 18-20) (Supplementary Table 1). Because our data and several of the included studies (3, 4, 19, 20) showed similar results when high CRP levels (e.g. >10 mg/L) were excluded or retained, we did not exclude CRP values higher than 10 mg/L in the meta-analysis. We pooled results of the relative risk of the highest quantile of CRP levels (vs. the reference group) from each study and calculated pooled relative risk using a random effect model (37). All P-values were based on two-sided tests and considered statistically significant if < 0.05. Analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC, USA).
Results
In both studies, breast cancer cases and non-cases were similar for most of the characteristics except that cases were more likely to have family history of breast cancer and history of benign breast disease (Table 1). Of note, the proportions were higher in the NHS than the WHS because the information used was updated biennially in the NHS versus a single baseline assessment in the WHS. Compared to the NHS, more women were nulliparous in the WHS although, among parous women, average parity was about 20% higher in WHS. More than 70% of the study population was postmenopausal at blood draw in the NHS while less than 60% was postmenopausal at blood draw/randomization in the WHS; among postmenopausal women, those in the WHS were more likely to be PMH users than those in the NHS. The baseline plasma CRP levels were higher in cases than controls (P = 0.05) in the NHS while the levels were similar between cases and non-cases in the WHS.
Table 1.
Characteristics of the Study Populations in the NHS and WHS.
| NHS | WHS | |||
|---|---|---|---|---|
|
|
|
|||
| Cases (N=943) | Controls (N=1221) | Cases (N=1919) | Non-cases (N=25981) | |
| Mean age, yrs | 57.3 | 58.3 | 55.6 | 54.6 |
| Mean BMI, kg/m2 | 25.4 | 25.5 | 25.7 | 26.0 |
| Mean physical activity, kcal/week | 1024.4 | 1123.9 | 987.3 | 986.9 |
| Mean alcohol consumption, gram/day | 5.6 | 5.4 | 4.8 | 4.1 |
| Current smoker, % | 12 | 12 | 12 | 12 |
| Age at menarche, ≥ 13 y, % | 49 | 51 | 45 | 48 |
| Nulliparous, % | 7 | 7 | 16 | 13 |
| Paritya, ≥ 3, % | 34 | 31 | 54 | 56 |
| Mean age at first birth, yrs | 25.0 | 25.0 | 24.9 | 24.2 |
| Postmenopausal women, % | 71 | 73 | 59 | 54 |
| Current PMH userb, % | 54 | 39 | 74 | 71 |
| Mean age at menopause, yrs | 48.4 | 47.9 | 47.9 | 47.1 |
| Family history of breast cancerc, % | 23 | 16 | 7.7 | 6.2 |
| History of benign breast diseasec, % | 55 | 42 | 44.1 | 34.8 |
| Median CRP (IQR), mg/L | 1.6 (0.6-3.5) | 1.4 (0.6-3.0) | 2.0 (0.8-4.3) | 2.0 (0.8-4.4) |
Among parous women.
Among postmenopausal women.
Updated every 2-year in the NHS but only baseline in the WHS.
Increasing plasma CRP levels were associated with a suggestively increased risk of breast cancer overall in the NHS (Q5 vs. Q1: RR = 1.27, 95%CI = 0.93, 1.73; Ptrend = 0.02) while no significant association was found in the WHS (Q5 vs. Q1: RR = 0.89, 95%CI = 0.76, 1.06; Ptrend = 0.38) (Table 2). Although a combined analysis of the two studies showed no association overall (Q5 vs. Q1: RR = 1.04, 95%CI = 0.74, 1.46; Ptrend = 0.52), significant between study heterogeneity was observed (Pheterogeneity = 0.01). In the NHS only, where circulating estradiol levels were available for a subset of the participants (N=292), a modest attenuation of the RR was observed after further adjusting for estradiol (e.g. Q5 vs. Q1 RR = 1.19, 95%CI = 0.71, 2.02 and RR = 1.10, 95%CI = 0.65, 1.87, before and after adjusting for estradiol respectively). Similar results were observed for risk of invasive breast cancer in each study and the combined analysis. Because women enrolled in the WHS were free of cardiovascular disease at baseline while women in the NHS could have heart disease, we performed a sensitivity analysis in the NHS by excluding those with a history of heart disease at blood draw; however, results were essentially unchanged (e.g. in all cases: Q5 vs. Q1: RR = 1.28, 95%CI = 0.93, 1.75; Ptrend = 0.02).
Table 2.
Pre-diagnostic Plasma CRP Levels and Risk of Breast Cancer Overall and Risk of Fatal Breast Cancer in the NHS and the WHS and the Combined Analysis.
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Trend-test P-value | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Risk of incident breast cancer overall | ||||||||
| All cases | NHS | Ca|Co | 192|245 | 158|242 | 161|243 | 200|246 | 232|245 | |
| RR(95%CI)a | 1.00Ref | 0.86 (0.64, 1.16) | 0.93 (0.69, 1.26) | 1.12 (0.83, 1.52) | 1.27 (0.93, 1.73) | 0.02 | ||
| WHS | Ca|Person-years | 401|89037 | 364|87230 | 384|86084 | 421|86008 | 349|85736 | ||
| HR(95%CI)b | 1.00Ref | 0.92 (0.79, 1.06) | 0.97 (0.84, 1.13) | 1.05 (0.90, 1.23) | 0.89 (0.76, 1.06) | 0.38 | ||
| Combined | 1.00Ref | 0.90 (0.79, 1.03) | 0.97 (0.84, 1.10) | 1.07 (0.93, 1.22) | 1.04 (0.74, 1.46) | 0.52 | ||
| P-hetc | 0.01 | |||||||
|
|
||||||||
| Risk of fatal breast cancer overall | ||||||||
| All cases | NHS | Ca|Co | 12|245 | 18|242 | 17|243 | 16|246 | 36|245 | |
| RR(95%CI)d | 1.00Ref | 1.35 (0.62, 2.94) | 1.25 (0.56, 2.79) | 1.10 (0.48, 2.53) | 2.50 (1.17, 5.33) | 0.004 | ||
| WHS | Ca|Person-years | 16|87377 | 16|85191 | 27|84066 | 28|84049 | 18|83587 | ||
| HR(95%CI)b | 1.00Ref | 0.97 (0.47, 2.00) | 1.61 (0.83, 3.12) | 1.35 (0.67, 2.70) | 1.12 (0.52, 2.42) | 0.97 | ||
| Combined | 1.00Ref | 1.13 (0.67, 1.92) | 1.45 (0.87, 2.42) | 1.24 (0.73, 2.11) | 1.68 (0.77, 3.67) | 0.33 | ||
| P-hetc | 0.03 | |||||||
Conditional logistic regression adjusted for family history of breast cancer, history of benign breast disease, BMI at blood collection (<21, 21-<23, 23-<25, 25-<30, ≥30kg/m2), age at menarche (<12, 12, 13, >13 years), parity and age at first birth (nulliparous, 1-2 children and <25, 1-2 children and 25-<30, 1-2 children and ≥30, >2 children and <25, >2 children and ≥25 years), alcohol (none, >0-<5, 5-<10, ≥10 g/day, missing), smoking (never/former/current), and physical activity (quartiles of weekly recreational activities mets-h/week). Matching variables were age, month of blood collection, time of day of blood draw, fasting status, menopausal status at blood collection and diagnosis and PMH use at blood collection.
Adjusted for the same variables in a and age (continuous), randomized treatment assignment (aspirin vs. placebo, vitamin E vs. placebo, and beta-carotene vs. placebo), menopausal status and postmenopausal hormone use (premenopausal, postmenopausal without hormone use, postmenopausal with hormone use, unknown or missing).
P-value for test between study heterogeneity of the trend.
Adjusted for family history of breast cancer, history of benign breast disease, BMI at blood collection, age at menarche, parity and age at first birth, alcohol, smoking, physical activity, age, month of blood collection, time of day of blood draw, fasting status, menopausal status at blood draw and diagnosis and PMH use at blood collection.
Study-specific quintile cut-offs (mg/L): NHS: 0.49, 1.02, 1.82, 3.56; WHS: 0.65, 1.46, 2.74, 5.17.
When stratified by tumor hormone receptor status, in the NHS, a slightly stronger positive association was observed for ER+/PR+ tumors (Q5 vs. Q1: RR = 1.40, 95%CI = 0.95, 2.08; Ptrend = 0.01) while increasing CRP levels were not significantly associated with risk for either ER-/PR- or ER+/PR- tumors (Ptrend = 0.21 and 0.67 for ER-/PR- and ER+/PR-, respectively; Pheterogeneity = 0.68) (Table 3). Associations were not different by tumor hormone receptor status in the WHS (Pheterogeneity = 0.86) and no significant association was noted in any tumor subtype.
Table 3.
Pre-diagnostic Plasma CRP Levels and Risk of Invasive Breast Cancer and by Tumor Subtypes in the NHS and the WHS and the Combined Analysis.
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Trend-test P-value | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Risk of invasive breast cancer and by tumor hormonal receptor subtypes | ||||||||
| Invasive | NHS | Ca|Co | 159|245 | 130|242 | 126|243 | 166|246 | 203|245 | |
| RR(95%CI)a | 1.00Ref | 0.84 (0.62, 1.13) | 0.82 (0.60, 1.13) | 1.10 (0.80, 1.50) | 1.30 (0.95, 1.79) | 0.01 | ||
| WHS | Ca|Person-years | 311|89037 | 289|87230 | 334|86084 | 339|86008 | 278|85736 | ||
| HR(95%CI)b | 1.00Ref | 0.92 (0.78, 1.09) | 1.06 (0.90,1.25) | 1.06 (0.89, 1.25) | 0.87 (0.72, 1.05) | 0.16 | ||
| Combined | 1.00Ref | 0.90 (0.78, 1.04) | 0.97 (0.77, 1.22) | 1.07 (0.92, 1.24) | 1.04 (0.70, 1.54) | 0.56 | ||
| P-hetc | 0.002 | |||||||
|
|
||||||||
| ER+/PR+ | NHS | Ca|Co | 83|245 | 72|242 | 69|243 | 85|246 | 108|245 | |
| RR(95%CI)a | 1.00Ref | 0.88 (0.61, 1.29) | 0.93 (0.63, 1.37) | 1.14 (0.77, 1.68) | 1.40 (0.95, 2.08) | 0.01 | ||
| WHS | Ca|Person-years | 209|89037 | 201|87230 | 228|86084 | 232|86008 | 196|85736 | ||
| HR(95%CI)b | 1.00Ref | 0.96 (0.78, 1.17) | 1.06 (0.87, 1.30) | 1.07 (0.87, 1.31) | 0.90 (0.72, 1.13) | 0.30 | ||
| Combined | 1.00Ref | 0.94 (0.79, 1.12) | 1.03 (0.86, 1.23) | 1.08 (0.90, 1.30) | 1.09 (0.71, 1.68) | 0.54 | ||
| P-hetc | 0.01 | |||||||
|
|
||||||||
| ER-/PR- | NHS | Ca|Co | 25|245 | 21|242 | 17|243 | 25|246 | 30|245 | |
| RR(95%CI)a | 1.00Ref | 0.83 (0.44, 1.56) | 0.72 (0.36, 1.42) | 1.10 (0.58, 2.09) | 1.22 (0.64, 2.33) | 0.21 | ||
| WHS | Ca|Person-years | 42|89037 | 40|87230 | 48|86084 | 42|86008 | 38|85736 | ||
| HR(95%CI)b | 1.00Ref | 0.95 (0.61, 1.49) | 1.25 (0.81, 1.95) | 1.10 (0.69, 1.77) | 1.01 (0.60, 1.69) | 0.96 | ||
| Combined | 0.91 (0.63, 1.31) | 1.01 (0.59, 1.72) | 1.10 (0.75, 1.61) | 1.09 (0.73, 1.63) | 0.55 | |||
| P-hetc | 0.27 | |||||||
|
|
||||||||
| ER+/PR- | NHS | Ca|Co | 18|245 | 18|242 | 15|243 | 20|246 | 15|245 | |
| RR(95%CI)d | 1.00Ref | 0.85 (0.42, 1.73) | 0.73 (0.34, 1.56) | 0.96 (0.46, 2.02) | 0.75 (0.34, 1.68) | 0.67 | ||
| WHS | Ca|Person-years | 40|89037 | 34|87230 | 31|86084 | 36|86008 | 29|85736 | ||
| HR(95%CI)b | 1.00Ref | 0.78 (0.49, 1.25) | 0.71 (0.43, 1.16) | 0.71 (0.43, 1.18) | 0.63 (0.37, 1.09) | 0.20 | ||
| Combined | 1.00Ref | 0.80 (0.54, 1.18) | 0.71 (0.47, 1.08) | 0.78 (0.52, 1.19) | 0.67 (0.43, 1.05) | 0.18 | ||
| P-hetc | 0.83 | |||||||
Adjusted for the same variables as Table 2 footnote d.
Adjusted for the same variables as Table 2 footnote b.
P-value for test between study heterogeneity of the trend.
Adjusted for the same variables in a except that parity and age at first birth was adjusted as 0-2 children and <25, 1-2 children and 25-<30, 1-2 children and ≥30, >2 children and <25, >2 children and ≥25 years.
Study-specific quintile cut-offs (mg/L): NHS: 0.49, 1.02, 1.82, 3.56; WHS: 0.65, 1.46, 2.74, 5.17.
When plasma CRP levels were modeled using clinical cut-points, increasing levels were associated with slightly but not significantly increased risk of breast cancer overall (3-10 vs. <1 mg/L: RR = 1.26, 95%CI = 0.97, 1.64; Ptrend = 0.10) in the NHS while no association was observed in the WHS (3-10 vs. <1 mg/L: RR = 1.02, 95%CI = 0.89, 1.16; Ptrend = 0.60). The combined analysis was null (RR = 1.08, 95%CI = 0.93, 1.26; Ptrend = 0.34) with no significant between study heterogeneity (Pheterogeneity = 0.17).
The associations between CRP and breast cancer risk did not vary significantly by menopausal status (Pinteraction = 0.15 and 0.13 in the NHS and WHS, respectively), BMI (comparable Pinteraction = 0.97 and 0.39) or aspirin use at the time of blood draw in either the NHS or the WHS (comparable Pinteraction = 0.48 and 0.80) (Supplementary Table 2). When stratified by menopausal status, despite a non-significant interaction in the NHS (Pinteraction = 0.15), a modestly positive association between plasma CRP and breast cancer risk was found among post-menopausal women (Q5 vs. Q1: RR = 1.35, 95%CI = 0.94, 1.95; Ptrend = 0.003); however, increasing CRP levels were associated with a non-significant decreased risk in WHS postmenopausal women (Q5 vs. Q1: RR = 0.82, 95%CI = 0.65, 1.02; Ptrend = 0.10; between study Pheterogeneity = 0.001). No association was observed among pre-menopausal women in either study. Although no significant interaction was seen, the CRP and breast cancer association was more pronounced among PMH users: a positive association was observed in the NHS (Q5 vs. Q1: RR = 1.41, 95%CI = 0.82, 2.42; Ptrend = 0.01) while an inverse association was seen in the WHS (Q5 vs. Q1: RR = 0.70, 95%CI = 0.53, 0.92; Ptrend = 0.04).
We assessed if the CRP and breast cancer association varied by years of follow-up (Supplementary Table 3). In the NHS, the positive association appeared stronger among participants with 5-8 years of follow-up (Q5 vs. Q1: RR = 1.40, 95%CI = 0.93-2.11; Ptrend = 0.01) while no association was found when restricting to cases diagnosed within 4 years. In the WHS, an overall null association was seen among cases diagnosed more than 5 years since baseline CRP measurement while a non-significant inverse association was observed when restricting to cases diagnosed within 5 years (Q5 vs. Q1: RR = 0.75, 95%CI = 0.53-1.05; Ptrend = 0.10).
Finally, increasing levels of pre-diagnostic CRP were significantly associated with increased risk of fatal breast cancer in the NHS (Q5 vs. Q1: RR = 2.50, 95%CI = 0.93-1.73; Ptrend = 0.02) but no significant dose-response relationship was found in the WHS (Q5 vs. Q1: RR = 1.12, 95%CI = 0.52, 2.42; trend test P-value = 0.97) (between study Pheterogeneity = 0.03) (Table 2).
In the meta-analysis of 11 prospective studies, a modest but statistically significant increased risk of breast cancer was observed among women in the highest CRP exposure group (highest vs. lowest category: RR = 1.26; 95%CI = 1.07, 1.49) (Figure 1). However, moderate between study heterogeneity was observed (I2 = 45%, 95%CI = 0, 80%).
Figure 1.
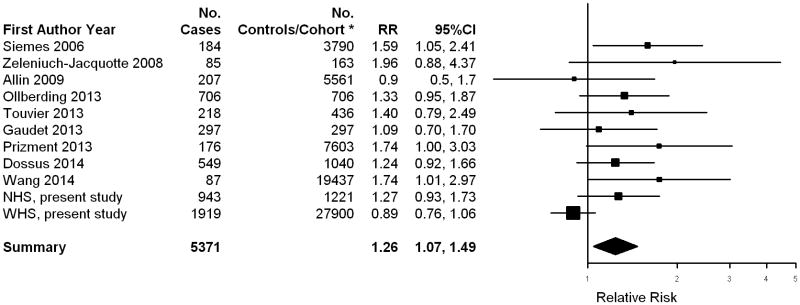
Relative Risk of Incident Breast Cancer Among Women in the Highest Category of CRP levels (vs. the Lowest Category of CRP Levels): A Meta-analysis of Prospective Studies. *Numbers of women in cohort at baseline are presented for prospective cohort studies (Siemes 2006, Allin 2009, Prizment 2013, Wang 2014 and WHS, present study), and numbers of controls are presented for nested case-control studies (all other studies).
Discussion
In the nested case-control study in the NHS, we found a modestly increased risk for overall, invasive, and hormone receptor positive breast cancer among women with the highest plasma CRP levels. On the other hand, in the WHS, no association was observed for both all cases combined and in the various case subgroups. The meta-analysis of prior prospective studies along with the NHS and the WHS showed a modest, statistically significant, positive association among women in the highest exposure group, but with moderate between study heterogeneity.
Of the 11 prospective studies contributing to the meta-analysis, the combined NHS and WHS provided 53% (2862/5371) of the total breast cancer cases. Five studies were not included in our meta-analysis because they did not use a high sensitivity CRP assay (38), or because the relative risk estimate needed was not available (2, 8, 9, 16). More specifically, one study with 1241 breast cancer cases was conducted before the availability of the high sensitivity assay, thus circulating CRP levels less than 10 mg/L were not measured precisely (38). They found that, compared to CRP <10 mg/L, increasing CRP levels were associated with a non-significantly decreased risk. Because we aimed to assess chronic low-grade inflammation and breast cancer risk, we excluded this study from the meta-analysis. Of the other four studies excluded, two reported a non-significant positive association (RR: 1.16 ∼ 1.32 per unit increase in SD or natural log CRP) (8, 9) and another two found a null association (2, 16). However, exclusion of these studies would not significantly change the meta-analysis results given their small sample size (total number of the cases of the four studies: 395). Among the prospective studies included in the meta-analysis, in addition to the NHS, four other studies reported a significant, modest to moderate, positive dose-response relationship (6, 17, 19, 20). The remaining reported either no association (3) or a non-significant or borderline significant positive association only among the highest CRP exposure group (4, 15, 18, 39).
We did not find consistent results between the WHS and NHS and these differences were statistically significant in some analyses. There are several possible reasons for this heterogeneity. First, differences in characteristics of the study populations may have contributed. The NHS had a higher proportional of post-menopausal women than the WHS, and, among postmenopausal women, WHS women were more likely than NHS women to use PMH. However, when stratified by menopausal status, results similar to the primary analysis remained in postmenopausal women in the NHS and the WHS, respectively while no association was observed in premenopausal women in both studies. Further, the association did not significantly vary by PMH use in both studies. In addition, the major factor correlated with CRP levels in this analysis was BMI (Spearman r: 0.35 ∼ 0.40); other factors that slightly correlated with CRP included age, PMH use and physical activity. The distribution of all these factors, expect PMH use, was similar between the two cohorts, and all the factors related to CRP were adjusted in the multivariable models. Secondly, the NHS had a shorter mean follow-up time after baseline CRP measurement than the WHS (average 4.5 and 8.5 yrs in the NHS and the WHS, respectively). However, results still varied by cohort when stratified by follow-up years. Finally, considering the large number of participants in both studies (especially WHS), any inter- and intra- laboratory differences may result in differential measurements of CRP over time. However, plasma CRP levels in both studies were measured using high sensitivity assay by the same laboratory, making the assay an unlikely source of the different results. Furthermore, given that the assigned interventions in the WHS (i.e. aspirin and Vitamin E) may influence CRP levels, it is possible that the baseline CRP may not necessarily reflect the levels after treatment. However, an earlier report of data from the randomized phase observed no association between CRP and breast cancer when stratified by either aspirin or Vitamin E treatment (28). Additionally, in our assessment incorporating data during the observational follow-up period, there was again no interaction between the aspirin treatment and CRP in breast cancer risk. Taken together, the source(s) of heterogeneity between these two cohort studies of female health professionals is not clear. Interestingly, the moderate between study heterogeneity in the meta-analysis appeared to be largely driven by WHS since no evidence of between study heterogeneity was found (I2 = 0%, 95%CI = 0, 66%) after excluding data from WHS.
The major strengths in our study include the prospective design, a large number of breast cancer cases, the detailed assessment by tumor hormone receptor status and the consistency in CRP assays across study. However, there are also several limitations. First, only a single measurement of CRP was available in each study. However, the intraclass correlation coefficient for CRP over a 4-year period was reported as 0.67 (40), suggesting that one measurement reasonably reflects relatively long-term exposure. Supporting this assertion is the positive association between CRP and heart disease in both of the cohorts (41, 42); when comparing the highest to the lowest quintile, the RR of a first cardiovascular event in the WHS was 2.3 (95%CI = 1.6, 3.4; Ptrend <0.0001) and the RR of coronary heart disease in the NHS was 1.6 (95%CI = 0.8, 3.1; Ptrend = 0.08). Second, other commonly measured inflammatory markers, such as interleukin-6, tumor necrosis factor alpha, or adipokines, were not available; incorporating additional inflammatory markers may provide insight into the independent and joint effect of CRP with other inflammatory markers in breast cancer etiology. Finally, circulating CRP has been considered to reflect a systemic inflammation but might not necessarily measure local tissue inflammation. A recent study reported a modest correlation between plasma and normal breast tissue (r: 0.2∼0.4) for leptin and adiponectin levels, respectively (43).
As there is some evidence of biological interaction between pathways of inflammation and estrogen synthesis (44), simultaneous evaluation of biomarkers reflecting inflammation and estrogen levels may provide further insights into the role of inflammation in breast cancer etiology. In one of the studies included in the meta-analysis and a subset of the NHS (18), estrogen levels were also included in the model in addition to other covariates; the association with CRP in each study was attenuated, but remained suggestively positive. Interestingly, a recent study reported an independent association between CRP and benign proliferative breast disease, a putative breast cancer precursor, after controlling for circulating estradiol, insulin and adiponectin (45).
In conclusion, our two studies, currently the largest prospective studies which have evaluated the CRP and breast cancer association, did not together support an important role of circulating CRP in the development of breast cancer overall, or by tumor hormone status. However, in a meta-analysis that combined results across 11 prospective studies, a modest, but statistically significant, positive association was observed for total breast cancer. Future prospective studies evaluating multiple biomarkers involved in estrogenic, adiposity and inflammatory pathways are warranted to better understand the underlying mechanism of chronic inflammation in breast cancer etiology. In addition, future studies that examine inflammatory markers in breast tissue in addition to circulating levels may be more relevant and could provide additional etiologic insights.
Supplementary Material
Acknowledgments
This study was supported by Research Grants CA186107 (Nurses' Health Study cohort infrastructure grant), CA87969 (M.J. Stampfer), CA49449 (S.E. Hankinson) and CA47988 (J.E. Buring and I-M Lee) from the National Cancer Institute and HL043851 (J.E. Buring), HL080467 (J.E. Buring) and HL099355 (J.E. Buring) from the National Heart, Lung, and Blood Institute. We would like to thank the participants and staff of the Nurses' Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY,LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. We acknowledge the dedicated participants of the Women's Health Study as well as the contribution of the entire staff of the Women's Health Study. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Conflict of interests: Dr. Paul Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Seimens. All other authors disclosed no potential conflict of interest.
References
- 1.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. The Journal of clinical investigation. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 3.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–24. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 4.Touvier M, Fezeu L, Ahluwalia N, Julia C, Charnaux N, Sutton A, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. American journal of epidemiology. 2013;177:3–13. doi: 10.1093/aje/kws359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allin KH, Bojesen SE, Johansen JS, Nordestgaard BG. Cancer risk by combined levels of YKL-40 and C-reactive protein in the general population. British journal of cancer. 2012;106:199–205. doi: 10.1038/bjc.2011.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 7.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer Institute. 2011;103:1112–22. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 9.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–4. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 10.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. Journal of the National Cancer Institute. 2013;105:1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. American journal of epidemiology. 2013;178:1256–64. doi: 10.1093/aje/kwt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. Jama. 2004;291:585–90. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 13.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer research. 2006;66:2483–7. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 14.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:690–5. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 15.Gaudet MM, Patel AV, Teras LR, Sun J, Campbell PT, Stevens VL, et al. Obesity-related markers and breast cancer in CPS-II Nutrition Cohort. International journal of molecular epidemiology and genetics. 2013;4:156–66. [PMC free article] [PubMed] [Google Scholar]
- 16.Amir E, Cecchini RS, Ganz PA, Costantino JP, Beddows S, Hood N, et al. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast cancer research and treatment. 2012;133:1077–88. doi: 10.1007/s10549-012-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ollberding NJ, Kim Y, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer prevention research (Philadelphia, Pa. 2013;6:188–95. doi: 10.1158/1940-6207.CAPR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeleniuch-Jacquotte A, Gu Y, Bruning PF, Bonfrer JM, Koenig KL, Arslan AA, et al. Re: C-reactive protein and risk of breast cancer. Journal of the National Cancer Institute. 2008;100:443–4. doi: 10.1093/jnci/djn016. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- 19.Prizment AE, Folsom AR, Dreyfus J, Anderson KE, Visvanathan K, Joshu CE, et al. Plasma C-reactive protein, genetic risk score, and risk of common cancers in the Atherosclerosis Risk in Communities study. Cancer Causes Control. 2013;24:2077–87. doi: 10.1007/s10552-013-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Li N, Chang S, Bassig BA, Guo L, Ren J, et al. A prospective follow-up study of the relationship between C-Reactive Protein and human cancer risk in the Chinese Kailuan Female Cohort. Cancer Epidemiol Biomarkers Prev. 2015 Feb;24(2):459–65. doi: 10.1158/1055-9965. Epub 2014 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albuquerque KV, Price MR, Badley RA, Jonrup I, Pearson D, Blamey RW, et al. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol. 1995;21:504–9. doi: 10.1016/s0748-7983(95)96935-7. [DOI] [PubMed] [Google Scholar]
- 22.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allin KH, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13:R55. doi: 10.1186/bcr2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Murri AM, Wilson C, Lannigan A, Doughty JC, Angerson WJ, McArdle CS, et al. Evaluation of the relationship between the systemic inflammatory response and cancer-specific survival in patients with primary operable breast cancer. British journal of cancer. 2007;96:891–5. doi: 10.1038/sj.bjc.6603682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasanisi P, Venturelli E, Morelli D, Fontana L, Secreto G, Berrino F. Serum insulin-like growth factor-I and platelet-derived growth factor as biomarkers of breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2008;17:1719–22. doi: 10.1158/1055-9965.EPI-07-0654. [DOI] [PubMed] [Google Scholar]
- 26.Ling J, Kumar R. Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer letters. 2012;322:119–26. doi: 10.1016/j.canlet.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669–78. doi: 10.1093/annonc/mdf125. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SM, Lin J, Cook NR, Lee IM, Manson JE, Buring JE, et al. C-reactive protein and risk of breast cancer. Journal of the National Cancer Institute. 2007;99:890–4. doi: 10.1093/jnci/djk202. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. Journal of the National Cancer Institute. 1995;87:1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. The New England journal of medicine. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Rifai N, Buring JE, Lee IM, Manson JE, Ridker PM. Is C-reactive protein specific for vascular disease in women? Annals of internal medicine. 2002;136:529–33. doi: 10.7326/0003-4819-136-7-200204020-00010. [DOI] [PubMed] [Google Scholar]
- 34.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 35.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American journal of epidemiology. 2008;167:653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TA, Bazzarre TL, Daniels SR, Fair JM, Fortmann SP, Franklin BA, et al. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107:645–51. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Van Hemelrijck M, Holmberg L, Garmo H, Hammar N, Walldius G, Binda E, et al. Association between levels of C-reactive protein and leukocytes and cancer: three repeated measurements in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev. 2011;20:428–37. doi: 10.1158/1055-9965.EPI-10-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dossus L, Jimenez-Corona A, Romieu I, Boutron-Ruault MC, Boutten A, Dupre T, et al. C-reactive protein and postmenopausal breast cancer risk: results from the E3N cohort study. Cancer Causes Control. 2014;25:533–9. doi: 10.1007/s10552-014-0355-9. [DOI] [PubMed] [Google Scholar]
- 40.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obesity research. 2003;11:1055–64. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. The New England journal of medicine. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 42.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. The New England journal of medicine. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 43.Llanos AA, Dumitrescu RG, Marian C, Makambi KH, Spear SL, Kallakury BV, et al. Adipokines in plasma and breast tissues: associations with breast cancer risk factors. Cancer Epidemiol Biomarkers Prev. 2012;21:1745–55. doi: 10.1158/1055-9965.EPI-12-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–9. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catsburg C, Gunter MJ, Chen C, Cote ML, Kabat GC, Nassir R, et al. Insulin, estrogen, inflammatory markers, and risk of benign proliferative breast disease. Cancer research. 2014;74:3248–58. doi: 10.1158/0008-5472.CAN-13-3514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


