Abstract
Background
Impairment in left ventricular (LV) systolic function has been described in heart failure with preserved ejection fraction (HFpEF), but its prognostic relevance is not known. We determined whether LV longitudinal strain (LS) is predictive of cardiovascular (CV) outcomes in HFpEF beyond clinical and conventional echocardiographic measures.
Methods and Results
LS was assessed by 2D speckle-tracking echocardiography at baseline in 447 HFpEF patients enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. At a median follow-up of 2.6 (IQR 1.5–3.9) years, 115 patients experienced the primary composite outcome of CV death, HF hospitalization, or aborted cardiac arrest. Impaired LS, defined as an absolute LS<15.8%, was present in 52% of patients and was predictive of the composite outcome (adjusted HR 2.14, 95% CI 1.26–3.66; p=0.005), CV death alone (adjusted HR 3.20, 95% CI 1.44–7.12; p=0.004), and HF hospitalization alone (adjusted HR 2.23, 95% CI 1.16–4.28; p=0.016) after adjusting for clinical and conventional echocardiographic variables. LS was the strongest echocardiographic predictor of the composite outcome. Exploratory analysis in a subset of 131 patients with follow-up LS assessed after 12–18 months demonstrated a trend towards improvement in LS associated with spironolactone in patients enrolled in the Americas but not in Russia or Georgia.
Conclusions
Impaired LV systolic function is a powerful predictor of HF hospitalization, CV death, or aborted cardiac arrest in HFpEF, independent of clinical predictors. Impaired LS represents a novel imaging biomarker to identify HFpEF patients at particularly high risk for CV morbidity and mortality.
Clinical Trial Registration Information
Clinicaltrials.gov. Identifier NCT00094302.
Keywords: Heart failure with preserved ejection fraction, systolic function, echocardiography, spironolactone, clinical trials
Introduction
Heart failure with preserved ejection fraction (HFpEF) is common, increasing in prevalence,1,2 and associated with rates of HF re-hospitalization and functional decline similar to patients with HF with reduced EF,3,4 and a higher risk of death compared to age-matched controls.5,6 Abnormal LV diastolic performance is an important pathophysiologic abnormality underlying HFpEF,7 but demonstrates limited specificity8 and sensitivity.9,10 During systole, the LV shortens in the longitudinal and circumferential planes and thickens in the radial plane. In patients with HFpEF, measures of systolic function are frequently abnormal when assessed by mitral annular systolic excursion11,12 and velocity12,13,14,15, midwall fractional shortening14,16, and longitudinal strain.17,18,19 Strain imaging in particular allows for quantitative assessment of myocardial deformation,20 appears to be a less load-dependent index of systolic function than LVEF,21 and is associated with clinical outcomes in HFrEF and LV dysfunction post-myocardial infarction. 22,23,24,25 Limited data are available regarding both the prognostic relevance of systolic dysfunction in HFpEF beyond clinical and conventional echocardiographic predictors, and the impact of treatment with an aldosterone antagonist on LV deformation. We hypothesized that worse LV longitudinal strain (LS) is associated with heightened risk for HF hospitalization and cardiovascular (CV) death in HFpEF, will provide incremental prognostic information beyond clinical and conventional echocardiographic measures, and will improve with aldosterone antagonist therapy.
Methods
Patient population
As previously described in detail,26 TOPCAT was a multicenter, international, randomized, double blind placebo-controlled trial testing the aldosterone antagonist spironolactone to reduce cardiovascular morbidity and mortality in 3,445 adults at least 50 years old with signs and symptoms of HF and an LVEF ≥45% per local site reading. Randomization was stratified by the presence of either one of the following inclusion criteria: at least one hospitalization in the prior 12 months for which HF was a major component of the hospitalization or, if no qualifying hospitalization, a B-type natriuretic peptide (BNP) in the prior 60 days ≥100 pg/ml or N-terminal pro-BNP (NT-proBNP) ≥360 pg/ml. All patients provided written informed consent and the study was approved by the local Institutional Review Board. Detailed baseline demographics and clinical characteristics of the trial population27 and the primary trial results28 have been previously published.
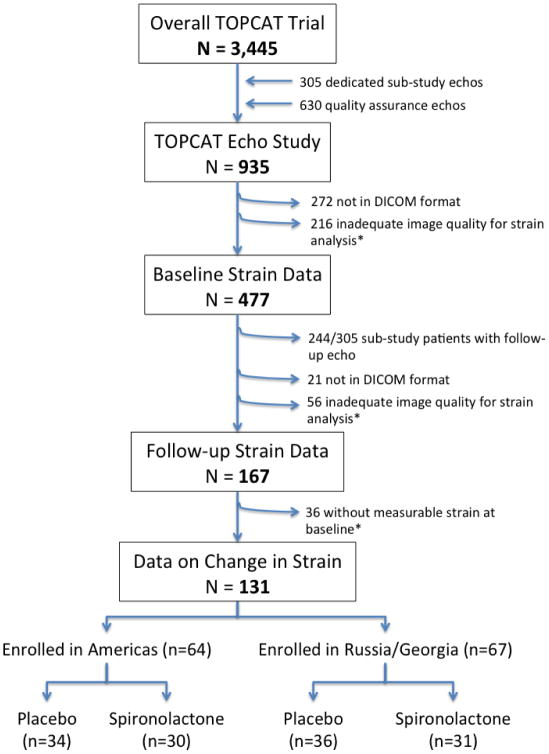
The design and baseline findings of the TOPCAT echocardiographic sub-study, including reproducibility metrics for conventional echocardiographic measures, have been previously described in detail.29 Strain analysis was performed on digitally acquired images in DICOM format with acceptable quality. Of 935 patients in the TOPCAT echo study, 663 (71%) were in DICOM format. Of those in DICOM format, 447 (67%) had adequate image quality for deformational analysis of LS by B-mode speckle tracking and are included in this report (Figure 1). Unacceptable image quality was defined as missing view, lack of a full cardiac cycle, more than 2 segment dropout, or significant foreshortening of the left ventricle. To determine whether LS values among the HFpEF patients studied differed significantly from elderly persons without HFpEF, we compared HFpEF patients aged 65–91 to matched community dwelling persons enrolled in the Atherosclerosis Risk in Communities (ARIC) study.30 ARIC is a prospective epidemiologic cohort study that originally enrolled 15,792 middle-aged subjects in 4 communities in the United States between 1987 and 1989. Between 2011 and 2013, 6,538 participants returned for a fifth study visit that included comprehensive echocardiography.31 The same echocardiography core laboratory served both the TOPCAT and ARIC studies.
Figure 1.
Consort diagram of the study population. *Unacceptable image quality was defined as missing view, lack of a full cardiac cycle, more than 2 segment dropout, or significant foreshortening of the left ventricle.
Echocardiographic Methods
Quantitative measures on all study echocardiograms were performed according to the American Society of Echocardiography recommendations by dedicated analysts at the core laboratory, blinded to clinical information and randomized treatment assignment as previously described for both the TOPCAT and ARIC studies.29,31,32,33 Digitally acquired baseline echocardiography images in DICOM format with acceptable image quality were uploaded to TomTec software (Munich, Germany) for deformational analyses (2D Cardiac Performance Analysis) as previously described.19 For deformation analysis, endocardial borders were traced at the end-diastolic frame in apical views, with end-diastole defined by the QRS complex or as the frame after mitral valve closure.19 The software tracks speckles along the endocardial border throughout the cardiac cycle. Peak LS was computed automatically, generating regional data from 6 segments and an average value for each view. For patients in sinus rhythm analyses were performed on a single cardiac cycle, while for patients in atrial fibrillation strain values were calculated as the average of 3 cardiac cycles. Peak average LS was measured in the apical 4 and apical 2 chamber views (in 6 segments from each view) and averaged. All strain measures were performed by a single reader at the echocardiography core laboratory. Intra-observer variability in our laboratory for LS, performed in 40 studies, is as follows: coefficient of variation: 8%, bias 0.40±1.48%, correlation coefficient: 0.71.
Outcomes
Clinical outcomes included CV death, HF hospitalization, and aborted sudden death during the follow-up period. All events were reported by the primary site investigator and independently adjudicated by the Clinical Endpoints Center. Definitions of these endpoints have been previously published.26
Statistical Analysis
LS is a negative value, but for ease of interpretation we have expressed LS as an absolute value. Abnormal LS was defined as a peak systolic absolute LS value <15.8%, which represents 2 standard deviations below the mean value for a healthy population of similar age as previously described.19 Strain values were compared between an equal number of patients with HFpEF in TOPCAT and community dwelling elderly persons without HF from the ARIC cohort by k:k matching for age (within 2 years), gender and race (white, black, or other). Matching was performed using a coarsened exact matching algorithm (STATA CEM command). Between group comparisons were made using a two-sample T-test. Comparisons were also made after additionally matching for history of hypertension, diabetes, coronary disease, BMI ≥30 kg/m2, and eGFR category (0–30, 30–60, 60–90, >90 mL/min per 1.73 m2).
To assess the prognostic relevance of baseline strain, the primary outcome was the composite of HF hospitalization, aborted sudden death, or CV death. Secondary endpoints assessed included CV death and HF hospitalization individually. The association of LS with the outcome variables of interest was assessed by time-to-event analysis using univariable and 2 multivariable Cox proportional hazards models adjusting for increasing number of covariates. Model 1 was developed from an initial set of 31 candidate variable predictors for the primary outcome in the echocardiography study as previously described in detail.34 Model 1 adjusted for age, gender, race, randomized treatment assignment (spironolactone versus placebo), randomization strata (qualifying hospitalization or elevated natriuretic peptide level), enrollment region (Americas versus Russia/Georgia), history of atrial fibrillation, core lab LVEF, heart rate, New York Heart Association class, history of stroke, creatinine, and hematocrit, in addition to LV mass which is prognostically relevant in the TOPCAT echo study34 and LV end-systolic volume index (LVESVi) as LV size may influence wall stress and strain. Septal E/E’, also prognostically relevant in this population,34 was additionally adjusted for in Model 2 given the prevalence of missing values for this variable. Based on event rates35,36 and prevalence of abnormal LS19 from previous HFpEF randomized trials, we anticipated 80% power to detect a hazard ratio of 1.6 associated with abnormal LS for the primary outcome. We had 80% power to detect a hazard ratio of 1.7 with the observed number of events and prevalence of abnormal LS in this study. The flexible continuous relationship of LS with the primary outcome and its components (HF hospitalization and CV death) was further assessed via a Cox model using restricted cubic splines. The relationship between LS and total HF hospitalizations during the follow-up period was assessed using a negative binomial model for recurrent events. The incremental value of LS when added to the clinical variables, LV mass, LVESVi, and E/E’ ratio was assessed by comparing the C-statistic of the predictive models without versus with LS, with all C-statistics obtained via leave-one-out cross validation. All analyses were performed using STATA version 12. Continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) associated with echo variables was assessed for the primary composite endpoint, HF hospitalization, and CV death at 3 years using time-to-event data.37 While LVEF criteria for HFpEF vary,7,38 an LVEF ≥55% is uniformly considered normal. Therefore, given the association of LS with LVEF, we performed a sensitivity analysis restricted to 354 patients with core lab LVEF≥55%. To assess the association of LS with outcomes after adjustment for natriuretic peptide level, we performed an additional analysis in 259 patients in whom data on either BNP or NT-proBNP was available, regardless of TOPCAT randomization strata (prior HF hospitalization or biomarker criteria).26 In this analysis, the Z score of the log-transformed BNP or NT-proBNP level was further included in multivariable Models 1 and 2 above. As differences in event rates were noted between patients enrolled in the Americas compared to Russia and Georgia,39 we also performed a sensitivity analysis stratified by region of enrollment (Americas [n=340] versus Russia/Georgia [n=107]).
For the association of baseline strain with outcomes, the primary analysis was performed using raw data, even when some patients had missing values. A sensitivity analysis was performed using multiple imputation for missing data. Given the arbitrary missing value pattern of the TOPCAT echo data, we employed multiple imputation by chained equations, an iterative imputation procedure (STATA mi impute chained). Imputation was performed for each echocardiographic measure with any missing data and was based on linear regression using 30 baseline clinical variables and 18 echocardiographic measures as predictor variables as previously described34 and was derived over 40 imputations.
LS measures were available both at baseline and at 12 (n=99) or 18 (n=32) months after randomization in 131 patients (Figure 1). Given the marked regional differences in patient characteristics and treatment effect noted in TOPCAT,39 we assessed the relationship between randomization to spironolactone and change in LS separately by geographic region (Americas vs Russia/Georgia) using linear regression adjusting for the baseline LS value. Additional analysis was performed adjusting for treatment strata and baseline characteristics that were unbalanced between treatment groups.
Results
As compared to the 2,998 patients in TOPCAT without strain data, the 447 patients with strain data were older, less frequently white, and less frequently enrolled in Russia or Georgia (24% vs 52% respectively; Supplemental Table 1). Co-morbidities such as diabetes, atrial fibrillation, prior coronary revascularization, and stroke were more prevalent. Similarly, compared to the 488 patients within the TOPCAT echocardiography study without strain measures, patients with strain data were less likely to be enrolled in Russia or Georgia, more frequently female, and more likely to have a history of atrial fibrillation (Supplemental Table 2). No significant differences in LV structure were noted, although LVEF was statistically higher (60±8 vs 59±8% among those with vs without adequate quality respectively) and LA volume larger (61±27 vs 56±22 ml).
Baseline Correlates of LS
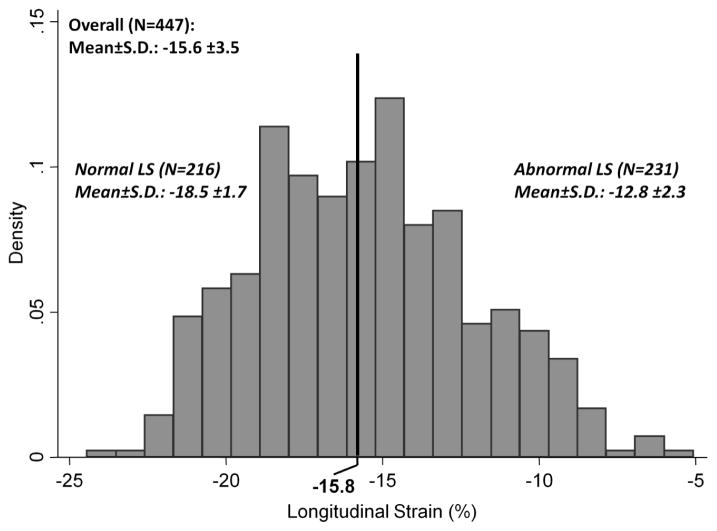
In the 447 TOPCAT patients in this analysis, the mean LS was 15.6±3.5% and was abnormal (absolute LS <15.8%) in 52% (Figure 2). There was a moderate correlation between LS and LVEF (r=0.55) in the entire cohort, and a large majority of patients with LVEF <55% demonstrated an abnormal LS. However, 66% of patients with abnormal LS had an LVEF >55%. In this group, the correlation between LS and LVEF (r=0.33) was more modest. Worse LS at baseline was associated with male gender, a higher prevalence of prior myocardial infarction, coronary revascularization, and history of atrial fibrillation, and with higher heart rate (Table 1). Worse LS was also associated with greater LV size, LV wall thickness, mass, and relative wall thickness (Table 2), worse LV longitudinal systolic function assessed by the TDI S‘, and elevated LV filling pressure, reflected in higher E/E’ ratio and greater LA size. Although not related to pulmonary pressure, worse LS was related to worse RV function by fractional area change and greater RV end-systolic area.
Figure 2.
Distribution of LV LS in the TOPCAT Echo study.
Table 1.
Baseline clinical charactersitics in the study population overall and by quartile of LV LS. LS is shown as absolute value.
Numbers represent mean ± S.D. for continuous variables and N (%) for categorical variables.
| Overall (n=447) | Quartile 1: <13.2 (n=111) | Quartile 2: 13.2 to 15.6 (n=112) | Quartile 3: 15.6 to 18.3 (n=112) | Quartile 4: >18.3 (n=112) | P for trend | |
|---|---|---|---|---|---|---|
| Age (years) | 70.3 ± 9.8 | 70.2 ± 9.5 | 69.8 ± 10.4 | 69.9 ± 9.1 | 71.2 ± 10.2 | 0.47 |
| Female | 240 (53.7%) | 49 (44.1%) | 58 (51.8%) | 65 (58.0%) | 68 (60.7%) | 0.008 |
| White | 359 (80.3%) | 88 (79.3%) | 93 (83.0%) | 92 (82.1%) | 86 (76.8%) | 0.62 |
| Enrollment in Russia/Georgia | 107 (23.9%) | 26 (23.4%) | 23 (20.5%) | 28 (25.0%) | 30 (26.8%) | 0.42 |
| Enrollment Strata: Prior Hospitalization | 289 (64.7%) | 68 (61.3%) | 78 (69.6%) | 74 (66.1%) | 69 (61.6%) | 0.90 |
| Co-morbidities | ||||||
| Hypertension | 411 (92.2%) | 104 (93.7%) | 104 (92.9%) | 101 (90.2%) | 102 (91.9%) | 0.48 |
| Myocardial Infarction | 115 (25.8%) | 44 (39.6%) | 25 (22.3%) | 24 (21.4%) | 22 (19.8%) | 0.001 |
| Coronary Revascularization | 130 (29.1%) | 39 (35.1%) | 47 (42.0%) | 24 (21.4%) | 20 (18.0%) | <0.001 |
| Stroke | 47 (10.5%) | 17 (15.3%) | 9 (8.0%) | 13 (11.6%) | 8 (7.2%) | 0.11 |
| Atrial Fibrillation | 193 (43.3%) | 65 (58.6%) | 54 (48.2%) | 41 (36.6%) | 33 (29.7%) | 0.0001 |
| Diabetes | 171 (38.3%) | 45 (40.5%) | 58 (51.8%) | 36 (32.1%) | 32 (28.8%) | 0.008 |
| Obesity | 262 (58.7%) | 60 (54.1%) | 75 (67.0%) | 61 (54.5%) | 66 (59.5%) | 0.86 |
| NYHA Functional Class | 0.16 | |||||
| 1 | 33 (7.4%) | 7 (6.3%) | 11 (9.8%) | 5 (4.5%) | 10 (9.1%) | |
| 2 | 241 (54.2%) | 68 (61.3%) | 58 (51.8%) | 63 (56.2%) | 52 (47.3%) | |
| 3 | 168 (37.8%) | 36 (32.4%) | 41 (36.6%) | 44 (39.3%) | 47 (42.7%) | |
| 4 | 3 (0.7%) | 0 (0.0%) | 2 (1.8%) | 0 (0.0%) | 1 (0.9%) | |
| Physical Characteristics | ||||||
| BMI (kg/m2) | 32.5 ± 6.9 | 31.5 ± 6.6 | 33.7 ± 6.9 | 31.7 ± 6.7 | 32. 9 ± 7.2 | 0.45 |
| Heart rate (bpm) | 68 ± 11 | 72 ± 12 | 68 ± 12 | 68 ± 13 | 64 ± 8 | <0.001 |
| Systolic blood pressure (mmHg) | 126 ± 15 | 125 ± 16 | 127 ± 16 | 128 ± 15 | 126 ± 14 | 0.44 |
| Diastolic blood pressure (mmHg) | 72 ± 11 | 73 ± 11 | 74 ± 11 | 71 ± 12 | 72 ± 10 | 0.09 |
| Laboratory Values | ||||||
| eGFR (mL/min per 1.73 m2) | 66 ± 22 | 65 ± 23 | 67 ± 22 | 66 ± 21 | 66 ± 20 | 0.84 |
| Creatinine (mg/dL) | 1.14 ± 0.34 | 1.19 ± 0.34 | 1.12 ± 0.35 | 1.12 ± 0.34 | 1.12 ± 0.35 | 0.15 |
| Hematocrit (%) | 38.9 ± 4.7 | 39.5 ± 4.5 | 39.2 ± 4.5 | 38.7 ± 4. 8 | 38.2 ± 4.9 | 0.03 |
Table 2.
Cardiac structure and function among the study population overall and by quartiles of LS. LS is shown as absolute value. Means and standard deviations provided. EDVi – LV end-diastolic volume indexed to BSA; EDVi – LV end-systolic volume indexed to BSA; LVEF - LV ejection fraction; LAV – left atrial volume; E wave – peak early diastolic transmitral flow velocity; A wave – peak late diastolic transmitral flow velocity; E’ septal – peak early diastolic mitral annular tissue velocity at septal mitral annulus; TR – tricuspid regurgitation; PVR – pulmonary vascular resistance; FAC – fractional area change.
| N | Overall (n=447) | Quartile 1: <13.2 (n=111) | Quartile 2: 13.2 to 15.6 (n=112) | Quartile 3: 15.6 to 18.3 (n=112) | Quartile 4: >18.3 (n=112) | P for trend | |
|---|---|---|---|---|---|---|---|
| LV Structure | |||||||
| LVEDVi (ml/m2) | 444 | 49.5 ± 16.0 | 53.6 ± 20.1 | 49.0 ± 15.1 | 48.7 ± 15.0 | 46.8 ± 12.3 | 0.002 |
| LVESVi (ml/m2) | 444 | 20.4 ± 10.2 | 26.0 ± 13.9 | 20.4 ± 8.7 | 18.5 ± 7.7 | 16.9 ± 6.4 | <0.001 |
| Mean wall thickness (cm) | 445 | 1.17 ± 0.20 | 1.26 ± 0.27 | 1.17 ± 0.18 | 1.13 ± 0.17 | 1.12 ± 0.14 | <0.001 |
| LV mass index (mg/m2) | 443 | 109 ± 30 | 124 ± 35 | 109 ± 26 | 103 ± 30 | 100 ± 25 | <0.001 |
| Relative wall thickness | 444 | 0.49 ± 0.11 | 0.52 ± 0.17 | 0.48 ± 0.09 | 0.48 ± 0.08 | 0.47 ± 0.08 | 0.002 |
| LV Hypertrophy | 443 | 216 (48.8%) | 71 (64.0%) | 57 (51.4%) | 44 (39.6%) | 44 (40.0%) | <0.001 |
| LV geometry | 443 | 0.002 | |||||
| Normal | 69 (15.6%) | 15 (13.5%) | 17 (15.3%) | 17 (15.3%) | 20 (18.2%) | ||
| Concentric remodeling | 158 (35.7%) | 25 (22.5%) | 37 (33.3%) | 50 (45.0%) | 46 (41.8%) | ||
| Eccentric hypertrophy | 38 (8.6%) | 19 (17.1%) | 5 (4.5%) | 5 (4.5%) | 9 (8.2%) | ||
| Concentric hypertrophy | 178 (40.2%) | 52 (46.8%) | 52 (46.8%) | 39 (35.1%) | 35 (31.8%) | ||
| LV Systolic Function | |||||||
| LVEF (%) | 447 | 59.9 ± 8.0 | 53.4 ± 9.4 | 59.1 ± 5.9 | 62.7 ± 5.9 | 64.4 ± 5.5 | <0.001 |
| TDI S’ (septal) (cm/sec) | 339 | 5.69 ± 1.52 | 4.90 ± 1.65 | 5.51 ± 1.50 | 5.79 ± 1.22 | 6.40 ± 1.32 | <0.001 |
| LV Diastolic Function | |||||||
| E/A ratio | 307 | 1.21 ± 0.65 | 1.33 ± 0.78 | 1.38 ± 0.80 | 1.12 ± 0.55 | 1.11 ± 0.51 | 0.007 |
| TDI E’ (septal) (cm/sec) | 339 | 5.96 ± 2.08 | 5.33 ± 2.12 | 6.22 ± 2.25 | 5.90 ± 1.90 | 6.31 ± 1.94 | 0.01 |
| E/E’ (septal) | 333 | 15.8 ± 6.9 | 18.7 ± 7.7 | 15.6 ± 6.7 | 15. 8 ± 6.5 | 13.7 ± 5. 9 | <0.001 |
| LAV (ml) | 434 | 61.0 ± 27.3 | 67.0 ± 22.6 | 66.9 ± 40.1 | 57.4 ± 21.1 | 53.0 ± 17.2 | <0.001 |
| LA diameter (cm) | 445 | 4.27 ± 0.63 | 4.43 ± 0.53 | 4.42 ± 0.73 | 4.18 ± 0.63 | 4.03 ± 0.50 | <0.001 |
| Diastolic Dysfunction Grade (Olmsted) | 256 | 0.12 | |||||
| Normal | 22 (8.6%) | 1 (2.4%) | 6 (10.3%) | 6 (9.0%) | 9 (10.1%) | ||
| Mild | 73 (28.5%) | 16 (38.1%) | 13 (22.4%) | 16 (23.9%) | 28 (31.5%) | ||
| Moderate | 113 (44.1%) | 12 (28.6%) | 25 (43.1%) | 38 (56.7%) | 38 (42.7%) | ||
| Severe | 48 (18.8%) | 13 (31.0%) | 14 (24.1%) | 7 (10.4%) | 14 (15.7%) | ||
| Pulmonary Vascular and RV | |||||||
| TR jet velocity (m/sec) | 278 | 2.79 ± 0.47 | 2.79 ± 0.49 | 2.80 ±0. 45 | 2.77 ±0. 44 | 2.79 ± 0.49 | 0.92 |
| RVFAC (%) | 362 | 0.49 ± 0.08 | 0.46 ± 0.08 | 0.47 ± 0.08 | 0.50 ± 0.07 | 0.52 ± 0.07 | <0.001 |
| RVEDA (cm2) | 362 | 19. 9 ± 6.3 | 20.7 ± 6.2 | 20.1 ± 6.5 | 20.0 ± 7.1 | 18.8 ± 5.2 | 0.04 |
| RVESA (cm2) | 362 | 10.2 ± 3.8 | 11.2 ± 3.8 | 10.7 ± 4.2 | 10.0 ± 4.0 | 9.1 ± 2.8 | <0.001 |
| Valvular Disease | |||||||
| ≥Moderate Mitral Regurgitation | 349 | 44 (12.6%) | 9 (10.5%) | 14 (16.1%) | 12 (14.1%) | 9 (9.9%) | 0.79 |
| Aortic Valve Peak Velocity (m/sec) | 359 | 1.50 ± 0.47 | 1.44 ± 0.55 | 1.51 ±0. 47 | 1.49 ± 0.37 | 1.55 ±0. 51 | 0.23 |
| Valve Disease^ | 432 | 66 (15.3%) | 19 (17.9%) | 19 (17.6%) | 17 (15.6%) | 11 (10.1%) | 0.10 |
Valve disease defined as mitral or aortic valve disease of moderate or greater severity, or presence of a prosthetic mitral or aortic valve.
LS in HFpEF and Matched Persons without HF
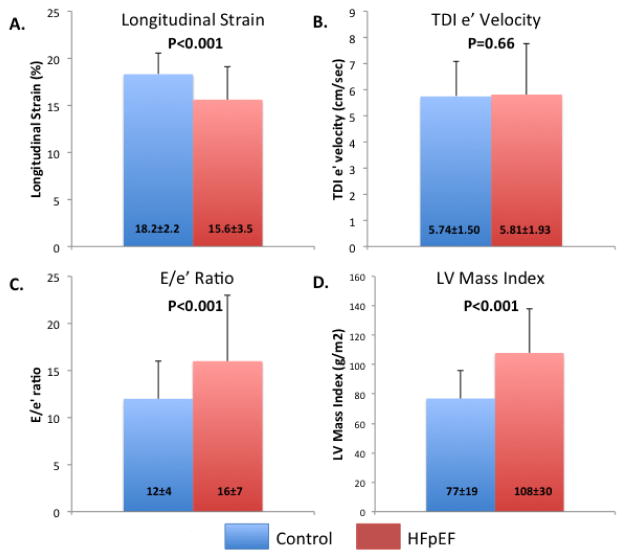
269 TOPCAT patients aged 65 to 91 years old were matched 1:1 with age-, gender-, and race-matched community-dwelling elderly individuals who were free of HF. E’ velocity did not differ between HFpEF and matched controls, while HFpEF patients demonstrated significantly lower LS, in addition to significantly higher E/e’ ratio and LVMi (Figure 3). LS remained significantly lower in HFpEF patients even when compared to community-based controls matched additionally for history of hypertension, diabetes, coronary disease, obesity, and eGFR level (15.8±3.4 vs 18.1±2.1%, p<0.0001; n=215 in each group).
Figure 3.
Comparison of LS (panel A), e’ (panel B), E/e’ (panel C), and LV mass index (panel D) between elderly TOPCAT patients with HFpEF (aged 65–91) and age-, gender-, and race-matched community dwelling persons without HF from the ARIC study.
LS and Incident Cardiovascular Events
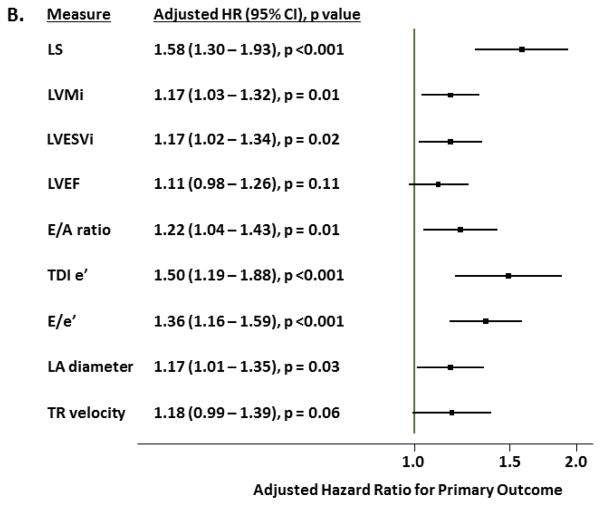
Over a median follow-up of 2.6 (IQR 1.5–3.9) years, 115 patients (26%) experienced the primary composite endpoint. HF hospitalization occurred in 78 (17%) and CV death occurred in 54 (12%). LS was associated with greater risk for the primary composite outcome, HF hospitalization alone, and CV death alone in unadjusted and fully adjusted analyses (Table 3). The relationship between LS and these clinical outcomes was nonlinear, with a linear relationship between LS and worse outcomes noted with values within the normal range which plateaued in the range of abnormal LS (Figure 4a). After adjusting for demographic, clinical, and conventional echocardiographic measures, abnormal LS was associated with a doubling of risk for the primary endpoint and each 1% absolute reduction in LS was associated with a 14% higher risk (Table 3). Per standard deviation change, LS was the strongest echocardiographic predictor of the primary outcome in multivariable adjusted models (Figure 4b). LS appeared to be more robustly predictive of CV death compared to HF hospitalization, with abnormal LS associated with a three-fold higher risk of CV death and a doubling of risk for HF hospitalization after multivariable adjustment. Impaired LS was also robustly associated with a greater number of HF hospitalizations in unadjusted and adjusted analysis. Similar findings were noted in analysis restricted to the 354 patients with LVEF≥55% (Supplemental Table 3), in a sensitivity analysis stratified by region of enrollment (Americas vs Russia and Georgia; Supplemental Table 4), and in an analysis using multiple imputation to account for missing data (Supplemental Table 5).
Table 3.
Association of LS with cardiovascular outcomes (primary composite outcomes, CV death, incident HF hospitalization, and total number of HF hospitalizations) in univariate and multivariable analysis (n=447). Multivariable analysis is adjusted for the following variables: Model 1: age, gender, race, randomization strata (prior HF hospitalization or biomarker criteria), region of enrollment (Americas versus Russia or Georgia), randomized treatment assignment, core lab LVEF, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, and hematocrit; Model 2: Model 1 + LV mass, LVESVi; Model 3: Model 2 + E/E’ ratio. For dichotomous analysis, abnormal LS was defined as LS > -15.8% (see text for details). LS is shown as absolute value.
| Normal LS (≥15.8) | Abnormal LS (<15.8) | Dichotomous | Continuous (per 1% ↓) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| N | Events | Rate | N | Events | Rate | HR (95% CI) | P | HR (95% CI) | P | |
| Primary | ||||||||||
| Unadjusted | 216 | 37 | 5.9 (4.2–8.1) | 231 | 78 | 13.5 (10.8–16.8) | 2.26 (1.53–3.34) | <0.001 | 1.13 (1.08–1.19) | <0.001 |
| Model 1 | 203 | 36 | 6.1 (4.4–8.4) | 226 | 77 | 13.6 (10.9–17.0) | 2.15 (1.34–3.45) | 0.001 | 1.14 (1.07–1.23) | <0.001 |
| Model 2 | 155 | 28 | 6.3 (4.3–9.1) | 164 | 58 | 14.7 (11.3–19.0) | 2.14 (1.26–3.66) | 0.005 | 1.14 (1.04–1.24) | 0.003 |
| CV death | ||||||||||
| Unadjusted | 216 | 15 | 2.2 (1.3–3.7) | 231 | 39 | 5.9 (4.3–8.1) | 2.67 (1.47–4.85) | 0.001 | 1.16 (1.08–1.25) | <0.001 |
| Model 1 | 203 | 15 | 2.4 (1.4–3.9) | 226 | 38 | 5.9 (4.3–8.1) | 3.12 (1.53––6.35) | 0.002 | 1.25 (1.13–1.38) | <0.001 |
| Model 2 | 155 | 12 | 2.5 (1.4–4.4) | 164 | 31 | 6.8 (4.8–9.7) | 3.20 (1.44–7.12) | 0.004 | 1.29 (1.14–1.46) | <0.001 |
| Incident HF Hosp | ||||||||||
| Unadjusted | 216 | 25 | 4.0 (2.7–5.9) | 231 | 53 | 9.1 (7.0–11.9) | 2.24 (1.39–3.60) | 0.001 | 1.12 (1.05–1.19) | 0.001 |
| Model 1 | 203 | 24 | 4.0 (2.7–6.0) | 226 | 53 | 9.3 (7.1–12.2) | 2.11 (1.19–3.74) | 0.011 | 1.11 (1.02–1.21) | 0.021 |
| Model 2 | 155 | 18 | 4.0 (2.5–6.4) | 164 | 39 | 9.8 (7.2–13.4) | 2.23 (1.16–4.28) | 0.016 | 1.08 (0.97–1.20) | 0.15 |
| Total HF Hosp | IRR (95% CI) | P | IRR (95% CI) | P | ||||||
| Unadjusted | 216 | - | - | 231 | - | - | 2.46 (1.36–4.46) | 0.003 | 1.17 (1.06–1.28) | 0.002 |
| Model 1 | 203 | - | - | 226 | - | - | 3.36 (1.68–6.72) | 0.001 | 1.19 (1.06–1.33) | 0.002 |
| Model 2 | 155 | - | - | 164 | - | - | 4.54 (1.95–10.6) | <0.001 | 1.17 (1.03–1.33) | 0.017 |
Figure 4.
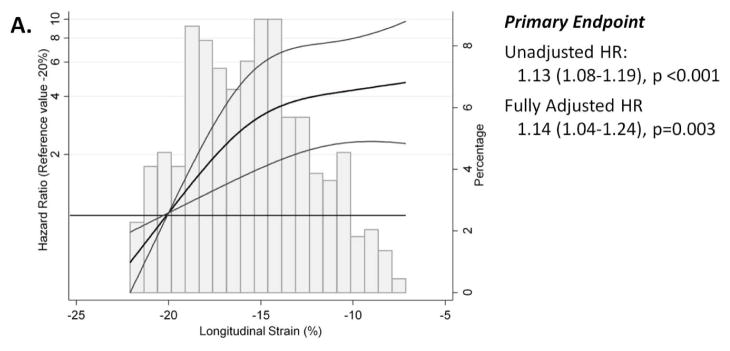
(A) Restricted cubic spline analysis demonstrating the unadjusted hazard ratio (black line) and 95% confidence limits (grey lines) for the primary composite endpoint of HF hospitalization, aborted cardiac arrest, or CV death (n=447; reference value: −20%). (B) Forest plot demonstrating the hazard ratio for the primary outcome, per standard deviation change in echocardiographic risk factors in HFpEF in multivariable adjusted models in the TOPCAT echocardiography study. Caption: For panel A, hazard ratios (HR) are per 1% absolute increase in LS. Histograms demonstrate the distribution of LS in the study population. Fully adjusted analysis (Model 2) is adjusted for age, gender, race, randomization strata (prior HF hospitalization or biomarker criteria), region of enrollment (Americas versus Russia or Georgia), randomized treatment assignment, core lab LVEF, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, hematocrit, LV mass, LVESVi, and E/E’ ratio. Values presented are a linear approximation. For panel B, risk associated with negative LVEF and e’ are shown. Covariates in multivariable model are the same as in panel (A) with the exception of LV mass, LVESVi, and E/E’ ratio. See text for further details.
Natriuetic peptide level was available in 259 patients, which was BNP in 207 patients (median 238, IQR 143–420 pg/ml) and NT-proBNP in 52 patients (median 802, IQR 431–1556 pg/ml). After log-transformation and standardization, natriuretic peptide level was modestly but significantly correlated with LS (r=0.22, p=<0.001). Among this subset of patients, LS remained significantly predictive of the primary composite endpoint after adjusting for natriuretic peptide level in addition to demographics, clinical predictors, LVEF, and LV mass index, whether modeled continously (HR 1.13 per 1% absolute decrease, 95% CI 1.03–1.24; p=0.013) or dichotomously (HR 2.15, 95% CI 1.13–4.10;p=0.022; Supplemental Table 6).
Incremental Value of LS in Predicting Risk of Incident Cardiovascular Events
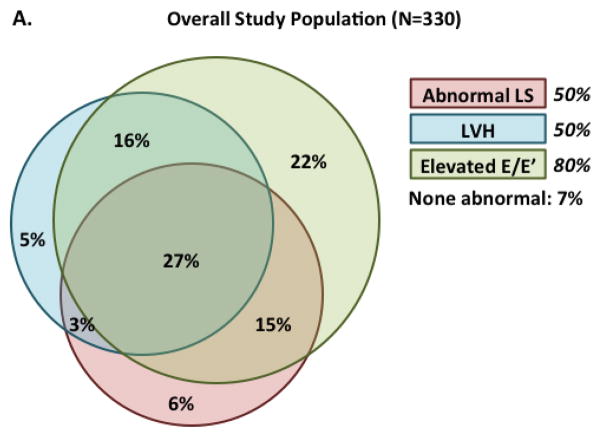
Among the 330 patients with complete data for LS, LV mass, and E/E‘, the majority (53%) of patients with abnormal LS also had LVH and elevated E/E‘, with an additional 30% demonstrating only elevated E/E‘ (Figure 5a). Abnormal LS, in the absence of abnormalities in LV mass or E/E‘, was present in only 6%. A similar pattern was noted among patients with LVEF ≥55% (Supplemental Figure). Greater number of abnormal measures (LS, LV mass, E/E‘) predicted higher risk of the primary outcome (Figure 5b).
Figure 5.
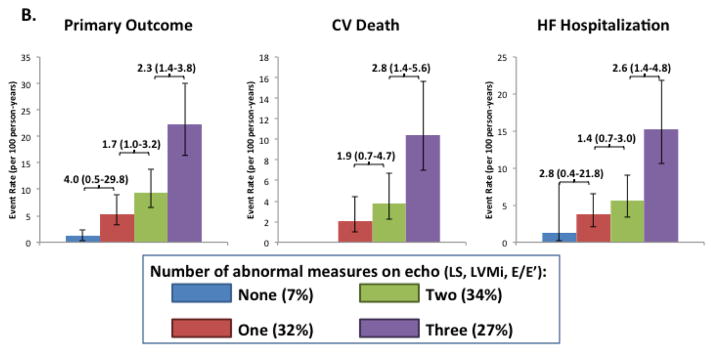
Venn diagram demonstrating the overlap between abnormal LS, LVH, and elevated E/E’ patients among patients will all 3 measures available (n=330). Panel (B) shows the event rates (per 100 person-years) among the 330 participants with all three measures of the primary composite endpoint (CV death, HF hospitalization, aborted cardiac arrest; 87 total events), CV death alone (32 total events), and of HF hospitalization alone (47 total events) based on the number of abnormal echo findings (abnormal LS, LVH, and elevated E/E’).
For prediction of the primary endpoint, addition of LS resulted in marginally significant improvement in the cross-validated C statistic beyond clinical predictors alone and in combination with measures of LV structure (LVMi and LVESVi), but not beyond these measures in addition to E/E‘ (Table 4). The IDI and NRI demonstrated incremental value of LS beyond these clinical and echocardiographic measures, again of marginal statistical significance. No significant improvement in risk prediction with LS was noted for incident HF hospitalization. In contrast, for prediction of CV death, LS did significantly improve the IDI and NRI beyond clinical variables and echocardiographic measures of LV structure and filling pressure. A net improvement in predicted risk of 37.2% (95% CI 7.9–54.1%, p = 0.012) for CV death at 3 years was noted, with an IDI of 7.9% (95% CI 1.2–17.2%, p = 0.024). Similar findings were noted in analysis restricted to the 249 patients with LVEF ≥55% (Supplemental Table 7).
Table 4.
Incremental value of LS in predicting cardiovascular outcomes (primary composite outcomes, CV death, incident HF hospitalization, and total number of HF hospitalizations) beyond clinical and echocardiographic variables (n=319). Clinical variables were: age, gender, race, randomization strata (prior HF hospitalization or biomarker criteria), region of enrollment (Americas versus Russia or Georgia), randomized treatment assignment, core lab LVEF, history of atrial fibrillation, heart rate, New York Heart Association class, history of stroke, creatinine, and hematocrit. Echocardiographic variables of LV structure were LV mass and LVESVi. Only individuals with no missing data for any clinical or echocardiographic variables were included.
| Base cross- validated C statistic | +LS cross- validated C statistic | P value | IDI (95% CI) | P value | NRI (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Primary (events =86) | |||||||
| Clinical | 0.69 (0.63–0.75) | 0.72 (0.66–0.77) | 0.069 | 4.2 (0.2–9.8) | 0.024 | 29.8 (0.0–40.4) | 0.048 |
| Clinical + structure | 0.70 (0.64–0.76) | 0.72 (0.67–0.78) | 0.059 | 3.9 (0.3–9.0) | 0.028 | 25.2 (2.6–39.8) | 0.032 |
| Clinical + structure + E/E’ | 0.71 (0.65–0.77) | 0.73 (0.67–0.78) | 0.098 | 2.7 (0.0–7.7) | 0.048 | 18.3 (-0.1–37.1) | 0.056 |
| CV death (events = 43) | |||||||
| Clinical | 0.64 (0.56–0.71) | 0.71 (0.63–0.79) | 0.038 | 8.8 (1.2–18.5) | 0.008 | 42.5 (8.2–55.7) | 0.016 |
| Clinical + structure | 0.67 (0.59–0.74) | 0.73 (0.65–0.80) | 0.064 | 8.6 (1.1–17.3) | 0.012 | 38.6 (8.5–52.8) | 0.004 |
| Clinical + structure + E/E’ | 0.66 (0.58–0.74) | 0.72 (0.65–0.80) | 0.068 | 7.9 (1.2–17.2) | 0.024 | 37.2 (7.9–54.1) | 0.012 |
| HF Hosp (events = 57) | |||||||
| Clinical | 0.71 (0.64–0.78) | 0.72 (0.65–0.80) | 0.42 | 2.0 (–0.4–8.2) | 0.12 | 15.1 (−17.5–33.4) | 0.26 |
| Clinical + structure | 0.72 (0.65–0.79) | 0.73 (0.66–0.80) | 0.35 | 2.1 (–0.3–7.5) | 0.14 | 15.0 (−12.4–34.8) | 0.20 |
| Clinical + structure + E/E’ | 0.74 (0.67–0.80) | 0.74 (0.67–0.81) | 0.79 | 0.9 (−0.4–5.4) | 0.19 | 10.5 (−14.9–29.2) | 0.32 |
LS and Treatment with Spironolactone
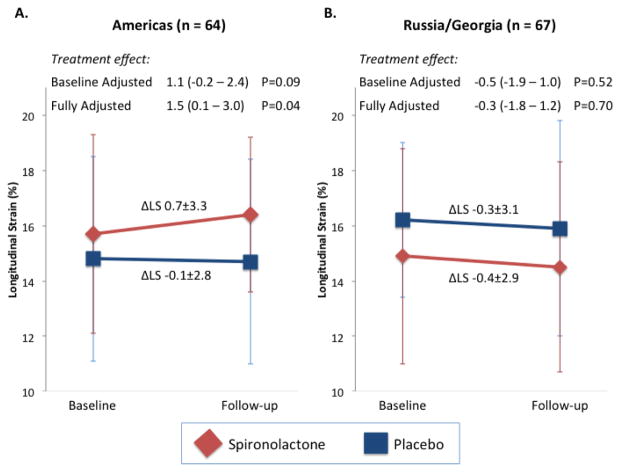
Of the 131 patients with LS at baseline and 12 or 18 months post-randomization, 64 were enrolled in the Americas and 67 were enrolled in Russia or Georgia. No significant difference was noted in the number of participant experiencing an interval HF hospitalization between the baseline and follow-up echo study by treatment arm in either region (Americas: 3 vs 2, and Russia/Georgia: 2 vs 0 in the placebo and spironolactone groups respectively). In the Americas, the 30 patients randomized to spironolactone were well matched to the 34 randomized to placebo with the exception of older age, more frequent white race, higher heart rate, and smaller LVEDVi in the placebo arm (Supplemental Table 8). Baseline LS was significantly higher in the spironolactone arm than the placebo arm, and demonstrated greater improvement at follow-up (Figure 6) which was of marginal significance in unadjusted analysis (p=0.09) and significant after adjustment for randomization strata and baseline characteristics differing between treatment arms (p=0.04). In contrast, no difference in change in LS was noted in Russia or Georgia between treatment-arms.
Figure 6.
Change in LS value from randomization to follow-up (12–18 months) by treatment arm (spironolactone versus placebo) among patients enrolled in (A) the Americas and (B) Russia and Georgia. P for interaction between randomized treatment assignment and change in LS by region=0.09. Baseline-adjusted analysis is based on an ANCOVA. Fully adjusted analysis adjusted for baseline characteristics that differed significantly between treatment arms by region. In the Americas, the multivariable model adjusted for randomization strata (prior hospitalization or natriuretic peptide level), age, race, heart rate, and LVEDVi. In Russia and Georgia, the multivariable model adjusted for randomization strata, history of hypertension, and the presence of significant valvular disease.
Discussion
There are three key novel findings of this analysis. First, among patients with HFpEF enrolled in the TOPCAT trial, LV longitudinal strain was worse than in community dwelling persons without HF matched for demographics and comorbidities, and was associated with a higher risk of the composite endpoint of HF hospitalization, CV death, or aborted sudden death, CV death alone, and HF hospitalization alone after adjusting for clinical and conventional echocardiographic measures. Second, a greater number of abnormalities in LS, filling pressure (E/e’), and LVH was associated with higher rates of the primary endpoint, CV death alone, and HF hospitalization alone. Furthermore, the addition of LS to other clinical and echocardiographic measures provided marginal incremental value in risk prediction for the primary outcome, and greater incremental value for the prediction of CV death. Similar findings were observed when restricting the population to those with LVEF ≥55% and after further adjustment for natriuretic peptide level. Third, among the subgroup of patients with LS measured at randomization and after 12–18 months, treatment with spironolactone was associated with a trend towards improvement in LS in the Americas, but not in Russia/Georgia. These findings suggest that systolic longitudinal dysfunction is important in prognosis of patients with HFpEF, and may also be relevant to disease pathophysiology.
Age-related changes in cardiac structure and function are well recognized. While several studies have explored the prevalence of LV systolic dysfunction in HFpEF, most have been limited in their ability to evaluate strain as a discriminating feature of HFpEF due to differences in age and comorbidity status in the control group.17,19,40 To our knowledge, this study is the largest to compare well-phenotyped elderly HFpEF patients with elderly persons in the community matched for key demographic feature and for comorbidities. LS, in addition to E/e’ ratio and LV mass index, were significantly lower in the HFpEF patients while e’ velocity was not. These findings confirm that, unlike e’, the observed impairments in LS are not just related to age and co-morbidities and highlight the importance of appropriate control groups in HFpEF studies. Systolic and diastolic dysfunction are inter-related due to abnormalities in myocyte calcium cycling, which has been implicated in hypertension-associated diastolic dysfunction and is detectable using strain imaging.41 Therefore, it is not surprising that systolic and diastolic dysfunction are both present in HFpEF, and that strain allows for the detection of abnormalities in the absence of an overt reduction in LVEF. Several studies have demonstrated abnormalities of LV longitudinal function among asymptomatic patients with common HFpEF risk factors such as hypertension42 and diabetes,43 often in concert with augmentation of LV circumferential deformation which may help preserve LVEF. Therefore, impaired longitudinal function is a more sensitive marked of impaired systolic performance that LVEF. In addition, strain appears to be a less load-dependent measure of LV systolic function compared to LVEF21 and – when measured using speckle-tracking – is independent of angle of incidence, unlike tissue Doppler based measures of LV longitudinal function.
To our knowledge, ours is the largest study to evaluate the prognostic utility of LS in HFpEF and the only study to demonstrate an independent relationship between LS and the composite of CV death, HF hospitalization, and aborted sudden death, in addition to CV death alone, HF hospitalization alone, and the total number of HF hospitalizations after adjusting for relevant demographic, clinical, laboratory, and conventional echocardiographic measures. While we did not account for multiple testing as these outcome measures are not independent of each other, all associations described in Table 3 would have remained significant at a Bonferroni-adjusted level of significance of p<0.013 accounting for four potential outcome measures, with the exception of the adjusted association of LS with incident HF hospitalization. In two small studies of ≤100 patients with HFpEF, LS was a significant univariate predictor of the composite endpoint of HF hospitalization or CV death.40,44 Neither study assessed the association of LS with the components of the composite or the incremental prognostic value of LS. In one study44 the independent prognostic relevance of LS was not assessed while in the other40 LS was not a significant independent predictor of outcomes. The reasons our results differ from that of Pellicori et al are unclear but may relate to the smaller sample size and shorter follow-up in that study or to differences in the HFpEF populations studied. Our findings of the prognostic importance of systolic dysfunction in HFpEF is concordant a prior study employing a different measure of systolic function – stress-corrected midwall fractional shortening – in an age-adjusted analysis.16
The risk associated with lower absolute LS was nonlinear. Patients with abnormal LS (LS ≤15.8%) were at the highest risk of all outcomes assessed, but the risk per 1% lower LS was greater at LS values within the normal range, where LVEF also tended to be well within the normal range. As noted in previous studies, LS was significantly correlated with LVEF, which may raise concern that LS is simply a more sensitive reflector of LVEF. While there is a lack of consensus on the LVEF criteria for HFpEF, an LVEF ≥55% is uniformly accepted as normal.7,38 TOPCAT entry required a site-reported LVEF ≥45% and the large majority of patients with LVEF <55% demonstrated impaired LS. However the majority of patients with impaired LS had an LVEF ≥55%, and 43% of patients with LVEF ≥55% had abnormal LS. Importantly, the unadjusted and adjusted association of LS with CV outcomes, and metrics for incremental value, were similar in the subgroup of patients with LVEF 55%, clearly demonstrating that LS provides functional and prognostic information distinct from LVEF.
The association of LS with clinical outcomes after adjusting for measures of LV structure and filling pressure is important as the majority of patients with abnormal LS also had elevated E/E’ with or without concomitant LVH, a finding which persisted in the subgroup of patients with LVEF ≥55%. Both LVH and elevated filling pressure are known prognostic markers in HFpEF.10,34 Incidence rates of the composite endpoint, CV death, and HF hospitalization increased in a stepwise fashion with greater number of abnormalities in these measures, suggesting that together they are useful to discriminate risk among HFpEF patients and identify those patients at higher risk for inclusion in therapeutic trials.
The incremental value of LS beyond relevant clinical, laboratory, and conventional echocardiographic measures was particularly robust in predicting CV death while LS demonstrated only margin incremental value in predicting the composite outcome and was not incremental in predicting HF hospitalization. The continuous NRI quantifies the predictive strength of the novel biomarker beyond, and accounting for correlations with, the existing predictors, with values above 60 considered strong, those around 40 considered intermediate, and values <20 considered weak.45 LS was associated with an NRI of approximately 40 for CV death, which compares favorably with established techniques such as PET myocardial perfusion imaging for predicting CV death (continuous NRI 0.5446), conventional echocardiography for predicting incident HF (continuous NRI 0.3247), and NT-proBNP for predicting incident CVD (continuous NRI 0.2048) when compared to clinical models. Concordant with the NRI, LS also significantly improved the IDI, which represents the absolute change in the difference in the mean predicted probabilities of patients experiencing versus not experiencing events. Other echocardiographic risk factors in TOPCAT, such as LVMi and E/E’ ratio, demonstrated greater incremental value in predicting HF hospitalization compared to CV death, highlighting the complementary information provided by LS.34 Indeed, the poorer performance of clinical characteristics alone or in combination with conventional echocardiographic measures in predicting CV death relative to HF hospitalization (C statistic: 0.64 vs 0.71 respectively [clinical variables only], 0.66 vs 0.74 respectively [clinical + conventional echo]; Table 4) may partially explain the greater incremental value of LS for CV death.
Spironolactone has been associated with improvement in LV filling pressure (E/e’ ratio) and LV mass in HFpEF,49,50,51 however its impact on LV deformation in HFpEF has not been previously evaluated. Prior trials in patients with HF risk factors, including hypertension with exertional intolerance,52 obesity,53 and metabolic syndrome,54 have demonstrated significant improvements in LS associated with spironolactone therapy. Patients in those studies tended to demonstrate a higher baseline LS, with a larger magnitude of effect of spironolactone on LS compared to our study. This may reflect more advanced, and less reversible, myocardial dysfunction and fibrosis in HFpEF, or between study differences in strain software and assessment. In our study, spironolactone was associated with a trend toward improvement in LS in the Americas, but not in Russia or Georgia. Marked regional differences have been noted in the TOPCAT trial in patient characteristics and, importantly, in spironolactone treatment effect on blood pressure, serum potassium, serum creatinine, and clinical endpoints.39 The etiology is unclear, but our finding of a marginal treatment effect on spironolactone in the Americas only is consistent with these other measures of spironolactone treatment effect.
Several limitations of this analysis should be noted. Strain analysis was only feasible in 48% of TOPCAT echocardiographic studies, due to non-DICOM imaging format, missing views, and poor image quality. This limited our power to assess associations and incremental value, particularly in multivariable analyses. In addition, as a portion of studies were echocardiograms obtained for clinical purposes and not according to protocol, certain conventional measures, particularly Doppler measures, were missing in a proportion of patients. This affected sample size for multivariable analyses adjusting for conventional echocardiographic measures, particularly E/E’. However, a sensitivity analysis using multiple imputation to account for missing data produced similar findings to the primary analysis (Supplemental Table 5). Given the limited number of patients with complete data necessary for assessment of incremental value of LS, we performed leave-one-out cross validation to determine model C statistics to obviate the risk of model over fitting. Finally, follow-up strain data was only available in a small subset of study participants and limited our power to detect a treatment effect related to spironolactone.
Conclusions
Systolic function reflected in LV longitudinal strain is impaired in HFpEF and is independently associated with risk of the composite endpoint of HF hospitalization, CV death, or aborted sudden death, as well as CV death alone, HF hospitalization alone, and total number of HF hospitalizations. LV LS provides incremental prognostic information for the composite endpoint and CV death alone beyond clinical, laboratory, and conventional echocardiographic risk markers. LV LS is a novel marker of heightened risk of CV morbidity and mortality in HFpEF, and may be important in the pathophysiology of this syndrome.
Supplementary Material
Acknowledgments
The authors wish to thank the staff and participants of the TOPCAT and ARIC studies for their important contributions.
Funding Sources: TOPCAT was funded by National Heart, Lung, and Blood Institute, National Institutes of Health (Bethesda, MD), Contract Number HHSN268200425207C. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The content of this paper does not necessarily represent the views of the sponsor or of the Department of Health and Human Services. The work for this manuscript was also supported by NHLBI grant 1K08HL116792-01A1 (A.M.S.) and AHA grant 14CRP20380422 (A.M.S.).
Footnotes
Disclosures: Dr A Shah reports receiving research support from Novartis, Gilead, and Actelion. Dr Pitt reports serving as a consultant for Pfizer, Bayer, Elli-Lilly, Novartis, and DaVinci therapeutics, and has a patent pending on site specific delivery of Eplerenone to the myocardium. Dr Pfeffer reports receiving research grants from Amgen, Celladon, Novartis, Sanofi Avantis, and Hamilton Health Sciences, Consulting for Abbot Vascular, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Fibrogen, Genzyme, GlaxoSmithKline, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Servier, and University of Oxford. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of MI with Novartis. Dr Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity.
References
- 1.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu P. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui H, Tsuchihashi M, Takeshita A. Mortality and readmission of hospitalized patients with congestive heart failure and preserved versus depressed systolic function. Am J Cardiol. 2001;88:530–3. doi: 10.1016/s0002-9149(01)01732-5. [DOI] [PubMed] [Google Scholar]
- 4.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–8. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S for the ALLHAT Collaborative Research Group. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–67. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 8.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–64. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy – CHARMES. J Am Coll Cardiol. 2007;49:687–94. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 11.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002;87:29.31. doi: 10.1136/heart.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip G, Wang M, Zhang Y, Fung JW, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefintion? Heart. 2002;87:121–5. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with "isolated" diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 14.Coiffi G, Senni M, Tarantini L, Faggiano P, Rossi A, Stefenelli C, Russo TE, Alessandro S, Furlanello F, de Simone G. Analysis of circumferential and longitudinal left ventricular systolic function in patients with non-ischemic chronic heart failure and preserved ejection fraction (from the CARRY-IN-HFpEF study) Am J Cardiol. 2012;109:383–389. doi: 10.1016/j.amjcard.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Vinereanu D, Nicolaides E, Tweddel A, Fraser AG. “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail. 2005;7:820–8. doi: 10.1016/j.ejheart.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular stiffening in hypertensive heart disease. Insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris DA, Boldt L-H, Eichstadt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5:610–20. doi: 10.1161/CIRCHEARTFAILURE.112.966564. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y-W, Tsai W-C, Su C-T, Lin C-C, Chen J-H. Evidence of left ventricular systolic dysfunction detected by automated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2009;15:782–9. doi: 10.1016/j.cardfail.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Clagget B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJV, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AM, Solomon SD. Myocardial Deformation Imaging: Current Status and Future Directions. Circulation. 2012;125:e244–8. doi: 10.1161/CIRCULATIONAHA.111.086348. [DOI] [PubMed] [Google Scholar]
- 21.Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283:H792–9. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 22.Cho G-Y, Marwick TH, Kim H-S, Kim M-K, Hong K-S, Oh D-J. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol. 2009;54:618–24. doi: 10.1016/j.jacc.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 23.Nahum J, Bensaid A, Dussault C, Macron L, Clemence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. doi: 10.1161/CIRCIMAGING.109.910893. [DOI] [PubMed] [Google Scholar]
- 24.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ, Bourgoun M, Foster E, Zareba W, Goldenberg I, McNitt S, Pfeffer MA, Moss AJ, Solomon SD. Dyssynchrony, Contractile Function, and Response to Cardiac Resynchronization Therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 25.Hung C-L, Verma A, Uno H, Shin S-H, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, Solomon SD. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;56:1812–22. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–72. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O’Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O’Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function in Heart Failure with Preserved Ejection Fraction: Baseline Findings from the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 31.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and Design of a Multicenter Echocardiographic Study to Assess the Relationship between Cardiac Structure and Function and Heart Failure Risk in a Biracial Cohort of Community Dwelling Elderly Persons: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Cardiovasc Imaging. 2014;7:104–15. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circ Heart Fail. 2014;7:740–51. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Ostergren J for the CHARM investigators and committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 36.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A for the I-PRESERVE investigators. N Engl J Med. 2008;359:2456–67. [Google Scholar]
- 37.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–42. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasan RS, Levy D. Defining diastolic heart failure: A call for standardized diagnostic criteria. Circulation. 2000;101:2118–21. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner J, Leweis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer N, McKinlay S, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 40.Pellicori P, Kallvikbacka-Bennett A, Khaleva O, Carubelli V, Costanzo P, Castiello T, Wong K, Zhang J, Cleland JGF, Clark AL. Global longitudinal strain in patients with suspected heart failure and a normal ejection fraction: does it improve diagnosis and risk stratification? Int J Cardiovasc Imaging. 2014;30:69–79. doi: 10.1007/s10554-013-0310-y. [DOI] [PubMed] [Google Scholar]
- 41.Shah SJ, Aistrup GL, Gupta DK, O’Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol. 2014;306:H88–100. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusma-Piccione M, Di Bella G, Saitta C, Saitta A. Left ventricular function in hypertension: New insights by speckle tracking echocardiography. Echocardiography. 2011;28:649–57. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 43.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (London) 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 44.Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography. 2014;00:1–8. doi: 10.1111/echo.12613. [DOI] [PubMed] [Google Scholar]
- 45.Pencina MJ, D’Agostino RB, Pencina KM, Janssens CJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–81. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorbala S, Di Carli MF, Beanlands RS, Merhige ME, Williams BA, Veledar E, Chow BJW, Min JK, Pencina MJ, Berman DS, Shaw LJ. Prognostic value of stress myocardial perfusion positron emission tomography: Results from a multicenter observational registry. J Am Coll Cardiol. 2013;61:176–84. doi: 10.1016/j.jacc.2012.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalogrepoulos AP, Georgiopoulou VV, deFilippi CR, Gottdiener JS, Butler J. Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults. JACC Cardiovasc Imaging. 2012:131–40. doi: 10.1016/j.jcmg.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsh P, Doolin O, Willeit P, Packard C, Macfarlane P, Cobbe S, Gudnason V, Di Angelantonio E, Ford I, Sattar N. N-terminal pro-B-type natriuretic peptide and the prediction of primary cardiovascular events: results from 15-year follow-up of WOSCOPS. Eur Heart J. 2013;34:443–50. doi: 10.1093/eurheartj/ehs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen H-D, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 50.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–42. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre-Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2014;20:560–8. doi: 10.1016/j.cardfail.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 53.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomized controlled trial of aldosterone blockade. Heart. 2013;99:320–6. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 54.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O’Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. JACC Cardiovasc Imaging. 2011;4:1239–49. doi: 10.1016/j.jcmg.2011.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.