Abstract
Background/Objectives
Mounting evidence supports a link between circadian disruption and metabolic disease. Humans with circadian disruption (e.g., night-shift workers) have an increased risk of obesity and cardiometabolic diseases compared to the non-disrupted population. However, it is unclear if the obesity and obesity-related disorders associated with circadian disruption respond to therapeutic treatments as well as individuals with other types of obesity.
Subjects/Methods
Here, we test the effectiveness of the commonly used bariatric surgical procedure, Vertical Sleeve Gastrectomy (VSG) in mouse models of genetic and environmental circadian disruption.
Results
VSG led to a reduction in body weight and fat mass in both ClockΔ19 mutant and constant-light mouse models (P < .05), resulting in an overall metabolic improvement independent of circadian disruption. Interestingly, the decrease in body weight occurred without altering diurnal feeding or activity patterns (P > .05). Within circadian-disrupted models, VSG also led to improved glucose tolerance and lipid handling (P < .05).
Conclusions
Together these data demonstrate that VSG is an effective treatment for the obesity associated with circadian disruption, and that the potent effects of bariatric surgery are orthogonal to circadian biology. However, since the effects of bariatric surgery are independent of circadian disruption, VSG cannot be considered a cure for circadian disruption. These data have important implications for circadian-disrupted obese patients. Moreover, these results reveal new information about the metabolic pathways governing the effects of bariatric surgery as well as of circadian disruption.
Introduction
More than 33% of Americans are obese and at risk for metabolic-associated diseases including heart disease, stroke and type-2 diabetes1. Despite the known health risk, relatively few treatment options are successful at producing substantial and sustained weight loss. Uniquely, bariatric surgery leads to significant and sustained weight loss. For example, patients receiving a Roux-en-Y Gastric Bypass lose ~30% body weight after surgery, and weight loss is maintained for periods of 10 years or more2. This and other bariatric surgical procedures, such as Vertical Sleeve Gastrectomy (VSG), additionally improve glucose and lipid metabolism independent of weight loss3 suggesting mechanisms of action which go beyond caloric restriction2.
Unlike other treatment options, bariatric surgery did not begin in animal models, but instead was pioneered in the clinical setting by surgeons. With increasing clinical data available, subsets of patients are observed to have very high or very low weight loss after surgery 4. However, it is unknown if certain sub-populations are associated with differing weight loss outcomes and little is known about the effect of bariatric surgery in specific obese populations.
An emerging factor associated with obesity and cardiometabolic disorders is circadian disruption. Humans who experience on-going disruptions to their day-night cycles, such as night-shift workers, have a greatly increased risk of obesity and diabetes5 and in a single published report have less weight loss 3, 6, and 12 months post-surgery compared to non-shift workers undergoing the same surgical procedure6. However, this study was small, did not include a substantial control group, and did not carefully characterize the circadian disruption of these patients. With the development of animal models for VSG, we can systematically determine the effects of surgery in obese animal models corresponding to different sub-populations of obese individuals, such as circadian disrupted patients.
Circadian disruption can be mimicked in animal models using both genetic and environmental perturbations that induce metabolic impairment. For example, the ClockΔ19 mutant mouse (hereafter, Clock) expresses a whole-body mutation in the core conical circadian gene, Clock, which results in a lengthened circadian period and often less circadian rhythmicity under constant darkness conditions. Accompanying this altered circadian rhythm is a spectrum of metabolic disorders including an increase in body fat, hyperphagia, hyperglycemia, hypoinsulinemia and hyperlipidemia, and a blunted diurnal feeding pattern7. Circadian disruption can also occur when behaviors of genetically-normal individuals become desynchronized from the environmental light:dark (LD) cycle. Such disruptions are common in humans (e.g., disruptions in the sleep/wake cycle and/or feeding/fasting patterns) and have also been associated with weight gain8-11. Using mouse models, environmental circadian disruption can be triggered by housing animals in constant light (LL). LL leads to arrhythmic activity, sleep, and feeding behaviors after approximately 4 weeks12, 13 and, when combined with a high-fat diet, leads to increased body weight and caloric intake, lowered energy expenditure, and impaired insulin sensitivity compared to animals maintained on a normal LD cycle14.
Despite the growing evidence demonstrating that circadian disrupted individuals are more likely to be obese, little is known about how they specifically respond to weight loss treatment, such as bariatric surgery. Indeed, the observed variation in weight loss outcomes after bariatric surgery may be a direct or indirect consequence of including patients with undocumented circadian disruption which, in turn, may have altered responses to surgical interventions. Therefore, the current research uses our established mouse model of VSG to test the metabolic effects of the surgery in two models of circadian disruption: a genetic model, the Clock mutant mouse, and an environmental model, a diet-induced obese mouse exposed to constant light. Determining if bariatric surgery is successful in an animal model of circadian disruption can provide critical insight into the clinical treatment of the circadian disrupted, obese subpopulation as well as drive future research on the mechanisms underlying bariatric surgery and obesity induced by circadian disruption.
Materials and Methods
Study Design
For the outlined experiments, sample size was determined based on previous validated mouse studies using the VSG model15. For all Sham surgery groups, n=10. For VSG groups, pre-surgery group sizes were 12-13. Some VSG mice did not recovery from the surgery, dropping group sizes to a minimum of 8/group. For data calculations, group sizes for VSG were as follows, Clock (n=13), WT (n=12), LD (n=9), and LL (n=8). VSG Primary endpoints were set in advance and included body weight, body composition, food intake, and glucose tolerance. The objective of the research was to determine if VSG could successfully treat circadian disruption-associated obesity and glucose tolerance. To create circadian disruption, we use two approaches: genetic (via the Clock mouse) and environmental (via LL exposure). In the case of the environmental disruption, mice were disrupted before the surgical intervention and remained on LL even after the VSG surgery. Similarly, all mice were maintained on the same diet before and after surgery. In general, mice recover quickly and well from the VSG surgery. However, we did note that mice in the LL surgery group showed slower recovery times compared to animals housed in the LD group. Given both VSG and circadian disruption have well-documented metabolic effects, we hypothesized that VSG may work through a similar mechanism. Thus, if circadian disruption-associated obesity impaired metabolism through a similar mechanism to which VSG improved metabolism, we should expect VSG to fail to reduce body weight and improve glucose in the genetic model and environmental disruption. For all collected measurements, except body weight and daily food intake measures, the experimenter was blinded to the experimental group. For measurement of body weight and food intake, it is necessary to know the group as an additional health check for recovery after the surgery.
Animals
Circadian disruption models were selected to encompass both genetic and environmental disruption. ClockΔ19 mutant mice were obtained from Northwestern University and bred in house at the University of Cincinnati to obtain homozygous and wild type littermate control mice. For the environmental disruption experiments, WT mice were ordered from the Jackson Laboratory (JAX) at 5-6 wk of age. All mice were singly housed and fed a 45% high-fat diet (45% fat, 4.54kcal/g, D12451 Research Diets, New Brunswick, NJ) both before and after surgery. For all studies, groups were age-matched and divided to ensure equal body weights among similar genotyped animals prior to surgery intervention. All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Cincinnati (approval number 05-04-1203).
Light protocols
Mice were housed in a standard vivarium room with a minimum of 80 lux during the light period. Standard 12L:12D (LD) consisted for 12-h of light followed by 12-h of dark. Constant light (LL) occurred in a neighboring room in which the lights were on continuously but with otherwise similar environmental conditions.
Automated behavioral measurements
Animals were singly housed, maintained on the appropriate light:dark cycle, and placed into an automated system equipped with metabolic chambers (TSE Systems International Group, Chesterfield, MO) to measure data in 10-min increments. Four weeks after surgery, energy expenditure (measured using indirect calorimetry and calculated as kcal/h per g of lean body mass), activity counts (via beam break), and feeding patterns were measured simultaneously over the course of 4-7 d. Data analysis began after animals acclimated to the metabolic chambers for at least 48 h. A meal bout was determined by at least 0.1 g of food consumed within a 10min span separated by at least a 10-min fast before and after.
Glucose Tolerance Test (GTT)
For the oral GTT, all animals were fasted for 6 h prior to an oral gavage of 200 ul Ensure Plus (Abbott Laboratories). For the i.p. GTT, all animals were fasted for 6 h prior to an i.p. injection of 25% dextrose at a dose of 8 ul/g. All glucose was measured by gluocometer prior to gavage/injection (time 0), and then 15, 30, 60, and 120 min post gavage/injection. Blood was collected from the tip of the tail by cutting a small amount of the tail and gently massaging the blood out. Animals were excluded from analysis based on pre-established criteria, i.e. if blood glucose did not rise significantly over the 2-h period, indicating a failed glucose injection.
Vertical Sleeve Gastrectomy
Surgery was performed as indicated previously16. Briefly, all mice were maintained on the high-fat diet at least 6 wk prior to surgery and exposed to liquid Osmolite before surgery. Surgery began when mice were 13-15 wk old. VSG mice had ~80% of the stomach resected along the major curvature while Sham mice had their stomach exposed and manipulated but not cut. Following surgery, mice were maintained on liquid Osmolite for 4 d, solid food was returned on the 4th d along with Osmolite, and on the 5th d mice were only provided solid food from then on. Metacam was provided daily for 4 d post-surgery for pain.
Lipid analysis
Lipid analysis was performed by the Metabolic Core at the University of Cincinnati. Blood samples from the tail were taken from animals during an ad libitum fed state as well as during fasting.
Body weight and food intake measurements
Body weight and food intake measurements were measured over the course of the experiment daily or 3x/wk. Food was measured by manually removing food from cage top to determine weight.
Macronutrient Preference
Food choice was assayed for seven days, eight weeks after surgery using a macronutrient selection paradigm in which three pure macronutrient diets (TD.02521 [carbohydrate], TD.02522 [fat], and TD02523 [protein] all Harlan Teklad, Indianapolis, IN) were presented simultaneously in separate containers.
Statistics
Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, California, USA). Statistical significance was determined either by two-way ANOVA followed by Bonferroni's multiple comparison post hoc test, or a repeated measures ANOVA followed by Bonferroni's multiple comparison post hoc test. Results were considered statistically significant when P < .05.
Results
GENETIC MODEL OF CIRCADIAN DISRUPTION
Body Weight and Fat Mass
As expected, Clock mutant mice were heavier (~5.4 g) than their wildtype (WT) littermates prior to surgery, P < .001. In response to VSG, both Clock and WT lost weight compared to their genotyped Sham controls (Figure 1A,B, P < .01). Interestingly, despite the Clock mouse starting at a higher baseline body weight and thus having more potential weight to lose, the two genotypes lost comparable weight following VSG, demonstrating independent effects of the surgery and circadian disruption. In both Clock and WT, weight loss was achieved almost exclusively through a reduction in fat mass (Figure 1C). There were no changes in lean mass in Clock Sham, Clock VSG, WT Sham, or WT VSG: 0.00 ± 0.15g, 0.04 ± 0.12g, −0.03 ± 0.10g, 0.01 ± 0.12g, respectively (P < .05).
Figure 1.
Body weight, fat mass, and food intake in a genetic model of circadian disruption with VSG. (A) Body mass (g) of Clock (purple) and WT (black) mice receiving either Sham surgery (solid line) or VSG (dotted line), expressed as days after surgery. (B) Absolute change in body mass. (C) Change in fat mass (g) as measured by NMR before and after Sham or VSG. (D) Total food intake (g) after the surgery. Data expressed as mean ± SEM, ** P < .01, *** P < .001.
Food Intake and Meal Patterns
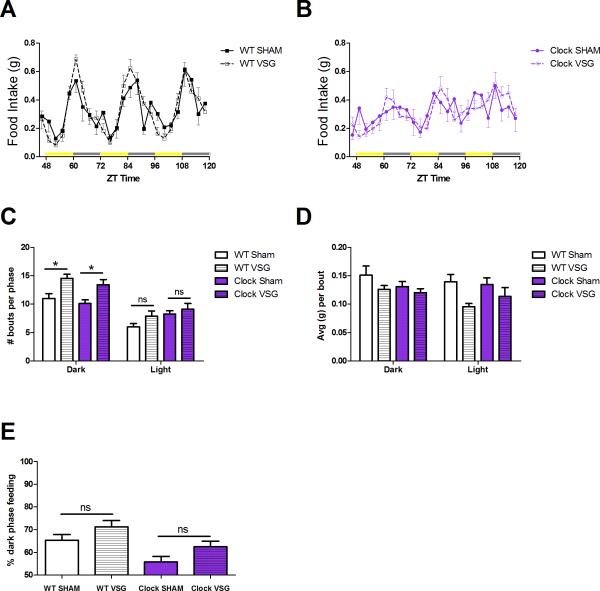
As previously described 7, Clock mice are hyperphagic and consume more calories than WT mice (P < .001). VSG reduced total caloric intake in both Clock and WT mice in an orthogonal fashion (Figure 1D, P < .001). Indeed, VSG normalized the Clock caloric intake to that of a WT Sham control, but did not lower caloric intake to that of the WT VSG control. Additionally, Clock mice displayed smaller day-night differences in food consumption compared to WT mice (Figure 2A,B). Our data support previous reports17, that VSG leads to changes in meal patterns, including an increase in the number of feeding bouts in the dark phase (Figure 2C, P < .05) and a trend toward smaller meals consumed during these bouts (Figure 2D). This effect on meal patterns occurred in both WT and Clock mice. In terms of diurnal feeding rhythms, there was a main effect of VSG (P < .05) and genotype (P < .01) to alter the percentage of dark phase feeding. However, there was no interaction between surgery and genotype (Figure 2E, P > .05).
Figure 2.
24-h feeding patterns. Feeding patterns of WT (A) and Clock (B) mice with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3 d. ZT time denotes hours after lights on. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total number of meal bouts per phase, grouped by bouts during the 12-h dark and 12-h light period. (D) The average amount of food consumed (g) per bout in the 12-h dark and 12-h light phase. (E) Percentage of dark phase feeding as measured by the total calories consumed during the 12-h dark divided by the total calories consumed during the 24-h period. No significant differences are denoted by “ns”. Data expressed as mean ± SEM, *P < .05.
Locomotor Activity and Activity Patterns
Both WT and Clock mice displayed a diurnal rhythm of activity while housed on a normal 12-light:12-dark cycle (Supplemental Figure 1A,B). There was no difference in average 24-hour activity among groups (Supplemental Figure 1C, P > .05), implying that the elevated body weight of Clock mice was due to increased food intake and/or reduced energy expenditure. Surgery had no effect on the diurnal activity patterns of either Clock or WT mice (Supplemental Figure 1D, P > .05); however, there was an effect of genotype to alter diurnal activity, which was lower in the Clock mice (P < .01).
Energy Expenditure
We used indirect calorimetry to assess energy expenditure (EE) in Clock and WT mice. To account for the differences in mass between genotypes and surgical groups, we normalized EE to g of lean mass as measured by NMR (kcal/hr* glean mass). Consistent with previous reports17, we found no main effect of VSG to alter EE in either Clock or WT mice (Supplemental Figure 1E,F, P > .05).
Glucose Analysis
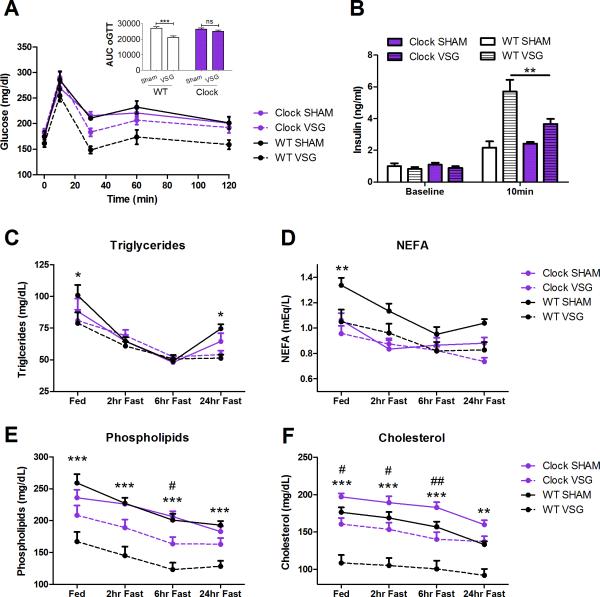
In response to an oral gavage of glucose, there was an overall main effect of surgery to improve glucose tolerance (Figure 3A, P < .05). However, Clock VSG mice did not differ from Clock Sham mice in glucose tolerance (Figure 3A, P < .05). Moreover, Clock VSG mice had less improvement than WT VSG mice (Figure 3A), perhaps due to the decreased insulin response in Clock VSG mice 10 min post glucose gavage (Figure 3B, P < .01). There was a main effect of VSG to lead to increased insulin levels at 10 min (P < .001) and an interaction with genotype (P < .05).
Figure 3.
Glucose homeostasis and lipid handling in a genetic model of circadian disruption with VSG. (A) Glucose tolerance curve and area under the curve (AUC) in response to an oral gavage of a mixed meal in both Clock (purple) and WT (black) mice receiving VSG (dotted line) and Sham (solid line) surgery. (B) Insulin levels (ng/mL) measured before the oral gavage and 10 min after. (C-F) Triglyceride, NEFA, phospholipids, and cholesterol in WT and Clock mice receiving Sham and VSG under various feeding/fasting conditions. * mark significant differences between WT surgery groups, # mark significant differences between Clock surgery groups. No significant differences are denoted by “ns”. Data expressed as mean ± SEM, *P < .05, **P < .01, ***P < .001, #P < .05, ##P < .01.
Lipid Analysis
Since Clock mice have hyperlipidemia, for the genetic circadian disruption experiments we also analyzed lipids in Clock and WT mice under Sham and VSG conditions. Triglycerides and NEFA were reduced in the WT with VSG (P < .05), but surgery had no effect in the Clock mouse in these same parameters (Figures 3C,D, P > .05). For triglycerides, there was an interaction (P < .05) between fasting state and animal group, with WT VSG having reduced triglycerides (P < .05) in the Fed and 24-h Fasted state compared to WT Sham. There was no difference in triglycerides comparing Clock VSG to Clock Sham at any feeding state (P > .05). For NEFA, there was a group effect (P < .01), with WT VSG having reduced NEFA (P < .05) in the Fed state compared to WT Sham. There was no difference in NEFA comparing Clock VSG to Clock Sham at any feeding state (P < .05).
Fasting levels of phospholipids and cholesterol were reduced in both Clock and WT mice following VSG (Figure 3E,F, P < .05). Although each genotype had a significant reduction, cholesterol and phospholipids were not reduced by the same magnitude in the Clock mouse with VSG compared to the WT with VSG. For phospholipids, there was a group effect (P < .001), with WT VSG having reduced phospholipids (P < .001) in all feeding states compared to WT Sham. Within the Clock, VSG only reduced phospholipids during the 6-h fasting time point (P < .05). For cholesterol, there was a group effect (P < .001) and interaction (P < .01), with WT VSG having reduced cholesterol (P < .01, P < .001) in all feeding states compared to WT Sham. Clock mice with VSG also had a reduction in phospholipids at all points except the 24-h fast, but to a lesser extent than within WT mice (P < .05, P < .01).
ENVIRONMENTAL MODEL OF CIRCADIAN DISRUPTION
Body Weight and Fat Mass
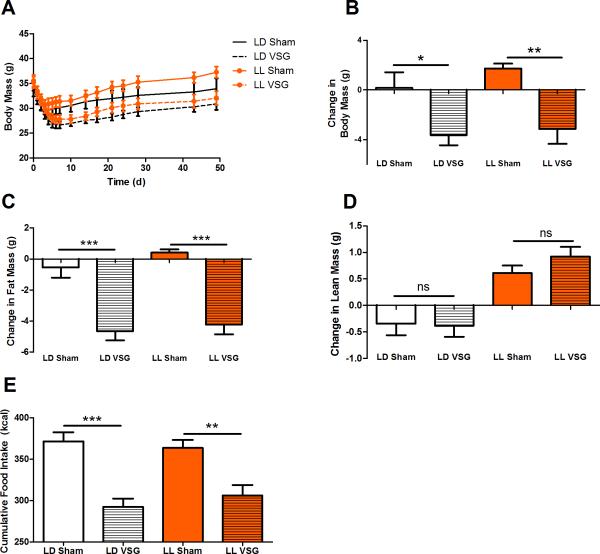
DIO mice exposed to constant light (LL) gained more body weight than those exposed to a normal light-dark schedule (LD, main effect of light exposure, P < .05; Figure 4A). In response to VSG, both LL and LD mice lost a comparable amount of weight (Figure 4B, P < .05), which can be accounted for by loss in fat mass specifically (Figure 4C, P < .001). There was also a main effect of the LL to increase both fat and lean mass independent of surgical condition (Figure 4C,D, P < .05).
Figure 4.
Body weight, fat mass, and food intake in an environmental model of circadian disruption with VSG. (A) Body mass (g) of DIO WT mice in LL (orange) and LD (black) receiving either Sham surgery (solid line) or VSG (dotted line). (B) Absolute change in body mass. (C) Change in fat mass (g) as measured by NMR before and after Sham or VSG. (D) Change in lean mass (g) as measured by NMR before and after Sham or VSG. (E) Total food intake (g) after the surgery. No significant differences are denoted by “ns”. Data expressed as mean ± SEM, * P < .05, ** P < .01, *** P < .001.
Food Intake and Meal Patterns
VSG similarly reduced caloric intake in both LL and LD models (Figure 4E, P < .01). Moreover, LL resulted in a blunting of the 24-h meal patterns (Figure 5A,B, P < .05), and affected meal patterns. Overall, there was a main effect of VSG to increase the number of feeding bouts (Figure 5C, P < .05) and a trend to decrease the amount consumed per bout (Figure 5D). LL led to a reduction in the diurnal organization of meals (P < .001) but there was no effect of VSG to alter diurnal meal organization (Figure 5E, P > .05).
Figure 5.
24-h feeding patterns. Feeding patterns of WT mice in LD (A) and LL (B) with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3 d. ZT time denotes h after lights on. HR denotes h into constant light protocol. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total number of meal bouts per phase, grouped by bouts during the 12-h dark and 12-h light period. (D) The average amount of food consumed (g) consumed per bout in the 12-h dark and 12-h light phase. (E) Percentage of dark phase feeding as measured by the total calories consumed during the 12-h dark divided by the total calories consumed during the 24-h period. No significant differences are denoted by “ns”. Data expressed as mean ± SEM, *P < .05.
Locomotor Activity and Activity Patterns
As expected, LL altered locomotor activity predominately by ablating diurnal activity patterns (Supplemental Figure 2A,B). Overall, there was no difference in total activity counts among the groups (Supplemental Figure 2C, P > .05). There was an effect of LL to cause a reduction in diurnal activity (P > .05) but there was no effect of the surgery to either increase or decrease dark-phase activity (Supplemental Figure 2D, P > .05).
Energy Expenditure
In agreement with the Clock data above, VSG did not alter EE (kcal/hr* glean mass) in WT mice under normal LD conditions (Supplemental Figure 2E, P < .05). In agreement with other reports14, mice housed in LL had decreased EE compared to those held in LD (P < .05). The combination of LL and VSG led to a further decrease in EE compared to LL Sham controls (Supplemental Figure 2F, P < .05). Moreover, the 24-h EE profiles of LL mice lacked circadian rhythmicity, unlike mice exposed to LD that displayed a clear, daily increase in energy expenditure.
Glucose Analysis
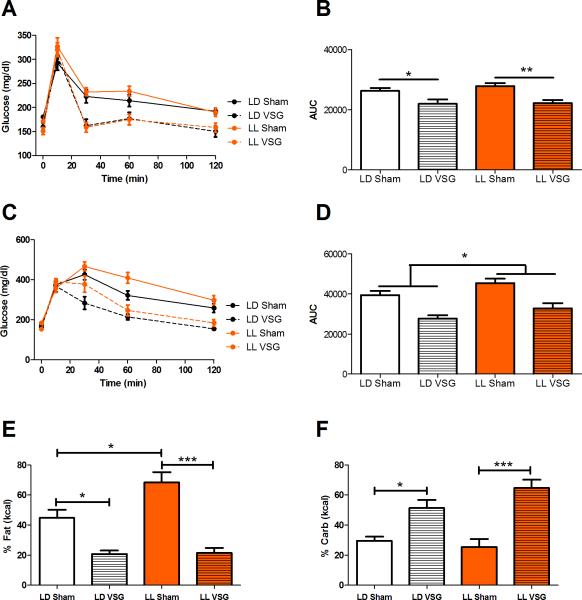
In response to an oral gavage of glucose, both LL VSG and LD VSG mice had similar improvements in glucose tolerance (Figure 6A,B, P < .05) compared to the response in Sham controls. Similarly, VSG improved glucose tolerance in response to an i.p injection of glucose, P < .05. In contrast to the oral gavage, an i.p. injection of glucose revealed a significant effect of LL to impair glucose tolerance regardless of surgery condition (Figure 6C,D, P < .05). The difference in glucose metabolism observed during an i.p. but not an oral gavage suggests that incretins may mitigate some glycemic effects of LL.
Figure 6.
Glucose homeostasis and macronutrient preference in an environmental model of circadian disruption with VSG. (A) Glucose tolerance curve in response to an oral gavage of a mixed meal (Ensure) in both LL (orange) and LD (black) housed mice receiving VSG (dotted line) and Sham (solid line) surgery. (B) Area under the curve (AUC) of the calculated glucose curves for the oral gavage. (C) Glucose tolerance curve in response to an i.p. glucose injection. (D) Area under the curve (AUC) of the calculated glucose curves for the i.p. injection. (E) Macronutrient preference for fat in LD and LL mice with surgery. (F) Macronutrient preference for carbohydrates in LD and LL mice with surgery. No significant differences are denoted by “ns”. Data expressed as mean ± SEM, *P < .05, **P < .01, ***P < .001.
Macronutrient preference
Since both mice housed in LL and those housed in LD had similar total kcal intake, for the environmental disruption experiments we additionally examined the macronutrient preference of the mice. Mice housed in LL and LD were evaluated for macronutrient preference by being provided a choice between 3 pure macronutrients: fat, protein, and carbohydrate. LL Sham mice consumed more calories from fat than LD Sham mice (Figure 6E, P < .05), an effect due to the circadian disruption. In agreement with previous reports18, VSG caused a profound reduction in fat preference and increase in carbohydrates consumption in LD and this was also observed in LL mice (P < .05). Preference for carbohydrates was unaffected by circadian disruption and was comparable between LL and LD Sham and between LL and LD VSG groups (Figure 6F, P > .05). These data demonstrate that VSG can overcome the macronutrient fat preference caused by environmental circadian disruption.
Discussion
Circadian disruption has been associated with adverse health conditions, including those related to obesity and diabetes. Despite the documented relationship between circadian disruption and obesity, no published data addresses the proportion of patients with a BMI ≥40 that also have circadian disruption. This could be in part due to the diverse causes of circadian disruption and the difficulties in assessing the degree of circadian disruption. Based on independent estimates of patients with Night-Eating Syndrome19, 20 and shift workers6, the population of circadian-disrupted individuals could be as high as 28% of the severely obese population, or ~20 million people in the United States. Thus, there is a need to understand the relationship and possible interactions between circadian disruption and obesity treatments.
To examine this relationship under controlled experimental conditions, we compared the effects of VSG in two mouse models of circadian disruption, the genetic Clock mutant mouse and an environmental model of constant light in a DIO WT mouse. Overall, our data indicate that VSG is effective at significantly decreasing body weight and food intake, and improving glucose tolerance within both of these models. However, given that the improvements from VSG are either similar or smaller in magnitude compared to mice with genetic or environmental circadian disruption, we conclude that mice with circadian disruption combined with VSG are not as metabolically fit as control mice receiving VSG. These data are in agreement with the observation that shift-workers tend to lose less excess body weight than non-shift workers after bariatric surgery6. Thus, while VSG is effective at improving overall metabolic health, by itself the surgery cannot completely eliminate the effects of circadian disruption and should not be considered a “cure” for metabolic impairments associated with circadian disruption. Indeed, this conclusion calls for further research on how to treat the circadian disrupted population.
Interestingly, VSG failed to cause significant changes in diurnal feeding or activity patterns in either genetic or environmental models of circadian disruption, and yet significant weight loss occurred. The Clock mouse, for example, continued to display a reduction in dark-phase feeding compared to WT mice after the surgery but lost significant weight. In the case of environmental LL exposure, WT mice continued to display both blunted activity and feeding patterns after VSG but still lost significant weight. Importantly, weight loss is possible within circadian-disrupted models and these changes in body weight occur even when the disrupted circadian rhythms in locomotor activity and feeding are not restored. While some research indicates that VSG can correct some kinds of circadian-disrupted feeding21, it remains unclear if the combination of bariatric surgery and meal pattern modification may be sufficient to completely alleviate metabolic impairments in an otherwise circadian-disrupted individual or animal model.
Most instances of human circadian disruption are due to voluntary activities and/or environmental disruption, making our animal model in constant light particularly relevant to the human experience. Within this model, we observed a difference in glucose tolerance between the oral and i.p. glucose tolerance tests, highlighting a potential interaction between incretins and the metabolic impact of circadian disruption. Indeed, recent reports suggest that incretins are altered with circadian misalignment22. It is possible that constant light could lead to an increase in incretin release that works to limit glucose intolerance.
Our data also indicate that macronutrient preference for fat increases in mice with LL-induced circadian disruption. These data are in agreement with a recent human study of simulated night work23, but contradict macronutrient preferences observed in human shift workers, which have an increased carbohydrate intake, not fat24. In our model, bariatric surgery completely reversed the tendency to consume more fat. Unlike the moderate changes in other metabolic endpoints, macronutrient preference was the only measure we found that was entirely reversed by bariatric surgery.
Using animal models of circadian disruption has great benefits, including the ability to amass significant numbers of animals with a specific type of circadian disruption and tightly controlled comparison groups to address metabolic questions. The current work focused on one particular source of obesity and metabolic dysregulation and examined the effects of a bariatric surgical procedure to improve a number of important metabolic endpoints, thus allowing us to ask specific, controlled questions that may be difficult to ask in a clinical setting.
Mechanistically, this study reveals that the metabolic benefit of bariatric surgery is downstream of the influence of clock biology on metabolism. Additionally, metabolic improvement from VSG is not dependent solely on the reorganization of feeding behavior. Together, these data suggest that another system(s) may be similarly altered in both circadian disrupted and control groups. One promising hypothesis points to alterations in the gut microbiota25. Gut microbiota populations are different in lean vs obese animals and bariatric surgery results in a microbiota population more similar to a lean control26. Intriguingly, circadian disruption also been shown to directly affect the microbiota27, 28 – indicating a possible mechanism of improvement that spans obese subpopulations.
The ability of VSG to improve a wide range of metabolic endpoints has two important implications. The first is clinical: These data support the use of bariatric surgical procedures in patients where circadian disruption contributes to increased body weight and poor metabolic regulation. Moreover, these data speak to the effectiveness of a specific treatment option within this distinct subpopulation of obese individuals, which have thus far been understudied for treatment purposes. The second is mechanistic: Given the powerful effects of bariatric surgery and circadian disruption to alter metabolic endpoints, one logical hypothesis is that these manipulations work through common underlying mechanisms. The current data do not support this hypothesis and indicate that most of the effects of bariatric surgery are orthogonal to those of circadian disruption. This is most notable as VSG does little to improve circadian behaviors yet still improves a variety of metabolic parameters.
Supplementary Material
Supplemental Figure 1. Activity and energy expenditure in a genetic model of circadian disruption with VSG. 24-h activity patterns in WT (A) and Clock (B) mice with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3 d. ZT time denotes hours after lights on. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total activity as measured by IR beam breaks over a 3-d period. (D) Percentage of dark phase activity as measured by the total number of beam breaks during the 12-h dark divided by the t 595 otal number of beam breaks during the 24-h period. (E) Energy expenditure (expressed as kcal/h per g of lean body mass) in WT or Clock mice (F) over the course of 3 d. No significant differences are denoted by “ns”. Data expressed as mean ± SEM.
Supplemental Figure 2. Activity and energy expenditure in an environmental model of circadian disruption with VSG. 24-h activity patterns of WT mice in LD (A) and LL (B) with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3-d. ZT time denotes h after lights. HR denotes h into contestant light protocol. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total 24-h activity as measured by IR beam breaks over a 3-d period. (D) Percentage of dark phase activity as measured by the total number of beam breaks during the 12-h dark divided by the total number of beam breaks during the 24-h period. (E) Energy expenditure (expressed as kcal/h per g of lean body mass) in mice held in LD or LL mice (F) over the course of 3 d. No significant differences are denoted by “ns”. Data expressed as mean ± SEM. ***P < .001.
Acknowledgments
Grant Support: This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (F32 DK097867-01, D.Arble; DK082480-01, D.Sandoval; and R01 DK093848-01, R.Seeley).
Footnotes
Author contributions: D.M.A. designed and performed experiments, analyzed data, and wrote the manuscript. D.A.S., F.W.T., S.C.W., and R.J.S. contributed to experimental design and edited the manuscript.
Conflict of Interest Statement: D.M.A has no conflicts to declare. D.A.S receives funding from Ethicon Endo-Surgery, Novo Nordisk, and Boehringer Ingelheim International. R.J.S receives funding from Ethicon Endo-Surgery, Ablaris Therapeutics, Inc., Novo Nordisk, Novartis, Angiochem, Eisai, Forest Pharmaceuticals, Givaudan, Zealand Pharmaceuticals, and Boehringer Ingelheim International.
Supplementary information is available on IJO's website.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- 2.Sandoval D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes (Lond) 2011;35(Suppl 3):S45–9. doi: 10.1038/ijo.2011.148. [DOI] [PubMed] [Google Scholar]
- 3.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–8. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessler M, Daud A, Kim T, DiGiorgi M. Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2007;3(4):480–4. doi: 10.1016/j.soard.2007.01.010. discussion 484-5. [DOI] [PubMed] [Google Scholar]
- 5.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53(2):103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 6.Ketchum ES, Morton JM. Disappointing weight loss among shift workers after laparoscopic gastric bypass surgery. Obes Surg. 2007;17(5):581–4. doi: 10.1007/s11695-007-9100-8. [DOI] [PubMed] [Google Scholar]
- 7.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Bertone ER, Stanek EJ, 3rd, Reed GW, Hebert JR, Cohen NL, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158(1):85–92. doi: 10.1093/aje/kwg117. [DOI] [PubMed] [Google Scholar]
- 9.Berkey CS, Rockett HR, Gillman MW, Field AE, Colditz GA. Longitudinal study of skipping breakfast and weight change in adolescents. Int J Obes Relat Metab Disord. 2003;27(10):1258–66. doi: 10.1038/sj.ijo.0802402. [DOI] [PubMed] [Google Scholar]
- 10.O'Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12(11):1789–96. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- 11.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8(3):267–9. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 13.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: V. Pacemaker structure: A clock for all seasons. J Comp Physiol A. 1976;106:333–355. [Google Scholar]
- 14.Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. Faseb J. 2013;27(4):1721–32. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 15.Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav. 2011;105(1):120–3. doi: 10.1016/j.physbeh.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–36. 2436, e1–3. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144(1):50–52. e5. doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rand CS, Macgregor AM, Stunkard AJ. The night eating syndrome in the general population and among postoperative obesity surgery patients. Int J Eat Disord. 1997;22(1):65–9. doi: 10.1002/(sici)1098-108x(199707)22:1<65::aid-eat8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Allison KC, Wadden TA, Sarwer DB, Fabricatore AN, Crerand CE, Gibbons LM, et al. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Obesity (Silver Spring) 2006;14(Suppl 2):77S–82S. doi: 10.1038/oby.2006.286. [DOI] [PubMed] [Google Scholar]
- 21.Colles SL, Dixon JB. Night eating syndrome: impact on bariatric surgery. Obes Surg. 2006;16(7):811–20. doi: 10.1381/096089206777822160. [DOI] [PubMed] [Google Scholar]
- 22.Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. 2012;96(4):689–97. doi: 10.3945/ajcn.112.037192. [DOI] [PubMed] [Google Scholar]
- 23.Cain SW, Filtness AJ, Phillips CL, Anderson C. Enhanced preference for high-fat foods following a simulated night shift. Scandinavian journal of work, environment & health. 2015 doi: 10.5271/sjweh.3486. [DOI] [PubMed] [Google Scholar]
- 24.de Assis MA, Kupek E, Nahas MV, Bellisle F. Food intake and circadian rhythms in shift workers with a high workload. Appetite. 2003;40(2):175–83. doi: 10.1016/s0195-6663(02)00133-2. [DOI] [PubMed] [Google Scholar]
- 25.Arble DM, Sandoval DA, Seeley RJ. Mechanisms underlying weight loss and metabolic improvements in rodent models of bariatric surgery. Diabetologia. 2014 doi: 10.1007/s00125-014-3433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812–27. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, et al. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Activity and energy expenditure in a genetic model of circadian disruption with VSG. 24-h activity patterns in WT (A) and Clock (B) mice with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3 d. ZT time denotes hours after lights on. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total activity as measured by IR beam breaks over a 3-d period. (D) Percentage of dark phase activity as measured by the total number of beam breaks during the 12-h dark divided by the t 595 otal number of beam breaks during the 24-h period. (E) Energy expenditure (expressed as kcal/h per g of lean body mass) in WT or Clock mice (F) over the course of 3 d. No significant differences are denoted by “ns”. Data expressed as mean ± SEM.
Supplemental Figure 2. Activity and energy expenditure in an environmental model of circadian disruption with VSG. 24-h activity patterns of WT mice in LD (A) and LL (B) with Sham (solid lines) and VSG (dotted lines) are plotted in 3-h increment over the course of 3-d. ZT time denotes h after lights. HR denotes h into contestant light protocol. Yellow bars on the x-axis represent lights on, grey bars represent lights off periods. (C) Total 24-h activity as measured by IR beam breaks over a 3-d period. (D) Percentage of dark phase activity as measured by the total number of beam breaks during the 12-h dark divided by the total number of beam breaks during the 24-h period. (E) Energy expenditure (expressed as kcal/h per g of lean body mass) in mice held in LD or LL mice (F) over the course of 3 d. No significant differences are denoted by “ns”. Data expressed as mean ± SEM. ***P < .001.