Abstract
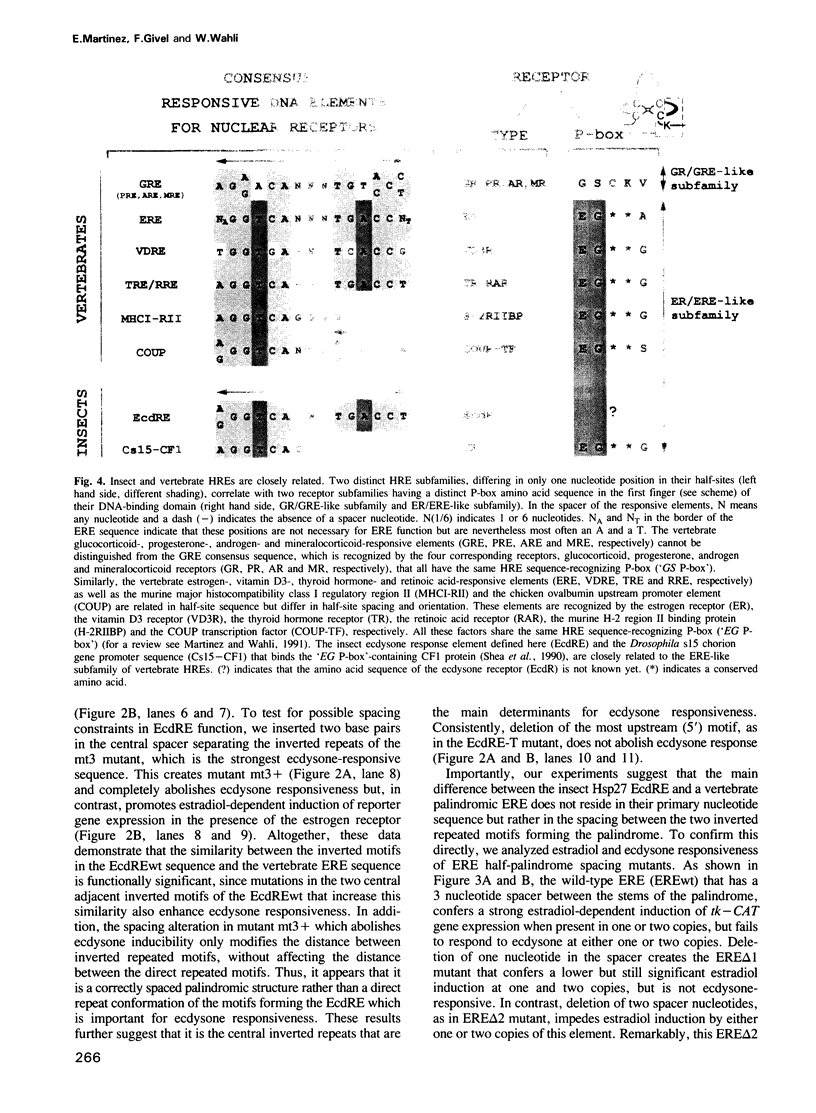
The ecdysone-responsive DNA sequence of the Drosophila hsp27 gene promoter contains four direct and inverted repeats reminiscent of those that compose the vertebrate palindromic estrogen response element (ERE) and the thyroid hormone/retinoic acid response element (TRE/RRE). Interestingly, a 3 bp substitution in the wild-type Hsp27 ecdysone response element (EcdRE) increases both its similarity with the vertebrate ERE and TRE/RRE and its capacity to confer ecdysone responsiveness to a heterologous promoter. Remarkably, increasing the spacing between the inverted repeats of this strong EcdRE by two nucleotides converts it into an ERE. Inversely, decreasing the spacing between the two inverted repeats of the vertebrate consensus palindromic ERE, from three to one nucleotide, converts it into a functional EcdRE. Thus, the only difference between an invertebrate EcdRE and a vertebrate palindromic ERE or TRE/RRE is in the spacing between the conserved inverted repeated motifs forming these palindromic HREs. The finding that the sequence motif 5'-GGTCA-3' present in the vertebrate ERE and TRE/RRE is also a functionally important characteristic of an invertebrate HRE, suggests that a common ancestor regulatory DNA sequence gave rise to all HREs known so far. We discuss the possibility that this progenitor motif is the GGTCA sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Harris B. A. Plasmids for the cloning and expression of full-length double-stranded cDNAs under control of the SV40 early or late gene promoter. Nucleic Acids Res. 1983 Oct 25;11(20):7119–7136. doi: 10.1093/nar/11.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. Two amino acids within the knuckle of the first zinc finger specify DNA response element activation by the glucocorticoid receptor. Cell. 1989 Jun 30;57(7):1131–1138. doi: 10.1016/0092-8674(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Henrich V. C., Sliter T. J., Lubahn D. B., MacIntyre A., Gilbert L. I. A steroid/thyroid hormone receptor superfamily member in Drosophila melanogaster that shares extensive sequence similarity with a mammalian homologue. Nucleic Acids Res. 1990 Jul 25;18(14):4143–4148. doi: 10.1093/nar/18.14.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Kaling M., Ryffel G. U. Synergism of closely adjacent estrogen-responsive elements increases their regulatory potential. J Mol Biol. 1988 Jun 5;201(3):537–544. doi: 10.1016/0022-2836(88)90635-3. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Gehring W. J. Sequence requirement for expression of the Drosophila melanogaster heat shock protein hsp22 gene during heat shock and normal development. Mol Cell Biol. 1986 Jun;6(6):2011–2019. doi: 10.1128/mcb.6.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Mader S., Kumar V., de Verneuil H., Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature. 1989 Mar 16;338(6212):271–274. doi: 10.1038/338271a0. [DOI] [PubMed] [Google Scholar]
- Martinez E., Givel F., Wahli W. The estrogen-responsive element as an inducible enhancer: DNA sequence requirements and conversion to a glucocorticoid-responsive element. EMBO J. 1987 Dec 1;6(12):3719–3727. doi: 10.1002/j.1460-2075.1987.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R., Schiller P., Amin J., Klapper H., Ananthan J., Voellmy R. Heat shock and ecdysterone activation of the Drosophila melanogaster hsp23 gene; a sequence element implied in developmental regulation. EMBO J. 1986 Jul;5(7):1667–1673. doi: 10.1002/j.1460-2075.1986.tb04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- Morganelli C. M., Berger E. M., Pelham H. R. Transcription of Drosophila small hsp-tk hybrid genes is induced by heat shock and by ecdysterone in transfected Drosophila cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5865–5869. doi: 10.1073/pnas.82.17.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauber U., Pankratz M. J., Kienlin A., Seifert E., Klemm U., Jäckle H. Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature. 1988 Dec 1;336(6198):489–492. doi: 10.1038/336489a0. [DOI] [PubMed] [Google Scholar]
- Oro A. E., Ong E. S., Margolis J. S., Posakony J. W., McKeown M., Evans R. M. The Drosophila gene knirps-related is a member of the steroid-receptor gene superfamily. Nature. 1988 Dec 1;336(6198):493–496. doi: 10.1038/336493a0. [DOI] [PubMed] [Google Scholar]
- Pignoni F., Baldarelli R. M., Steingrímsson E., Diaz R. J., Patapoutian A., Merriam J. R., Lengyel J. A. The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell. 1990 Jul 13;62(1):151–163. doi: 10.1016/0092-8674(90)90249-e. [DOI] [PubMed] [Google Scholar]
- Ponglikitmongkol M., White J. H., Chambon P. Synergistic activation of transcription by the human estrogen receptor bound to tandem responsive elements. EMBO J. 1990 Jul;9(7):2221–2231. doi: 10.1002/j.1460-2075.1990.tb07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddihough G., Pelham H. R. Activation of the Drosophila hsp27 promoter by heat shock and by ecdysone involves independent and remote regulatory sequences. EMBO J. 1986 Jul;5(7):1653–1658. doi: 10.1002/j.1460-2075.1986.tb04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddihough G., Pelham H. R. An ecdysone response element in the Drosophila hsp27 promoter. EMBO J. 1987 Dec 1;6(12):3729–3734. doi: 10.1002/j.1460-2075.1987.tb02707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M., Nauber U., Jäckle H. Three hormone receptor-like Drosophila genes encode an identical DNA-binding finger. EMBO J. 1989 Oct;8(10):3087–3094. doi: 10.1002/j.1460-2075.1989.tb08460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves W. A., Hogness D. S. The E75 ecdysone-inducible gene responsible for the 75B early puff in Drosophila encodes two new members of the steroid receptor superfamily. Genes Dev. 1990 Feb;4(2):204–219. doi: 10.1101/gad.4.2.204. [DOI] [PubMed] [Google Scholar]
- Shea M. J., King D. L., Conboy M. J., Mariani B. D., Kafatos F. C. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 1990 Jul;4(7):1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]






