Abstract
Adaptation to spatially varying environments has been studied for decades, but advances in sequencing technology are now enabling researchers to investigate the landscape of genetic variation underlying this adaptation genome wide. In this review, we highlight some of the decades-long research on local adaptation in Drosophila melanogaster from well-studied clines in North America and Australia. We explore the evidence for parallel adaptation and identify commonalities in the genes responding to clinal selection across continents as well as discuss instances where patterns differ among clines. We also investigate recent studies utilizing whole-genome data to identify clines in D. melanogaster and a number of other systems. Although connecting segregating genomic variation to variation in phenotypes and fitness remains challenging, clinal genomics is poised to increase our understanding of local adaptation and the selective pressures that drive the extensive phenotypic diversity observed in nature.
Keywords: latitudinal cline, local adaptation, spatially varying selection
The clinal genomic framework
Despite vast phenotypic and genetic diversity in the tree of life, species often appear precisely adapted to their local environment, suggesting strong selection for DNA variants that underlie local adaptation. Evolutionary biologists have long sought to connect this genetic variation to variation in phenotypes and fitness within natural populations. One fruitful approach has been to sample individuals along geographic transects—such as latitude, longitude, or altitude—that vary predictably in abiotic (e.g., temperature, precipitation, ultraviolet radiation) and biotic (e.g., species biodiversity, levels of competition) conditions. Evaluation of variation along such transects enables the identification of clines broadly defined as a predictable geographic gradient in a measurable genotypic (e.g., allozyme or allele frequencies) or phenotypic (e.g., body size, thermal tolerance) character [1]. Two types of clines—those situated along discrete environments and those along continuous environments—have been historically evaluated theoretically and empirically (Box 1).
Box 1. Genetic models underlying discrete- and continuous-environment clines.
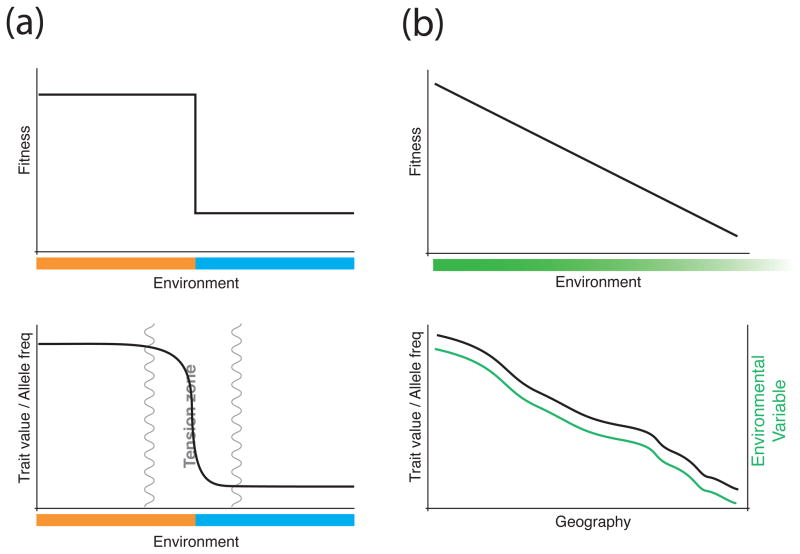
Two major types of clinal patterns have historically been studied in natural populations; clines where two discrete environments meet in a tension zone (or sometimes a hybrid zone), and clines where populations are locally adapted along a continuous environments (Figure I). Clines in tension zones are often sharp, narrow, and centered on an ecotone—the transition between two biomes. The exact shape of discrete-environment clines is determined by a balance between selection against maladapted alleles—either due to intrinsic or extrinsic incompatibilities in the tension zone—and dispersal distance [3,4,128]. Individuals in the tension zone should therefor have lower fitness relative to their “pure” counterparts in the tails of the cline. Discrete-environment clines have been well studied both theoretically [4,5,128–130] and empirically, with some of the best examples coming from studies of threespine sticklebacks [131,132], mice [133,134], Heliconius butterflies [135,136], and fire-bellied toads [4,5]. The genetic model underlying discrete-environment clines can be contrasted with that of continuous-environment clines—clines arising due to adaptation to continuously varying local environments—the sole focus of this review. Relative to discrete-environment clines, continuous-environment clines are found in a single species where populations are connected by high levels of gene flow. In contrast to the stepped fitness function of discrete-environment clines, fitness optima of continuous-environment clines gradually shift with the environmental gradient, and selection favors locally adapted alleles at all positions along the geographic transect. While continuous-environment clines are often broader than their discrete-environment counterparts, their shape should parallel changes in the environment—leading to sharp clines under certain environmental conditions. In continuous-environment clines, causative variants are expected to closely track their environmental selection pressures, while clines of neutral variants should not. Despite these expectations, distinguishing causal variants from background noise remains a challenge. The underlying genetic model of continuous-environment clines suggests that these clinal variants will have a quantitative genetic basis. Whether all variants underlying such quantitative traits will track the environmental gradient equally well remains an outstanding theoretical and empirical question.
Figure I.
A simplified fitness landscape contrasting discrete- and continuous-environment clines, as well as their expected shapes. A) In discrete-environment clines, the fitness landscape (top; pattern shown for the orange population, blue population would be a mirror image) is often represented by a step function with two fitness optima, where alleles from one species are selected against as they introgress away from their home population. Consequently, the slope of the resulting trait/allele frequency cline (bottom) is relatively shallow in the tails and transitions sharply through the tension zone, though the exact shape is dependent on the strength of selection and dispersal distance. B) The fitness landscape of a continuous-environment cline (top; pattern shown for leftmost population) represents a shifting fitness optimum along a continuous environmental gradient. The resulting trait/allele frequency cline (bottom, black line) may be less steep than a discrete-environment cline and should closely track the environmental selection pressure (green line).
Sampling along clines provides unique benefits, and can potentially attenuate some of the confounding effects of demography, which may be difficult to control for when sampling populations from patchy landscapes. For example, gene flow should be more predictable along clines, thus making it easier to identify adaptive from non-adaptive differentiation [2]. Clines are often predictable and replicable to a degree that variation sampled from patchy landscapes is not: for example, a cline along a coastal latitudinal transect can potentially be replicated on multiple continents. Such patterns of differentiation repeated among clines provide evidence of parallel adaptation. Finally, properties of a cline—such as the width, slope, and shape—can also inform inferences about underlying demographic and selective forces [1–5].
Although adaptation to spatially varying selection has been evaluated for decades using phenotypic data and genetic data from a small number of candidate loci, the recent abundance of whole genome data provides an opportunity to discover novel causative variants—beyond those previously identified by candidate gene studies. Moreover, the discovery of novel clines allows researchers to ask fundamental questions about natural selection and the genetic basis of adaptation: what are the genomic targets of spatially varying selection, and how do they facilitate adaptation to the local environment? What are the molecular mechanisms underlying local adaptation? How widely distributed across the genome are loci with alleles under clinal selection, and what does this imply about the genetic basis of adaptive traits? And, as homologous characters may exhibit parallel responses to similar underlying selection pressures, how often does adaptation occur in parallel—within and between species—among clines? Here, we highlight some of the decades-long research on local adaptation in Drosophila melanogaster from a group of particularly well-studied clines in North America and Australia. We explore the evidence for parallel adaptation, and identify commonalities in the genes responding to clinal selection among continents. We also highlight cases where patterns are not repeatable among clines. Finally, we explore recent studies utilizing whole-genome data that have just begun to identify the targets of selection along clines in D. melanogaster and in other species.
Phenotypic, genetic, and genomic variation in Drosophila melanogaster clines
Expansion of D. melanogaster out of equatorial Africa
Decades of careful study has made D. melanogaster the most extensively explored system for elucidating phenotypic, genetic, and genomic divergence among natural populations. Genetic data suggest that D. melanogaster expanded out of its native range in equatorial Africa into Eurasia approximately 10,000 to 20,000 years ago, and that this expansion was associated with a severe population bottleneck [6]. Changes in climatic conditions during the late Pleistocene period likely facilitated migration out of Africa [7,8], but adaptation to a number of ecological factors that vary with latitude has been required in the derived high-latitude populations that now extend as far north as Finland (64°N) and as far south as Tasmania (43°S) [9]. More recently, D. melanogaster invaded North America and Australia, and was first collected within only the last 150 years on each continent; populations of D. melanogaster along the eastern coasts of North America and Australia have since been independently sampled and evaluated for decades [9,10] (Table 1).
Table 1.
Clinal patterns among American D. melanogaster populations
| Trait/genetic marker | Location | Clinal pattern | Reference | |
|---|---|---|---|---|
| Genome wide | Eastern N.A. | Higher sequence diversity and most negative Tajima’s D in lowest latitude population, In(3R)P at highest frequency in the lowest latitude population, | [50] | |
| Eastern N.A. | Higher sequence diversity and lower ratio of X-to autosomal-linked variation in low latitude population; greater skew toward high-frequency alleles in high latitude population; In(3R)Mo, In(2L)t, In(2R)NS, In(3L)Payne, In(3R)Payne frequencies decrease with latitude (verify with Andy) | [52] | ||
| Eastern N.A. | Differential gene expression—highly expressed genes in high latitude populations of D. melanogaster are also highly expressed in high latitude populations of D. simulans | [12] | ||
| Genes | Adh | Eastern N.A. and Western S.A. | Adh-F increases with | [33,36,38] |
| Aldh | Eastern N.A. | Aldh-Phe increases with latitude | [39] | |
| cpo | Eastern N.A. | Various clinal SNPs | [40,41] | |
| Est6 | Eastern N.A. | Est61.00, Est63, and Est6-S increase with latitude | [30,33,138] | |
| EstC | Ontario, CA; Hamilton, CA; MA, USA; TX, USA | EstC3 allele generally decreases with latitude | [33] | |
| G6pd | N.A | G6pd-F and G6pd increase with latitude | [30,31,33] | |
| Gpdh | Eastern N.A., Western South America | Gpdh-S increases with latitude | [30,38] | |
| InR | Eastern N.A. | InRshort increases with latitude. | [139,140] | |
| LapD | Ontario, CA; Hamilton, CA; MA, USA; TX, USA | LapD3 allele generally decreases with latitude | [33] | |
| Odh | N.A | Odh-S decreases with latitude | [30,32] | |
| Pgd | N.A | Pgd-F increases with latitude | [30,31,33] | |
| Tpi | N.A | Tpi-F increases with latitude | [141] | |
| Inversions | In(2L)t, In(2R)NS, In(3L)Payne, In(3R)Payne | N.A. | Frequency decreases with latitude | [47,48] |
| In(2L)t, In(3L)Payne, In(3R)Payne, In(3R)Mo | N.A. and S.A. | Frequency decreases with latitude | [46] | |
| Transposable Elements | Family: Rt1b, invader4, pogo, Doc, S-element, BS, 1360 | Eastern N.A. | Families differ | [72,142] |
| Phenotype | Cell membrane plasticity | Eastern N.A. | Highest in high latitude population | [143] |
| Diapause incidence and ovariole number | Eastern N.A. | Increases with latitude | [144] | |
| Different organs | Western S.A. | Size increase with latitude | [23] | |
| Egg Size | Western S.A. | Increases with latitude | [26] | |
| Ethanol tolerance | Western N.A. | Ethanol resistance increases with latitude | [145] | |
| Female fresh weight and ovariole number | America (tropical and temperate) | Increases with latitude | [24] | |
| Lifetime fecundity | Eastern N.A. | Decreases with latitude | [27] | |
| Life span, heat and cold resistance | Eastern N.A. | Increases with latitude | [27] | |
| Nightime locomotor activity | Eastern N.A. | Increases with latitude | [11] | |
| Per-capita fecundity | Eastern N.A. | Varies with age | [144] | |
| Sleep bout duration | Eastern N.A. | Decreases with latitude | [11] | |
| Wing area, cell size, and cell number | Western S.A. | Increases with latitude | [21] | |
| Wing length and bristle number | Eastern N.A. | Increases with latitude | [22] |
North American and Australian clines
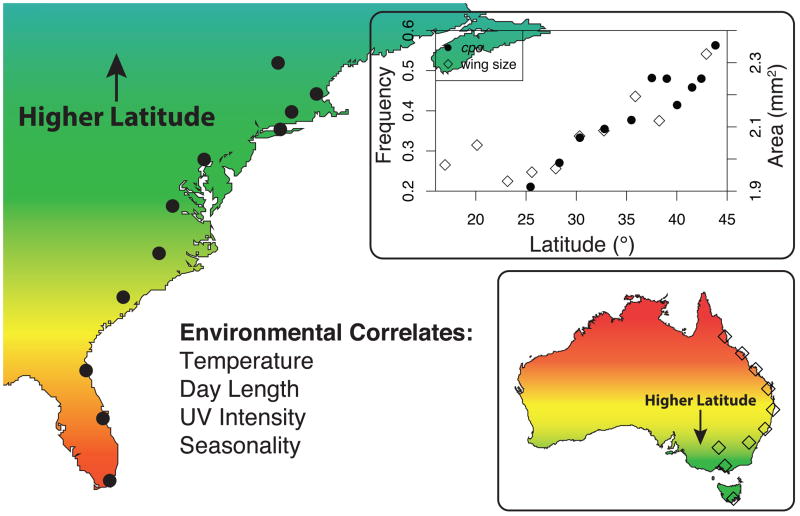
Although clines in individual traits or alleles are typically treated separately, for simplicity we refer to the collective of clines along the same transect as a singular entity (“the cline”). The North American cline along the Atlantic seaboard has been heavily sampled over 18° of latitude from southern Florida, USA (25°N) to Vermont and Maine, USA (44°N), although recent studies have extended this cline by 22° latitude in the south to include a population of D. melanogaster from Panama City, Panama (9°N) [11,12]. The Australian cline has been heavily sampled over 28° of latitude from northern Queensland (15°S) to Tasmania (43°S). Although both clines are coastal, longitude varies more than 10° along the North American cline, but remains relatively constant in Australia (Refs. [13,14]; Figure 1). Environmental conditions vary greatly—though often predictably—along these clines. High-latitude populations on both continents consistently experience lower mean temperatures, greater variance in temperatures across seasons, and reduced ultraviolet (UV) light exposure [10,15–17]. As an example, over the last 30 years mean monthly temperatures averaged 24.1°C in southern Florida (Station ID 087020) and 5.5°C in central Vermont (Station ID 431360) [18]. Perhaps even more notable, the average maximum monthly temperature during this period in Vermont (12.6°C) was 5.9°C lower than the average minimum monthly temperature in Florida (18.5°C). Many other environmental factors vary along the North American and Australian clines that generate spatial differentiation of phenotypes and genotypes, and it should be noted that many of these factors also co-vary with altitude, such that similar clines are found along individual mountain ranges [19,20].
Figure 1.
Phenotypes and alleles often respond to clinal selection in predictable ways. For example, both the allele frequency of the SNP corresponding to amino acid residue 356 in cpo sampled from North America (circles; modified from Schmidt et al., 2008), and the wing area of flies sampled from eastern Australia (diamonds; modified from James et al., 1995) increase with latitude. Selection on these traits could be mediated by multiple environmental factors that correlate with latitude.
Phenotypic variation
Phenotypic differentiation along latitudinal transects has been shown for a variety of traits in D. melanogaster, and many patterns are recapitulated among continents (Table 1). One of the most striking patterns is the size difference in flies from different locations: populations of flies from high latitudes generally display larger body size and a concomitant increase in wing size [13,21], bristle number [22], and organ size [23]. Ovariole number [24,25] and egg size also increase with latitude [26], though lifetime fecundity decreases with latitude [27]. Adaptation to colder temperature reveals itself as phenotypic differentiation in a number of traits not directly related to size. For example, in the eastern United States, populations of D. melanogaster from higher latitudes display greater diapause incidence at low temperatures [25], and have greater cold tolerance [27]. High-latitude populations of D. melanogaster in Australia also have greater cold tolerance, but lower heat tolerance, than their lower latitude conspecifics [10,28]. In contrast to the North American cline, the incidence of diapause expression [29] and the number of ovarioles [26] display nonlinear associations with latitude in eastern Australia, which illustrates the fact that not all differentiation occurs in parallel among continents. Finally, recent work has shown a strong cline for the length of time that flies sleep: high-latitude populations of D. melanogaster in North America sleep for smaller bouts of time, and this pattern is reflected in higher nighttime locomotor activity in these populations [11].
Genetic variation
For decades researchers used single markers to elucidate clinal differentiation and spatial variation in allele frequencies. This approach revealed multiple markers with variation that tracked the clines, including a number with the same allele at higher frequency at the same latitude in the Northern and Southern hemispheres. Some examples include: alcohol dehydrogenase (Adh), α-glycerol-3-phosphate dehydrogenase (Gpdh), glucose-6-phosphate dehydrogenase (G6pd), esterase-6 (Est-6), octanol dehydrogenase (Odh), 6-phosphogluconate dehydrogenase (Pgd), and others [30–33] (Table 1). Perhaps the most heavily explored locus in D. melanogaster has been Adh, the first step in the ethanol detoxification pathway. The Adh-F allele codes for high catalytic activity of ADH, but this increase in activity trades off with enzyme stability at higher temperatures [34,35]. Not surprisingly, the Adh-F allele is found at a higher frequency in cooler high-latitude populations, and differentiation has occurred in parallel along clines in North and South America, Europe-Africa, Asia, and Australia [36–38]. A similar pattern of clinal differentiation exists for the acetaldehyde dehydrogenase locus (Aldh)—the second step in the ethanol detoxification pathway—with the derived allele segregating at a higher frequency in high-latitude North American populations [39].
Although researchers have identified many instances of clinal variation in allele frequencies (Table 1), this genetic variation has rarely been connected to variation in fitness, or to causal clinal selection pressures. A notable exception is the gene couch potato (cpo), which has been shown to underlie adaptation to seasonality in D. melanogaster from North America [40]. Geographic variation in the incidence of diapause, or reproductive quiescence, has been demonstrated to vary predictably with latitude: female flies sampled at higher latitudes exhibit diapause at higher frequency than females sampled at lower latitudes [25]. Using QTL mapping and genetic complementation, cpo was identified as the causative locus underlying diapause incidence; furthermore, the frequencies of single nucleotide polymorphisms (SNPs) in cpo were strongly correlated with latitude [40,41] (Figure 1). Clinal patterns in cpo suggest that increased incidence of diapause in high-latitude flies is strongly linked to clinal selection pressures along the North American cline. Indeed, diapause has been directly connected to fitness: in D. melanogaster population cages the frequency of flies expressing the diapause phenotype increased over time after exposure to both starvation and cold stress [42].
This clear picture of clinal adaptation of cpo—and its relationship to the diapause phenotype in North America—becomes cloudy when evaluated along the eastern Australian cline. The cline in cpo allele frequencies in Australia resembles that found in North America (Figure 1). However, when placed at conditions found to induce diapause in previous studies (i.e., 12°C and short day length [43]), Australian flies display reduced—but not arrested—egg maturation at pre-vitellogenic stages [44]. Delays in egg maturation were also nonlinear in Australia, with ovarian dormancy increasing towards both ends of the cline. This is in contrast to the North American cline where high-latitude populations have the highest and low-latitude populations have the lowest incidence of ovarian dormancy under similar conditions [25]. Thus, the clear association between cpo genotype and diapause phenotype in North America does not exist in Australia. This highlights a key point: even some of the strongest patterns of clinal adaptation observed in nature may not be repeatable. The factors that lead to such discrepancies provide fruitful avenues for future research. These may be biological in nature—for example, interactions with genetic background—or an artifact of sampling. Because tropical populations below 25°N in the Americas have gone underexplored, we simply do not know whether inclusion of these populations would strengthen or lessen support for parallel adaptation among clines.
In addition to single markers, clinal variation has also been detected in larger genomic regions. Due to dramatically reduced recombination in heterokaryotypic individuals (those carrying one copy of each arrangement), inverted regions can contain many variants that all segregate together. A number of the major cosmopolitan inversions including In(2L)t, In(2R)NS, In(3L)Payne, In(3R)Payne, and In(3R)Mo vary predictably with latitude [45–48], and may house genetic factors involved in climatic adaptation [10]. Indeed, very recent analysis has shown strong differentiation between tropical and temperate populations of D. melanogaster: populations in northern Australia carry the inverted arrangement of In(3R)Payne at high frequencies, while those in the south do not [49]. Because of the very low levels of recombination in inversions, implicating any single variant within them as the causal one can be extremely difficult. Patterns of differentiation across chromosome 3R combined with gene ontology analysis suggest that large areas of the third chromosome—rather than any single variant—are under climatic selection, and implicate entire gene families such as developmental and stimulus-response-related genes in clinal adaptation [49].
Genomic variation
Whole genome analyses of D. melanogaster populations have made it possible to identify both large- and fine-scale clinal genomic patterns beyond those identified by candidate gene studies [50–54]. Low-latitude populations on multiple continents display greater sequence diversity, more negative values of Tajima’s D, and a lower ratio of X-to autosome variation [50,52]. Whole genome data continue to support inferences made using smaller datasets: for example, chromosome 3R is the most strongly differentiated region of the genome due to In(3R)Payne [50]. This pattern is recapitulated in populations of D. melanogaster from Australia, indicating clinal adaptation of this region has occurred in parallel on both continents [51].
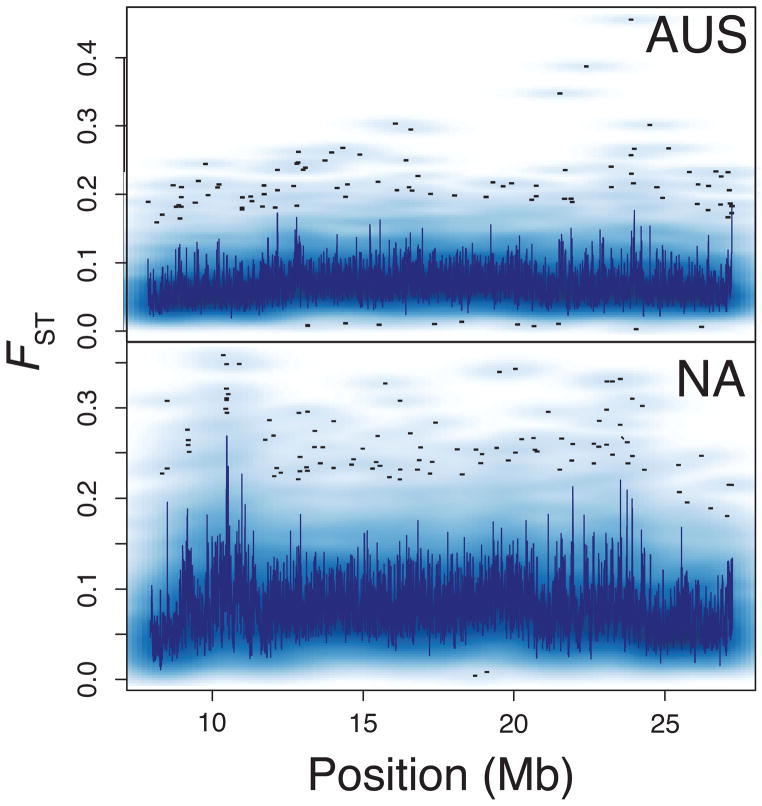
To identify signatures of adaptation, strongly differentiated regions of the genome between the samples are identified, and differentiated regions that overlap among multiple clines—both within and among species—provide evidence of parallel adaptation (Figure 2). For example, in northern and southern populations of D. melanogaster from the eastern United States and eastern Australia, the most highly differentiated sites in the genome (approximately the top 60% of FST values) cluster by environment rather than by continent: at these differentiated sites, populations from Maine more closely resemble populations from Tasmania than they do their lower latitude American conspecifics [52]. Importantly, parallelism is found even among less-differentiated alleles in North American and Australian populations of D. melanogaster, suggesting that many polymorphic sites are targets of clinal selection [52] (Figure 2). One cautionary note is that migrants from Europe or Africa founded the North American and Australian populations, likely bringing along both the high- and low-latitude adapted alleles [55]. Therefore, any neutral variants linked to the causal variants in the migrant population could also show patterns of clinal selection simply because of their proximity to recently selected sites. Differentiation at both cpo and at key circadian rhythm genes also supports parallel adaptation in North American [50] and Australian [51] populations of D. melanogaster. Strikingly, clinal variation in circadian genes has previously been identified in salmonids [56,57], passerine birds [58], and plants [59,60] in addition to D. melanogaster [11,61–63; but see 64].
Figure 2.
Genome-wide SNP data from the endpoints of the Australian (top) and North American (bottom) cline (modified from Reinhardt et al., 2014). Patterns of differentiation along chromosome arm 3R highlight exceptionally differentiated SNPs—potential targets of clinal selection. The dark blue line represents median estimates of FST, the density at a position is indicated by the intensity of the blue cloud, and black dots represent outliers.
Many genes are differentially expressed between high- and low-latitude populations of both D. melanogaster and D. simulans in North America [12]. Of the genes that are differentially expressed in both species, the majority differ in the same direction—that is, highly expressed genes at high latitudes in D. melanogaster are also highly expressed in high-latitude populations of D. simulans [12]. Although gene expression is a plastic trait that can be affected by the temperature at which flies are raised, genes that are differentially expressed between high- and low-latitude North American populations at specific temperatures also tend to be differentially expressed between Australian populations exposed to similar temperatures [12,65]. Additional studies are needed to strengthen the evidence for parallel adaptation of gene expression between clines within and between species. Regardless, exploring parallel patterns of gene expression will be a fruitful enterprise for discovering novel clines and for identifying the causative alleles underlying quantitative trait variation and local adaptation.
Copy number variants (CNVs) are an important source of functional genomic variation [66] and have been shown to vary with respect to latitude in populations of D. melanogaster from both North America [67] and Australia [54]. Analysis of genes within differentiated CNVs suggests that CNVs can respond to spatially varying selection pressures such as pesticides [54,67]. Likewise, the movement of transposable elements (TEs) represents another important source of genetic variation: TEs have been implicated in resistance to viral infection [68] and insecticides in D. melanogaster [69] and resistance to insecticides in the mosquito Culex pipiens [70]. Members of many TE families exhibit clinal variation in their relative frequencies with respect to latitude in D. melanogaster [71], and recently clines on multiple continents were identified in a TE from the invader4 family, which is associated with shorter development time in D. melanogaster [72]. Parallel clines were found in both North America and Australia, and similar differences are seen across the Europe-Africa cline, though not within Europe [72]. The flanking regions of this element show signatures of recent positive selection suggesting selection for shorter development times at higher latitudes [72]. These early results, assessing only a limited number of TE families, suggest that clinal analysis of TEs across the genome are needed.
Although all of the genes involved in clinal adaptation are not known, we can speculate as to their identity by combining phenotypic and genomic data, and specifically by identifying regions of the genome exhibiting parallel differentiation among continents. Among populations of D. melanogaster, recent studies found strong enrichment of many important biological functions including genes involved in embryonic development, larval development, transcriptional regulation, eye development, signaling, and immunity [50–52]. Parallel differentiation among continents has been identified in genes associated with D. melanogaster wing morphogenesis [52], suggesting a connection to well-characterized clines in wing size [13,21,73]. More generally, 31% of the genes that are differentiated between endpoint populations in North America [50] are also differentiated in Australia [51]. In contrast, some functions—for example, genes associated with pigmentation during development—that are enriched in North America are not enriched in Australia [52]. Unlike pigmentation during development, pigmentation of the thoracic trident has been shown to strongly correlate with latitude in populations of D. melanogaster from Australia [74]. Moreover, abdominal pigmentation is correlated with latitude in India [75], and with altitude in sub-Saharan African populations of D. melanogaster [76]. Connections between genomic and phenotypic pigmentation clines within Australian D. melanogaster suggests that parallelism among continents is not a necessary requirement to glean key inferences about the targets of clinal selection. Regardless, future concordance among studies in the functional categories of genes that show signatures of parallel adaptation will help provide candidates for future experimentation and analysis [50–52].
The current state of clinal genomics outside D. melanogaster
Despite innovations in whole-genome sequencing technologies, there currently exist relatively few studies investigating the impact of clinal selection on genomic variation. There are, for example, currently no published clinal genomic studies for other Drosophila species despite much work describing clines in multiple phenotypes and genetic markers. Many of these patterns resemble those seen in D. melanogaster, including clinal variation in body size traits in D. subobscura [77], D. simulans [78], and D. serrata [79]; temperature tolerance in D. simulans [80] and D. serrata [79]; pigmentation in D. yakuba [81] and D. simulans [82]; and inversions in D. subobscura [83–85]. There are of course also many examples of traits that are not clinal in these other species, or where the pattern of variation is different from those found in D. melanogaster. Still, the parallelism observed between D. melanogaster and D. simulans in the clinality of gene expression hints at the utility of species comparisons when attempting to identify the genes that are generally important for clinal adaptation [12].
It is perhaps not surprising that genome-wide patterns of clinal variation in humans and Arabidopsis thaliana are among the best-studied cases outside of Drosophila, given the wealth of geographically stratified genomic data available for both. Although data for these two species and others have not always been collected with clinal variation in mind, their results nevertheless represent pertinent examples of how genomic data can inform our understanding of environmental adaptation. In humans, clines in body size [86–88], skull and brain morphology [89], HLA allele frequency [90], skin pigmentation [91,92], salt sensitivity [93], and susceptibility to hypertension [94] have all been described. Instead of identifying the causal variants underlying many of these well-characterized phenotypic clines, studies utilizing genome-wide SNPs in humans have primarily focused on characterizing population structure across continents, where genomic variation is strongly correlated with geography [95–98]. While migration may explain much of the observed population structure in humans [95], adaptation to local environmental conditions is also recognized as an important factor influencing human genomic differentiation [99]. Indeed, SNP frequencies correlate with gradients in precipitation, temperature, and solar radiation across multiple geographic regions, as do SNPs associated with thermal tolerance, pigmentation, disease resistance, and life at high altitude [100–103]. It is clear that identifying the genomic basis of many clinal patterns in humans is both attainable and of importance.
In A. thaliana, multiple fitness-related life history traits—such as growth rate, flowering time, and seed dormancy—correlate with latitude [104]. Moreover, genome-wide SNP frequencies are associated with both geography and a host of important climatic gradients such as temperature, precipitation, isothermality, aridity, and day length [105–107]. Evidence points to local adaptation shaping much of this genomic variation: for example, variation in climate data explains more variation in nonsynonymous SNPs than predicted by chance [107]. Moreover, relative to synonymous SNPs, nonsynonymous SNPs were enriched among the loci most strongly associated with precipitation, humidity, temperature, and day length [106]. Finally, alleles associated with high fitness also tend to be locally abundant alleles that co-vary with climatic factors [105].
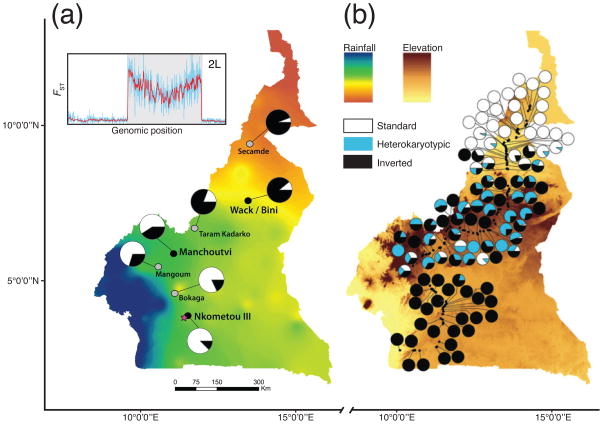
In addition to D. melanogaster and other model systems, recent studies exploring genomic patterns of local adaptation have surveyed a number of non-model organisms. Studies of the malaria vector, Anopheles gambiae, have revealed extensive clinal variation along an aridity gradient in Cameroon [108]. In A. gambiae, the frequency of a large polymorphic inversion on chromosome 2L covaries with both latitude and aridity (Figure 3a). Moreover, SNPs within the inversion are strongly differentiated between northern and southern populations, whereas SNPs in collinear regions of the genome appear nearly panmictic (Figure 3a). Interestingly, a parallel cline has been observed in a distantly related mosquito, A. funestus, which exhibits clinal variation in the frequency of an inversion on chromosome 3R along the same latitudinal transect in Cameroon (Figure 3b). This degree of parallelism provides an excellent opportunity to study the link between inversion polymorphisms and adaptation to the environment. Genome-wide clinal variation has also been identified in Medicago truncatula, an annual legume, sampled throughout the Mediterranean [109]. SNPs were correlated with gradients in several abiotic factors such as mean annual temperature, precipitation in the wettest month, and isothermality. Moreover, patterns of nucleotide diversity surrounding clinal SNPs suggest a history of positive selection shaping the evolution of some of these loci [109]. Genome-wide SNPs from Atlantic salmon (Salmo salar) sampled along a cline in Eastern Canada are correlated with temperature, river properties, geological variables, and longitude [110]. Similarly, strongly differentiated SNPs from Atlantic herring (Clupea harangus) sampled around the Baltic Sea were identified along a salinity gradient [111,112]. Finally, SNPs are correlated with temperature in populations of the Black Cottonwood, (Populus trichocarpa) sampled along a latitudinal transect in North America [113]. These examples of genome-wide clinal variation demonstrate the diversity of climate factors influencing genomic differentiation.
Figure 3.
Parallel clines in two distantly related mosquitoes along a latitudinal transect in Cameroon. A) Pies show the frequency of the standard (2L+a, white) and inverted (2La, black) arrangement of inversion 2La in A. gambiae. The inset displays genomic differentiation surrounding the 2La inversion (shaded area) as FST —plotted over 200-kb windows (red) and 20-kb windows (blue)—between high and low latitude populations (modified from Cheng et al., 2012). B) The karyotype frequency of the standard homozygote (white), heterozygote (blue), and inverted homozygote (black) arrangement of inversion 3Ra in A. funestus (reproduced with permission from Ayala, Guerrero, & Kirkpatrick, 2013).
The future of clinal genomics
In D. melanogaster and other model systems, whole-genome data from clines will make it possible to identify individual regions of the genome that are differentiated along with phenotypic traits. However, the most impressive genomic patterns of clinal differentiation in D. melanogaster come from studies limited to sampling the endpoints of clines—two geographic locations that often differ dramatically in many environmental factors. Sampling only the endpoints of a cline potentially limits the power of these studies to link the targets of selection with any particular environmental factor. Moreover, correlations between axes of environmental variation—such as the correlation between day length and temperature—can obscure both the true targets and agents of selection. Statistical methods that can appropriately handle correlated environmental factors have recently been developed, and promise to illuminate many loci responding to selection via a complex environment [114–116]. However, there is still a need for novel population genetic theory that extends classic models of clines with two fitness optima to models incorporating shifting optima over a continuous landscape—the biological reality of environmental gradients in nature (Box 1).
Fine-scale sampling from multiple geographic locations along climatic gradients—and the development of statistical approaches for dealing with such data—will help to parse genomic patterns of adaptation that may be eclipsed by differentiation at larger geographic scales. Likewise, fine-scale sampling may also be more effective at revealing the unique environmental variables associated with a particular pattern of genomic variation (frequency of a SNP, TE, inversion, etc.), as patterns of variation might be expected to become increasingly distinct when sampled at smaller spatial scales. Future studies should reap the benefits of joint approaches that identify connections between both outlier loci and their environmental correlates.
In addition to sampling fine-scale spatial genomic variation, future studies should also consider variation along a temporal scale. Researchers have recently shown that changes in allele frequencies can occur across seasons within populations of D. melanogaster [117]. Determining the extent to which the loci under seasonal selection align with those under spatially varying selection will provide an additional avenue for identifying the targets of climatic selection and patterns of parallel adaptation.
Perhaps the most promising avenue for future clinal genomic studies involves harnessing known phenotypic clines in order to characterize both their genetic determinants and to discover novel clines correlated with similar geographic axes. Related approaches have been successfully applied to understand the genetic basis of both skin pigmentation and adaptation to life at high altitudes in humans. Skin pigmentation is one of the most noticeable human phenotypes, and is strongly correlated with latitude and UV intensity. Both candidate-gene approaches and genome-wide scans have yielded numerous loci associated with variation in pigmentation [118–121]. Likewise, the genetic basis of adaptation to life at high altitudes has been well-characterized in populations of Tibetans displaying high-altitude phenotypes [100,102,103,122]. Still, these studies have primarily contrasted highland Tibetans with closely related lowland Han Chinese populations—similar to sampling only the endpoints of a cline. Future studies that take advantage of gradients in altitude promise to identify novel clines associated with this important geographic axis.
Finally, more data is clearly needed in non-model systems, and once available, the same approaches that have been useful in model systems can be employed with relative ease. For example, clinal patterns of phenotypic differentiation have very recently been characterized in monkeyflowers [123], Ivyleaf morning glories [124], Sakhalin firs [125], Anolis lizards [126], and Japanese sika deer [127]. Connecting these recent examples of phenotypic variation to patterns of genomic variation will clearly be a fruitful avenue for future exploration. Furthermore, the availability of well-assembled genomes in systems that have previously been found to have phenotypic or single-marker clines will be one of the most promising developments in the near term. As the province of genomics expands and matures, clinal genomics is poised to deliver one of the most valuable avenues to understand adaptation to spatially varying environments.
Acknowledgments
We thank David Begun and Daniel Schrider for their helpful comments. Discussions with Alisa Sedghifar and Nicolas Svetec improved this paper, as did comments from two anonymous reviewers. Funding for JRA came from the Indiana University Genome, Cell, and Developmental Biology Training Grant from the National Institutes of Health (T32-GM007757) and the National Science Foundation Graduate Research Fellowship (1342962). BSC was supported by a National Research Service Award from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (F32-AI114176).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Endler JA. Geographic variation, speciation, and clines. Princeton University Press; 1977. [PubMed] [Google Scholar]
- 2.Endler JA. Natural selection in the wild. Princeton University Press; 1986. [Google Scholar]
- 3.Slatkin M. Gene flow and selection in a cline. Genetics. 1973;75:733–756. doi: 10.1093/genetics/75.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szymura JM, Barton NH. Genetic analysis of a hybrid zone between the fire-bellied toads, Bombina bombina and B. variegata, near Cracow in Southern Poland. Evolution. 1986;40:1141–1159. doi: 10.1111/j.1558-5646.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 5.Szymura JM, Barton NH. The genetic structure of the hybrid zone between the fire-bellied toads Bombina bombina and B. variegata: comparisons between transects and between loci. Evolution. 1991;45:237–261. doi: 10.1111/j.1558-5646.1991.tb04400.x. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Stephan W. Inferring the demographic history and rate of adaptive substitution in Drosophila. PLoS Genet. 2006;2:e166. doi: 10.1371/journal.pgen.0020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David J, Capy P. Genetic variation of Drosophila melanogaster natural populations. Trends Genet. 1988;4:106–111. doi: 10.1016/0168-9525(88)90098-4. [DOI] [PubMed] [Google Scholar]
- 8.Lachaise D, et al. Evolutionary Biology. 22. 1988. Historical biogeography of the Drosophila melanogaster species subgroup; pp. 159–225. [Google Scholar]
- 9.Keller A. Drosophila melanogaster’s history as a human commensal. Curr Biol. 2007;17:R77–R81. doi: 10.1016/j.cub.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann AA, Weeks AR. Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica. 2007;129:133–47. doi: 10.1007/s10709-006-9010-z. [DOI] [PubMed] [Google Scholar]
- 11.Svetec N, et al. Evidence that natural selection maintains genetic variation for sleep in Drosophila melanogaster. BMC Evol Biol. 2015;15 doi: 10.1186/s12862-015-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, et al. Parallel gene expression differences between low and high latitude populations of Drosophila melanogaster and D. simulans. PLoS Genet. 2015;11:e1005184. doi: 10.1371/journal.pgen.1005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James AC, et al. Cellular basis and developmental timing in a size cline of Drosophila melanogaster. Genetics. 1995;140:659–666. doi: 10.1093/genetics/140.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sezgin E, et al. Single-locus latitudinal clines and their relationship to temperate adaptation in metabolic genes and derived alleles in Drosophila melanogaster. Genetics. 2004;168:923–931. doi: 10.1534/genetics.104.027649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angilletta M. Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press; 2009. [Google Scholar]
- 16.Cooper BS, et al. Acclimation of thermal physiology in natural populations of Drosophila melanogaster: A test of an optimality model. J Evol Biol. 2010;23:2346–2355. doi: 10.1111/j.1420-9101.2010.02095.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann AA, et al. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J Therm Biol. 2003;28:175–216. [Google Scholar]
- 18.Menne MJ, et al. United States Historical Climatology Network daily temperature, precipitation, and snow data 2014 [Google Scholar]
- 19.Bastide H, et al. Pigmentation in Drosophila melanogaster reaches its maximum in Ethiopia and correlates most strongly with ultra-violet radiation in sub-Saharan Africa. BMC Evol Biol. 2014;14:179. doi: 10.1186/s12862-014-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klepsatel P, et al. Similarities and differences in altitudinal versus latitudinal variation for morphological traits in Drosophila melanogaster. Evolution. 2014;68:1385–98. doi: 10.1111/evo.12351. [DOI] [PubMed] [Google Scholar]
- 21.Zwaan BJ, et al. Cellular of wing size variation in Drosophila melanogaster: A comparison of latitudinal clines on two continents. Heredity. 2000;84:338–347. doi: 10.1046/j.1365-2540.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- 22.Coyne Ja, Beecham E. Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics. 1987;117:727–737. doi: 10.1093/genetics/117.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azevedo RBR, et al. Temperature modulates epidermal cell size in Drosophila melanogaster. J Insect Physiol. 2002;48:231–237. doi: 10.1016/s0022-1910(01)00168-8. [DOI] [PubMed] [Google Scholar]
- 24.David JR, Bocquet C. Evolution in a cosmopolitan species: genetic latitudinal clines in Drosophila melanogaster wild populations. Experientia. 1975;31:164–166. doi: 10.1007/BF01990682. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt PS, et al. Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution. 2005;59:1721–1732. [PubMed] [Google Scholar]
- 26.Azevedo R, et al. Thermal evolution of egg size in Drosophila melanogaster. Evolution. 1996;50:2338–2345. doi: 10.1111/j.1558-5646.1996.tb03621.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt PS, Paaby AB. Reproductive diapause and life-history clines in North American populations of Drosophila melanogaster. Evolution. 2008;62:1204–1215. doi: 10.1111/j.1558-5646.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann AA, et al. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett. 2002;5:614–618. [Google Scholar]
- 29.Lee SF, et al. Molecular basis of adaptive shift in body size in Drosophila melanogaster: functional and sequence analyses of the Dca gene. Mol Biol Evol. 2011;28:2393–402. doi: 10.1093/molbev/msr064. [DOI] [PubMed] [Google Scholar]
- 30.Bubliy O, et al. Geographic variation of six allozyme loci in Drosophila melanogaster: an analysis of data from different continents. Hereditas. 1999;130:25–32. doi: 10.1111/j.1601-5223.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 31.Oakeshott JG, et al. Geographic variation in G6pd and Pdg allele frequencies in Drosophila melanogaster. Heredity. 1983;50:67–72. doi: 10.1038/hdy.1983.7. [DOI] [PubMed] [Google Scholar]
- 32.Oakeshott JG, et al. Latitudinal variation in octanol dehydrogenase and acid phosphatase allele frequencies in Drosophila melanogaster. Theor Appl Genet. 1983;65:191–6. doi: 10.1007/BF00308064. [DOI] [PubMed] [Google Scholar]
- 33.Singh RS, et al. Genetic differentiation between geographically distant populations of Drosophila melanogaster. Genetics. 1982;101:235–256. doi: 10.1093/genetics/101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milkman R. Further evidence of thermostability variation within electrophoretic mobility classes of enzymens. Biochem Genet. 1976;14:383–387. doi: 10.1007/BF00484776. [DOI] [PubMed] [Google Scholar]
- 35.Anderson SM, McDonald JF. Biochemical and molecular analysis of naturally occurring Adh variants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983;80:4798–4802. doi: 10.1073/pnas.80.15.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry A, Kreitman M. Molecular analysis of an allozyme cline: Alcohol dehydrogenase in Drosophila melanogaster on the east coast of North America. Genetics. 1993;134:869–893. doi: 10.1093/genetics/134.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David JR, et al. Alcohol tolerance and ADH gene frequencies in European and African populations of Drosophila melanogaster. Genet Sel Evol. 1986;18:405–416. doi: 10.1186/1297-9686-18-4-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oakeshott JG, et al. Alcohol dehydrogenase and glycerol-3-phosphate dehydrogenase clines in Drosophila melanogaster on different continents. Evolution. 1982;36:86–96. doi: 10.1111/j.1558-5646.1982.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 39.Fry JD, et al. A worldwide polymorphism in Aldehyde dehydrogenase in Drosophila melanogaster: Evidence for selection mediated by dietary ethanol. Evolution. 2008;62:66–75. doi: 10.1111/j.1558-5646.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt PS, et al. An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:16207–16211. doi: 10.1073/pnas.0805485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogni R, et al. The intensity of selection acting on the couch potato gene-spatial-temporal variation in a diapause cline. Evolution. 2014;68:538–548. doi: 10.1111/evo.12291. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt PS, Conde DR. Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila Melanogaster. Evolution. 2006;60:1602–1611. [PubMed] [Google Scholar]
- 43.Saunders DS, et al. Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc Natl Acad Sci U S A. 1989;86:3748–3752. doi: 10.1073/pnas.86.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SF, et al. Polymorphism in the couch potato gene clines in eastern Australia but is not associated with ovarian dormancy in Drosophila melanogaster. Mol Ecol. 2011;20:2973–2984. doi: 10.1111/j.1365-294X.2011.05155.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu Rev Ecol Evol Syst. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapun M, et al. Inference of chromosomal inversion dynamics from Pool-Seq data in natural and laboratory populations of Drosophila melanogaster. Mol Ecol. 2014;23:1813–27. doi: 10.1111/mec.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knibb W. Chromosome inversion polymorphisms in Drosophila melanogaster II. Geographic clines and climatic associations in Australasia, North America and Asia. Genetica. 1982;58:213–221. [Google Scholar]
- 48.Mettler LE, et al. Inversion clines in populations of Drosophila melanogaster. Genetics. 1977;87:169–176. doi: 10.1093/genetics/87.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rane ARV, et al. Genomic evidence for role of inversion 3RP of Drosophila melanogaster in facilitating climate change adaptation. Mol Ecol. 2015;4:2423–2432. doi: 10.1111/mec.13161. [DOI] [PubMed] [Google Scholar]
- 50.Fabian DK, et al. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol Ecol. 2012;21:4748–4769. doi: 10.1111/j.1365-294X.2012.05731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolaczkowski B, et al. Genomic differentiation between temperate and tropical Australian populations of Drosophila melanogaster. Genetics. 2011;187:245–60. doi: 10.1534/genetics.110.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt Ja, et al. Parallel geographic variation in Drosophila melanogaster. Genetics. 2014;197:361–73. doi: 10.1534/genetics.114.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrider DR, et al. Rates and genomic consequences of spontaneous mutational events in Drosophila melanogaster. Genetics. 2013;194:937–54. doi: 10.1534/genetics.113.151670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner TL, et al. Genomic analysis of adaptive differentiation in Drosophila melanogaster. Genetics. 2008;179:455–73. doi: 10.1534/genetics.107.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kao JY, et al. Population genomic analysis uncovers African and European admixture in Drosophila melanogaster populations from the southeastern United States and Caribbean Islands. Mol Ecol. 2015 doi: 10.1111/mec.13137. [DOI] [PubMed] [Google Scholar]
- 56.O’Malley KG, Banks Ma. A latitudinal cline in the Chinook Salmon (Oncorhynchus tshawytscha) Clock gene: evidence for selection on PolyQ length variants. Proc Biol Sci. 2008;275:2813–21. doi: 10.1098/rspb.2008.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Malley KG, et al. Clock polymorphism in Pacific salmon: evidence for variable selection along a latitudinal gradient. Proc Biol Sci. 2010;277:3703–14. doi: 10.1098/rspb.2010.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnsen A, et al. Avian Clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol Ecol. 2007;16:4867–80. doi: 10.1111/j.1365-294X.2007.03552.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, et al. Disentangling the roles of history and local selection in shaping clinal variation of allele frequencies and gene expression in Norway Spruce (Picea abies) Genetics. 2012;191:865–81. doi: 10.1534/genetics.112.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keller SR, et al. Local adaptation in the flowering-time gene network of balsam poplar, Populus balsamifera. Mol Biol Evol. 2012;29:3143–3152. doi: 10.1093/molbev/mss121. [DOI] [PubMed] [Google Scholar]
- 61.Costa R, et al. A latitudinal cline in a Drosophila Clock gene. Proc Biol Sci. 1992;250:43–49. doi: 10.1098/rspb.1992.0128. [DOI] [PubMed] [Google Scholar]
- 62.Kyriacou CP, et al. Clines in clock genes: fine-tuning circadian rhythms to the environment. Trends Genet. 2008;24:124–132. doi: 10.1016/j.tig.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Sawyer LA, et al. The period gene Thr-Gly polymorphism in Australian and African Drosophila melanogaster populations: Implications for selection. Genetics. 2006;174:465–480. doi: 10.1534/genetics.106.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weeks AR, et al. In search of clinal variation in the period and clock timing genes in Australian Drosophila melanogaster populations. J Evol Biol. 2006;19:551–557. doi: 10.1111/j.1420-9101.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 65.Levine MT, et al. Whole-genome expression plasticity across tropical and temperate Drosophila melanogaster populations from eastern Australia. Mol Biol Evol. 2011;28:249–256. doi: 10.1093/molbev/msq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schrider DR, Hahn MW. Gene copy-number polymorphism in nature. Proc Biol Sci. 2010;277:3213–21. doi: 10.1098/rspb.2010.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrider DR, et al. Detecting highly differentiated copy-number variants from pooled population sequencing. Pacific Symp Biocomput. 2013;18:334–355. [PMC free article] [PubMed] [Google Scholar]
- 68.Magwire MM, et al. Successive increases in the resistance of Drosophila to viral infection through a transposon insertion followed by a duplication. PLoS Genet. 2011;7:e1002337. doi: 10.1371/journal.pgen.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt JM, et al. Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet. 2010;6:e1000998. doi: 10.1371/journal.pgen.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darboux I, et al. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae) Cell Microbiol. 2007;9:2022–2029. doi: 10.1111/j.1462-5822.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 71.González J, et al. Genome-wide patterns of adaptation to temperate environments associated with transposable elements in Drosophila. PLoS Genet. 2010;6:33–35. doi: 10.1371/journal.pgen.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ullastres A, et al. Exploring the phenotypic space and the evolutionary history of a natural mutation in Drosophila melanogaster. Mol Biol Evol. 2015 doi: 10.1093/molbev/msv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKechnie SW, et al. A clinally varying promoter polymorphism associated with adaptive variation in wing size in Drosophila. Mol Ecol. 2010;19:775–784. doi: 10.1111/j.1365-294X.2009.04509.x. [DOI] [PubMed] [Google Scholar]
- 74.Telonis-Scott M, et al. The molecular genetics of clinal variation: A case study of ebony and thoracic trident pigmentation in Drosophila melanogaster from eastern Australia. Mol Ecol. 2011;20:2100–2110. doi: 10.1111/j.1365-294X.2011.05089.x. [DOI] [PubMed] [Google Scholar]
- 75.Das A. Abdominal pigmentation in Drosophila melanogaster females from natural Indian populations. J Zool Syst Evol Res. 1995;33:84–87. [Google Scholar]
- 76.Pool JE, Aquadro CF. The genetic basis of adaptive pigmentation variation in Drosophila melanogaster. Mol Ecol. 2007;16:2844–2851. doi: 10.1111/j.1365-294X.2007.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huey RB, et al. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- 78.Arthur AL, et al. Investigating latitudinal clines for life history and stress resistance traits in Drosophila simulans from eastern Australia. J Evol Biol. 2008;21:1470–1479. doi: 10.1111/j.1420-9101.2008.01617.x. [DOI] [PubMed] [Google Scholar]
- 79.Hallas R, et al. Clinal variation in Drosophila serrata for stress resistance and body size. Genet Res. 2002;79:141–148. doi: 10.1017/s0016672301005523. [DOI] [PubMed] [Google Scholar]
- 80.Van Heerwaarden B, et al. Complex patterns of local adaptation in heat tolerance in Drosophila simulans from eastern Australia. J Evol Biol. 2012;25:1765–1778. doi: 10.1111/j.1420-9101.2012.02564.x. [DOI] [PubMed] [Google Scholar]
- 81.Matute DR, Harris A. The influence of abdominal pigmentation on desiccation and ultraviolet resistance in two species of Drosophila. Evolution. 2013;67:2451–2460. doi: 10.1111/evo.12122. [DOI] [PubMed] [Google Scholar]
- 82.Capy P, et al. Thoracic trident pigmentation in natural populations of Drosophila simulans: a comparison with D. melanogaster. Heredity. 1988;61:263–268. [Google Scholar]
- 83.Balanya J, et al. Global genetic change tracks global climate warming in Drosophila subobscura. Science. 2006;313:1773–1775. doi: 10.1126/science.1131002. [DOI] [PubMed] [Google Scholar]
- 84.Balanyà J, et al. Evolutionary pace of chromosomal polymorphism in colonizing populations of Drosophila subobscura: an evolutionary time series. Evolution. 2003;57:1837–1845. doi: 10.1111/j.0014-3820.2003.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 85.Prevosti A, et al. Colonization of America by Drosophila subobscura: Experiment in natural populations that supports the adaptive role of chromosomal-inversion polymorphism. Proc Natl Acad Sci U S A. 1988;85:5597–600. doi: 10.1073/pnas.85.15.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinstein KJ. Body proportions in ancient Andeans from high and low altitudes. Am J Phys Anthropol. 2005;128:569–85. doi: 10.1002/ajpa.20137. [DOI] [PubMed] [Google Scholar]
- 87.Roberts DF. Body weight, race and climate. Am J Phys Anthropol. 1953;11:533–558. doi: 10.1002/ajpa.1330110404. [DOI] [PubMed] [Google Scholar]
- 88.Katzmarzyk PT, Leonard WR. Climatic influences on human body size and proportions: ecological adaptation and secular trends. Am J Phys Anthropol. 1998;106:483–503. doi: 10.1002/(SICI)1096-8644(199808)106:4<483::AID-AJPA4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 89.Bakken TE, et al. A geographic cline of skull and brain morphology among individuals of European ancestry. Hum Hered. 2011;72:35–44. doi: 10.1159/000330168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith T, et al. Altitude, language, and class I HLA allele frequencies in Papua New Guinea. Am J Phys Anthropol. 1994;95:155–68. doi: 10.1002/ajpa.1330950204. [DOI] [PubMed] [Google Scholar]
- 91.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 92.Lao O, et al. Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Ann Hum Genet. 2007;71:354–369. doi: 10.1111/j.1469-1809.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 93.Thompson EE, et al. CYP3A variation and the evolution of salt-sensitivity variants. Am J Hum Genet. 2004;75:1059–1069. doi: 10.1086/426406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young JH, et al. Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet. 2005;1:e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- 96.Novembre J, et al. Genes mirror geography within Europe. Nature. 2008;456:98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paschou P, et al. Maritime route of colonization of Europe. Proc Natl Acad Sci U S A. 2014;111:9211–6. doi: 10.1073/pnas.1320811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suo C, et al. Natural positive selection and north–south genetic diversity in East Asia. Eur J Hum Genet. 2012;20:102–110. doi: 10.1038/ejhg.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barreiro LB, et al. Natural selection has driven population differentiation in modern humans. Nat Genet. 2008;40:340–5. doi: 10.1038/ng.78. [DOI] [PubMed] [Google Scholar]
- 100.Beall CM, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hancock AM, et al. Adaptations to climate-mediated selective pressures in humans. PLoS Genet. 2011;7:e1001375. doi: 10.1371/journal.pgen.1001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simonson TS, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 103.Yi X, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Debieu M, et al. Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS One. 2013;8:e61075. doi: 10.1371/journal.pone.0061075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fournier-Level A, et al. A map of local adaptation in Arabidopsis thaliana. Science. 2011;334:86–9. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- 106.Hancock AM, et al. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334:83–6. doi: 10.1126/science.1209244. [DOI] [PubMed] [Google Scholar]
- 107.Lasky JR, et al. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Mol Ecol. 2012;21:5512–29. doi: 10.1111/j.1365-294X.2012.05709.x. [DOI] [PubMed] [Google Scholar]
- 108.Cheng C, et al. Ecological genomics of Anopheles gambiae along a latitudinal cline: a population-resequencing approach. Genetics. 2012;190:1417–32. doi: 10.1534/genetics.111.137794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoder JB, et al. Genomic signature of adaptation to climate in Medicago truncatula. Genetics. 2014;196:1263–75. doi: 10.1534/genetics.113.159319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vincent B, et al. Landscape genomics in Atlantic salmon (Salmo salar): searching for gene-environment interactions driving local adaptation. Evolution. 2013;67:3469–87. doi: 10.1111/evo.12139. [DOI] [PubMed] [Google Scholar]
- 111.Lamichhaney S, et al. Population-scale sequencing reveals genetic differentiation due to local adaptation in Atlantic herring. Proc Natl Acad Sci U S A. 2012;109:19345–50. doi: 10.1073/pnas.1216128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Günther T, Coop G. Robust identification of local adaptation from allele frequencies. Genetics. 2013;195:205–20. doi: 10.1534/genetics.113.152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evans LM, et al. Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nat Genet. 2014;46:1089–1096. doi: 10.1038/ng.3075. [DOI] [PubMed] [Google Scholar]
- 114.Coop G, et al. Using environmental correlations to identify loci underlying local adaptation. Genetics. 2010;185:1411–1423. doi: 10.1534/genetics.110.114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frichot E, et al. Testing for associations between loci and environmental gradients using latent factor mixed models. Mol Biol Evol. 2013;30:1687–99. doi: 10.1093/molbev/mst063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poncet BN, et al. Tracking genes of ecological relevance using a genome scan in two independent regional population samples of Arabis alpina. Mol Ecol. 2010;19:2896–2907. doi: 10.1111/j.1365-294X.2010.04696.x. [DOI] [PubMed] [Google Scholar]
- 117.Bergland AO, et al. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 2014;10:e1004775. doi: 10.1371/journal.pgen.1004775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beleza S, et al. Genetic architecture of skin and eye color in an African-European admixed population. PLoS Genet. 2013;9:e1003372. doi: 10.1371/journal.pgen.1003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Graf J, et al. Single nucleotide polymorphisms in the MATP gene are associated with normal human pigmentation variation. Hum Mutat. 2005;25:278–284. doi: 10.1002/humu.20143. [DOI] [PubMed] [Google Scholar]
- 120.Han J, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Valverde P, et al. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 122.Huerta-Sánchez E, et al. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature. 2014;512:194–197. doi: 10.1038/nature13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kooyers NJ, et al. Replicate altitudinal clines reveal that evolutionary flexibility underlies adaptation to drought stress in annual Mimulus guttatus. New Phytol. 2015;206:152–165. doi: 10.1111/nph.13153. [DOI] [PubMed] [Google Scholar]
- 124.Campitelli BE, Stinchcombe JR. Natural selection maintains a single-locus leaf shape cline in Ivyleaf morning glory, Ipomoea hederacea. Mol Ecol. 2013;22:552–564. doi: 10.1111/mec.12057. [DOI] [PubMed] [Google Scholar]
- 125.Ishizuka W, et al. Use of intraspecific variation in thermal responses for estimating an elevational cline in the timing of cold hardening in a sub-boreal conifer. Plant Biol. 2014;17:177–185. doi: 10.1111/plb.12214. [DOI] [PubMed] [Google Scholar]
- 126.Muñoz MM, et al. Untangling intra- and interspecific effects on body size clines reveals divergent processes structuring convergent patterns in Anolis Lizards. Am Nat. 2014;184:636–646. doi: 10.1086/678084. [DOI] [PubMed] [Google Scholar]
- 127.Kubo MO, Takatsuki S. Geographical body size clines in Sika Deer: path analysis to discern amongst environmental influences. Evol Biol. 2015;42:115–127. [Google Scholar]
- 128.Barton NH. Multilocus clines. Evolution. 1983;37:454–471. doi: 10.1111/j.1558-5646.1983.tb05563.x. [DOI] [PubMed] [Google Scholar]
- 129.Fitzpatrick BM. Alternative forms for genomic clines. Ecol Evol. 2013;3:1951–1966. doi: 10.1002/ece3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gompert Z, Buerkle A. Bayesian estimation of genomic clines. Mol Ecol. 2011;20:2111–2127. doi: 10.1111/j.1365-294X.2011.05074.x. [DOI] [PubMed] [Google Scholar]
- 131.Jones FC, et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hohenlohe PA, et al. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 2010;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Payseur BA, et al. Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice. Evolution. 2004;58:2064–2078. doi: 10.1111/j.0014-3820.2004.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 134.Tucker PK, et al. Abrupt cline for sex chromosomes in a hybrid zone between two species of mice. Evolution. 1992;46:1146–1163. doi: 10.1111/j.1558-5646.1992.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 135.Mallet J, Barton NH. Strong natural selection in a warning-color hybrid zone. Evolution. 1989;43:421–431. doi: 10.1111/j.1558-5646.1989.tb04237.x. [DOI] [PubMed] [Google Scholar]
- 136.Mallet J, et al. Estimates of selection and gene flow from measures of cline width and linkage disqualirium in Heliconius hybrid zones. Genetics. 1990;124:921–36. doi: 10.1093/genetics/124.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ayala D, et al. Reproductive isolation and local adaptation quantified for a chromosome inversion in a malaria mosquito. Evolution. 2013;67:946–958. doi: 10.1111/j.1558-5646.2012.01836.x. [DOI] [PubMed] [Google Scholar]
- 138.Oakeshott JG, et al. Latitudinal relationships of esterase-6 and phosphoglucomutase gene frequencies in Drosophila melanogaster. Heredity. 1981;47:385–396. doi: 10.1038/hdy.1981.99. [DOI] [PubMed] [Google Scholar]
- 139.Paaby AB, et al. Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol Ecol. 2010;19:760–774. doi: 10.1111/j.1365-294X.2009.04508.x. [DOI] [PubMed] [Google Scholar]
- 140.Paaby AB, et al. A highly pleiotropic amino acid polymorphism in the Drosophila insulin receptor contributes to life-history adaptation. Evolution. 2014;68:3395–3409. doi: 10.1111/evo.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oakeshott JG, et al. Population genetics of the metabolically related Adh, Gpdh and Tpi polymorphisms in Drosophila melanogaster I. Geographic variation in Gpdh and Tpi allele frequencies in different continents. Genetica. 1984;63:21–29. [Google Scholar]
- 142.González J, et al. Genome-wide patterns of adaptation to temperate environments associated with transposable elements in Drosophila. PLoS Genet. 2010;6:e1000905. doi: 10.1371/journal.pgen.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cooper BS, et al. Thermal adaptation of cellular membranes in natural populations of Drosophila melanogaster. Funct Ecol. 2014;28:886–894. doi: 10.1111/1365-2435.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schmidt PS, et al. Geographic variation in diapause incidence, life-history traits, and climatic adaptation in Drosophila melanogaster. Evolution. 2005;59:1721–1732. [PubMed] [Google Scholar]
- 145.Cohan FM, Graf JD. Latitudinal cline in Drosophila melanogaster for knockdown resistance to ethanol fumes and for rates of response to selection for further resistance. Evolution. 1985;39:278–293. doi: 10.1111/j.1558-5646.1985.tb05666.x. [DOI] [PubMed] [Google Scholar]