Abstract
We examined associations between observational dampness scores and measurements of microbial agents and moisture in three public schools. A dampness score was created for each room from 4-point-scale scores (0–3) of water damage, water stains, visible mold, moldy odor, and wetness for each of 8 room components (ceiling, walls, windows, floor, ventilation, furniture, floor trench, and pipes), when present. We created mixed microbial exposure indices (MMEIs) for each of 121 rooms by summing decile ranks of 8 analytes (total culturable fungi; total, Gram-negative, and Gram-positive culturable bacteria; ergosterol; (1→3)-β-D-glucan; muramic acid; and endotoxin) in floor dust. We found significant (P ≤ 0.01) linear associations between the dampness score and culturable bacteria (total, Gram-positive, and Gram-negative) and the MMEIs. Rooms with dampness scores greater than 0.25 (median) had significantly (P < 0.05) higher levels of most microbial agents, MMEIs, and relative moisture content than those with lower scores (≤0.25). Rooms with reported recent water leaks had significantly (P < 0.05) higher dampness scores than those with historical or no reported water leaks. This study suggests that observational assessment of dampness and mold using a standardized form may be valuable for identifying and documenting water damage and associated microbial contamination.
Keywords: Dampness, Mold, Assessment, Microbial, School, Building
Introduction
The World Health Organization (WHO) has estimated that 10 to 50% of indoor environments in Europe, North America, Australia, India, and Japan are affected by dampness (WHO, 2009a). Excess moisture in damp indoor environments can promote microbial proliferation and result in occupants’ exposure to various microbial agents such as intact spores and cell fragments containing toxins, inflammatory substances, and allergens (AIHA, 2005; IOM, 2004a; WHO, 2009b). Indoor dampness has been recognized as a public health hazard by many researchers and authoritative entities such as the Institute of Medicine, WHO, ASHRAE, the American Industrial Hygiene Association, the National Institute for Occupational Safety and Health (NIOSH), and the Environmental Protection Agency. Dampness- and mold-related health effects include development and exacerbation of asthma, current asthma, hypersensitivity pneumonitis, bronchitis, respiratory infections, eczema, and various upper and lower respiratory symptoms (IOM, 2004b; Mendell et al., 2011; WHO, 2009b).
Indications of dampness and mold have been used as an exposure surrogate of dampness-related microbial and other agents in large epidemiologic studies due to low cost and easy application (Bornehag et al., 2001, 2004; Jaakkola et al., 2002). The definition of dampness/mold in most studies was based on the presence or absence without considering the severity and extent of damage, which may result in misclassification of exposure that could mask true associations with health outcomes. However, Haverinen et al. (2001, 2003) defined dampness in varying degrees using additional observational information about location, severity, and extent of damage as well as questionnaire information about duration of exposure to dampness. In our previous studies, we created observational dampness scores by grading dampness/mold and related damage with a 4-point scale (0–3) based on the size of an affected area (Cox-Ganser et al., 2009; Park et al., 2004). In a study of buildings in a college, we calculated an individual exposure index for dampness/mold based on observational assessment performed by trained industrial hygienists and time that occupants spent in multiple rooms obtained from a questionnaire (Park et al., 2004). We found positive and linear associations between occupants’ respiratory and non-respiratory symptoms and this exposure index. In a study of hospital buildings, we found that observational scores for dampness and mold were positively and linearly associated with post-hire onset asthma and work-related lower respiratory tract symptoms in employees (Cox-Ganser et al., 2009).
The observational method of assessing dampness and mold may be used proactively to prevent severe water damage and identify needs for remediation and building maintenance. From a building maintenance perspective, an observational assessment may be the most practical screening tool to continuously monitor buildings and identify dampness-related issues. Nonetheless, indoor environmental quality (IEQ) professionals, building owners, or facility managers tend to rely on results of costly environmental air sampling to attempt to identify dampness-related problems. This trend may be partly due to lack of evidence of associations between observed dampness and objective measures of fungi, bacteria, and moisture content (Choi et al., 2014; IOM, 2004a). In this study, we examined whether observational dampness scores were associated with various objective measurements of microbial agents and moisture content in three school buildings.
Materials and methods
Environmental survey
We conducted environmental surveys at three public schools (primary, middle, and high) in a school district in the northeastern US in May, 2006. The primary school was originally built in 1973, the middle school in 1952, and the high school in 1961. Annexes to the schools had been built over the years up to 1993.
Assessment of dampness and mold
Three trained building engineers from an indoor environmental quality consultant company contracted by NIOSH inspected all accessible rooms in each school for all signs of dampness, visible mold, and mold odor using a standardized observational assessment form (Park et al., 2004) with minor modification. The building engineers were sent the assessment form in advance and trained together prior to the site visits. Each of 8 components [floor, walls, windows, ceiling, furniture, pipes, ventilation, and floor trench (a subgrade channel to route heating pipes to perimeter heating terminals)] when present in a room was graded on a 4-point scale (0–3) for water damage, water stains, visible mold (density and area), and wetness. Mold odor was graded once upon first entry into a room. Water damage was defined as disintegration of the surface material such as peeling paint, wood decay, corrosion, or buckling of ceiling or floor tiles consistent with water leaks, and water stains was defined as discoloration of surface material. Wetness was defined as the presence of wet materials or standing or saturated water on surface material. Based on the size of an affected area, water damage, water stains, visible mold, and wetness were scored as ‘0’ for none, ‘1’ for <0.19 m2 (2 ft2), ‘2’ for 0.19–3.07 m2 (2–33 ft2), and ‘3’ for >3.07 m2 (33 ft2). Visible mold density and moldy odor were recorded as ‘0’ for none, ‘1’ for slight, ‘2’ for moderate, and ‘3’ for heavy. If the area component consisted of multiple structural materials (e.g., brick, concrete, or gypsum board for the walls), the engineers evaluated all the dampness-related factors for each of the materials.
To obtain a composite observational dampness score for each room (Equation 1), we first created a visible mold variable by multiplying the score of the visible mold density with the score of the visible mold area. We averaged the scores over all identified room components for each of the 4 dampness-related factors and then summed these scores across the four factors (water damage, water stains, visible mold, and wetness) and the score for mold odor. If a room component consisted of multiple structural materials, we graded the four factors for each material of the component. When calculating a dampness score for the room (Equation 1), we used a total score summed over the multiple materials for the component.
| (1) |
where f indicates factor and c room component; the factors included water damage, water stains, visible mold, and wetness; and the room component included ceiling, floor, walls, furniture, ventilation, windows, floor trench, and pipes. The formula in the parenthesis included ‘m’ which indicates that the number of components for each room varies (maximum number is 8) and that the calculated score is an average score over the components.
To obtain information on location and time of water leaks and remediation at the schools, the building engineers interviewed facility maintenance workers or long-tenured workers in each of the schools using a questionnaire.
Measurements of microbes and moisture
We collected samples of floor dust from all accessible classrooms and offices and from other areas or rooms such as corridors, conference rooms, or the library in the three schools using a backpack vacuum sampler (Pro-Team Inc., Boise, ID, USA). The dust was collected onto 6.7-μm pore size polyethylene filter socks (Midwest Filtration Company, Fairfield, OH, USA) attached to a crevice tool. For each room, we used a different crevice tool that was pre-cleaned by brushing in water with detergent and then dried. For each sample, a total of 1.813 m2 of floor surface was vacuumed for 10 min [18 meters of floor perimeter two inches from the walls (0.813 m2) for 8 min plus the floor of the staff workstation (1 m2) for 2 min].
We analyzed dust samples for culturable fungi, culturable bacteria, ergosterol (a principal sterol in fungal cell walls), (1→3)-β-D-glucan (a fungal cell wall component), muramic acid (a marker for peptidoglycan which is a cell wall component of bacteria), and endotoxin (a component of the outer membrane of Gram-negative bacteria). We sent dust aliquots for analyses of culturable fungi and bacteria to EMLab P&K (formerly, Environmental Microbiology Laboratory, Inc., San Bruno, CA, USA) and ergosterol and muramic acid to the University of Lund in Sweden. For culturable fungi, fungal colonies were cultured on malt extract, cellulose, and dichloran 18% glycerol agars at room temperature for 7–10 days. Serially diluted samples were cultivated onto trypticase soy agar for total bacteria, colistin nalidixic acid agar for Gram-positive bacteria, and MacConkey agar for Gram-negative bacteria. Colonies of culturable microbes were counted and results were reported as colony-forming units (CFU) per gram of dust. Ergosterol and muramic acid were analyzed with gas chromatography-tandem mass spectrometry (GC-MS-MS) and reported as nanograms per gram of dust (Sebastian and Larsson, 2003). We analyzed endotoxin with the kinetic Limulus amoebocyte lysate (LAL) assay with parallel-line estimation (Milton et al., 1992) and reported in endotoxin units (EU) per gram of dust. We analyzed (1→3)-β-D-glucan with the (1→3)-β-D-glucan-specific LAL assay kit (Glucatell®; Associates of Cape Cod Inc., Falmouth, MA, USA) using the kinetic chromogenic assay (Shogren and Park, 2011) and reported in nanograms per gram of dust.
We created mixed microbial exposure indices (MMEIs) using decile ranks of each of the microbial agents’ concentration (per g dust). For each microbial agent, concentrations from all rooms (n = 121) were coded as 1 to 10 corresponding to the decile rankings from low to high. Using these decile rankings, we created three mixed microbial exposure indices. The first MMEI (I) was created by summing the decile ranks over all 8 microbial agents (total culturable fungi, total culturable bacteria, Gram-positive bacteria, Gram-negative bacteria, (1→3)-β-D-glucan, ergosterol, muramic acid, and endotoxin) for each room. For the second MMEI (II), we categorized the 8 agents into three groups: (i) a fungi group (total culturable fungi, (1→3)-β-D-glucan, and ergosterol); (ii) a total bacteria group (total culturable bacteria and muramic acid); and (iii) a Gram-negative bacteria group (culturable Gram-negative bacteria and endotoxin). Then, we summed the highest decile rank within the group over the three groups. For the third MMEI (III), we replaced the highest decile ranking of the total bacteria group used in the MMEI (II) with that of Gram-positive bacteria.
Relative moisture content in multiple components of each room such as walls, floor, and furniture was measured in search mode (referred to as a pinless meter) with a Protimeter (Model MMS POL5800; GE Sensing, Billerica, MA, USA), which provides a relative moisture reading (range: 0–1000). The maximum value of the relative moisture content measured on the area components within a room was used for statistical analysis.
Statistical analyses
The distribution of concentrations of culturable microbes and their biomasses in floor dust and relative moisture content were right skewed, and thus, we used common log-transformed values as outcome variables in all regression models. Observational dampness scores were not normally distributed, and a large fraction of rooms investigated were scored as zero values. Thus, we used ranks of the scores as an outcome variable in nonparametric Kruskal–Wallis analysis of variance (ANOVA) models. Using this nonparametric ANOVA with pairwise multiple comparisons with the Dunn’s procedure (Elliott and Hynan, 2011; Ruxton and Beauchamp, 2008), we examined whether the dampness scores differed by school or floor of building. To examine differences in the microbial concentrations and relative moisture content between schools, we performed parametric multiple comparisons with Scheffé’s correction.
To examine whether the observational dampness score was associated with quantitative measurements of microbes and relative moisture content, we used general linear regression models adjusted for a school effect. This was performed because the school variable had a significant, independent effect on levels of microbial agents. First, we examined whether linear associations existed between the continuous observational dampness score (both including and excluding zero score rooms) and measured levels of microbial agents (per g of dust as well as per m2). To show the linear relationships with nonzero dampness scores, we presented partial regression plots for culturable microbes and MMEI. Second, we examined associations between a binary dampness score variable and microbial levels. To create the binary dampness score variable, we categorized the evaluated rooms into two groups—‘damp rooms’ with higher dampness scores than 0.25 (the median) and relatively ‘dry rooms’ with lower scores (≤0.25). We compared adjusted means of individual microbial levels, MMEIs (I, II, and III), and moisture content between damp and dry rooms. In the second sets of models, we replaced the adjusting variable of school with two variables (one for gypsum-boarded walls and another for carpeted floor) to examine whether the presence of gypsum board or carpets in each room significantly affected MMEIs. For these models, we created two three-level variables—one for gypsum-boarded walls and the other for carpeted floor. If the walls were fully boarded with gypsum or the floor was fully carpeted, they were categorized into ‘full.’ If there was no gypsum board on the walls (or no carpet on the floor), they were categorized into ‘no.’ And if the walls or the floor were partially covered with gypsum board or carpet, respectively, they were categorized into ‘partial.’ We performed sensitivity analysis for different composite scores of dampness and mold using two other calculation methods: (i) summing all individual scores across the multiple structural materials, the area components, and the factors; (ii) applying this same method except for using a maximum value among scores of multiple structural materials on the same component of a room.
Our study was not designed to evaluate interengineer differences in assessment, and we used general linear models stratified by engineer to examine whether the associations between dampness scores and measurements of microbial agents were similar for each of the three engineers.
We examined whether observational dampness scores (outcome variable) were associated with water leaks reported by building maintenance employees or long-tenured workers. We first categorized the investigated rooms into three groups based on the last date of reported water leaks: January through May in 2006 (the year of the survey) as a ‘recent leak,’ before 2006 as an ‘historical leak,’ and no reports as ‘no leak.’ We examined differences in dampness scores among the groups using nonparametric Kruskal–Wallis ANOVA. Using linear regression models adjusted for the school effect, we further examined whether rooms with recent water leaks had higher levels of measured microbes and relative moisture content than the rest of the rooms. We also examined correlations between measurements of microbial agents and maximum relative moisture content of a room.
All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC, USA), and we considered p ≤ 0.05 as statistically significant.
Results
Characteristics of school buildings
Approximately 60% (129/219) of rooms inspected for dampness and mold were classrooms or offices (Table 1). We collected 125 floor dust samples for microbial analyses mainly from classrooms or offices (79%). Gypsum board was the most common type of interior wall surface in the primary (78%) and middle schools (75%), while there were more rooms with concrete masonry block walls than gypsum board in the high school. Carpeting was predominant in the primary school (79%), while tile floor was more prevalent (59%) than carpeted floor in the middle and high schools. Information from the interviews indicated that all water leaks (15 rooms) in the primary school occurred prior to 2006 (between 1976 and 2004) and were mainly from pipe failures. In comparison, more than 50% of water leaks in the middle and high schools occurred in 2006, and water intruded mainly through roofs, walls, or windows during heavy rain. All roof leaks were reported to have had been repaired and damaged building materials replaced in all three schools.
Table 1.
Characteristics of inspected rooms in school buildings
| Number (%) of rooms inspecteda by school
|
|||
|---|---|---|---|
| Primary (n = 78) | Middle (n = 77) | High (n = 64) | |
| Room use | |||
| Classroom | 30 (38.5) | 26 (33.8) | 28 (43.8) |
| Office | 18 (23.0) | 12 (15.6) | 15 (23.4) |
| Otherb | 30 (38.5) | 39 (50.6) | 21 (32.8) |
| Room floor | |||
| Basement | 1 (1.3) | 16 (20.8) | N/A |
| 1st floor | 66 (84.6) | 44 (57.1) | 54 (84.4) |
| 2nd floor | 11 (14.1) | 17 (22.1) | 10 (15.6) |
| Interior wall surfacec | |||
| Gypsum board | 61 (78.2) | 58 (75.3) | 43 (67.2) |
| Concrete masonry block | 38 (48.7) | 48 (62.3) | 49 (76.6) |
| Flooring typec | |||
| Carpet | 55 (70.5) | 23 (29.9) | 21 (32.8) |
| Tile | 28 (35.9) | 46 (59.7) | 38 (59.4) |
| Reported water leaksd | |||
| Recent leak | 0 (0.0) | 10 (13.0) | 7 (10.9) |
| Historical leak | 15 (19.2) | 9 (11.7) | 5 (7.8) |
| No leak | 63 (80.8) | 58 (75.3) | 52 (81.3) |
| Rooms for dust collectione | 38 (48.7) | 39 (50.6) | 44 (68.8) |
N/A: Not applicable – no basement existed.
A total of 219 rooms were inspected.
‘Other’ included corridor, library, lounge, and conference room.
The two most frequently used materials for walls or floors are only presented. Other materials included wood, concrete, tile, carpet, deck, and sheet for wall surface; and wood, concrete, slab, and sheet for flooring type. Some rooms had more than one type of walls or flooring.
‘Recent leak’: occurred from January to May in 2006; ‘Historical leak’: occurred before 2006; ‘No leak’: no documentation of water leaks.
N = 121. Of rooms with dust samples, 121 rooms were evaluated for dampness and mold.
Distribution of observational dampness scores
Overall, 63% of the rooms had some sign of dampness and mold (Table 2) which occurred most frequently in ceilings (n = 96, 44%), followed by windows (n = 42, 19%), and walls (n = 30, 14%). Fifty-two percent of the rooms had water stains, 31% water damage, and 17% moldy odor. Among the rooms with moldy odor (n = 37), 51% were in the primary school (n = 19), followed by the middle school (n = 13, 35%), and the high school (n = 5, 14%). Water stains, water damage, and moldy odor were the major contributors to the observational dampness scores. Visible mold was found only in the middle school (n = 5, 2%), and wetness in the middle (n = 3, 1%) and primary (n = 2, <1 %) schools.
Table 2.
Distribution of observational dampness scores
| Factor | N of rooms > zero scoresa (%) | Dampness score
|
||||
|---|---|---|---|---|---|---|
| Median | 75th percentile | Max | Mean | STD | ||
| Dampness scoreb | 138 (63) | 0.25 | 0.83 | 6.25 | 0.56 | 0.81 |
| Water stain | 113 (52) | 0.17 | 0.33 | 2.00 | 0.23 | 0.32 |
| Water damage | 68 (31) | 0.00 | 0.20 | 1.00 | 0.13 | 0.23 |
| Moldy odor | 37 (17) | 0.00 | 0.00 | 3.00 | 0.19 | 0.46 |
| Visible mold | 5 (2) | 0.00 | 0.00 | 0.80 | 0.01 | 0.06 |
| Wetness | 5 (2) | 0.00 | 0.00 | 0.67 | 0.01 | 0.06 |
N, number; STD, standard deviation.
A total of 219 rooms were inspected.
Dampness score of each room: summation of all 5 average factor scores. Average factor score: average of the raw scores over all available area components within a room for each dampness-related factor (see text).
Observational dampness scores in the middle school were significantly (P ≤ 0.05) higher than those in the high school but not significantly (P > 0.05) different from those in the primary school (Table 3). Basements had the highest mean dampness score which was significantly higher than the 1st floor. There was no difference in dampness scores between rooms with carpeted and vinyl tile floor.
Table 3.
Observational dampness scores by school and floor
| N of rooms > zero scores (%) | Observational dampness score
|
||||
|---|---|---|---|---|---|
| Median | Mean (s.d.) | Pairwise comparisona
|
|||
| Pair | P-value | ||||
| School | |||||
| Primary (P) | 78 (62.8) | 0.25 | 0.50 (0.69) | P-M | NS |
| Middle (M) | 77 (71.4) | 0.50 | 0.78(1.05) | M-H | <0.05 |
| High (H) | 64 (53.1) | 0.18 | 0.36 (0.48) | H-P | NS |
| Room floor | |||||
| Basement (0) | 17 (88.2) | 1.00 | 1.48 (1.58) | 0–1 | <0.01 |
| 1st floor (1) | 164 (58.5) | 0.20 | 0.45 (0.67) | 1–2 | NS |
| 2nd floor (2) | 38 (71.1) | 0.71 | 0.64 (0.55) | 2-0 | NS |
Mean = arithmetic mean; s.d., standard deviation; NS, not significant, P > 0.05.
Kruskal–Wallis nonparametric analysis of variance with a multiple comparison test (Dunn’s method).
Concentrations of microbial agents
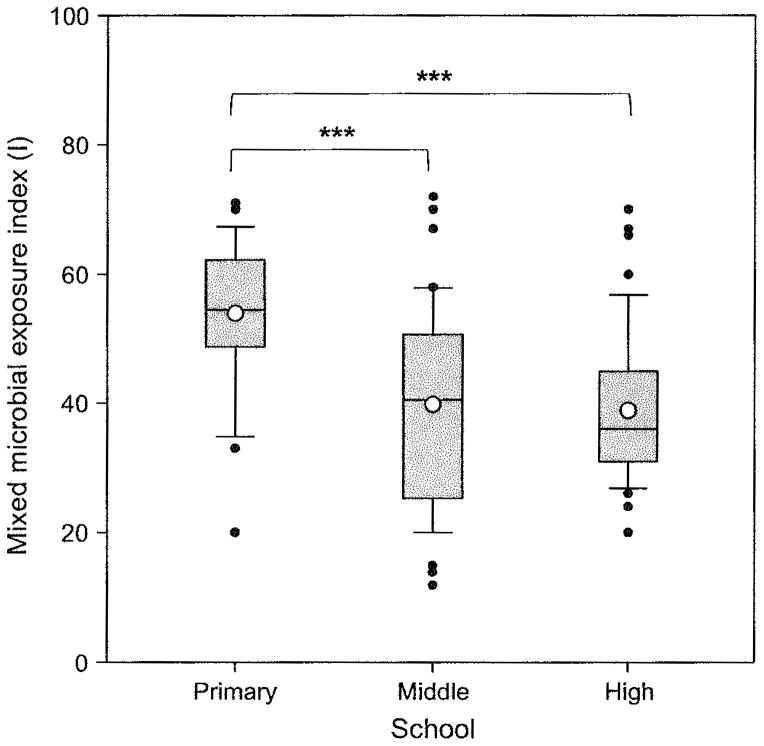
Geometric means (GMs) of microbial agents in the primary school were significantly higher than those in the middle or high school, except for culturable bacteria and endotoxin (Table 4). Rooms with carpeted floor had two- to fourfold higher GMs of culturable microbes and biomass [all P-values <0.01, except for total bacteria (P = 0.07) and Gram-positive bacteria (P = 0.14)] than those with vinyl tile floors only. In the models with three-level variables (no/partial/full coverage) for gypsum board on walls and carpet on floor, and a binary variable for dampness, rooms with either the partially or fully carpeted floor had significantly (P < 0.001) higher MMEIs (I, II, and III) than those without carpet on the floor, but there were no differences between partially and fully carpeted floor. The presence of gypsum board on walls of the rooms did not affect MMEIs. The primary school had significantly higher arithmetic means (AMs) of MMEIs (I, II, and III) than the middle or the high school (Figure 1). Moisture content was significantly (P < 0.01) higher in the high and middle schools than the primary school, but not different between the middle and high schools.
Table 4.
Geometric means of microbial agents in floor dust and relative moisture content by school
| Overall GM (GSD) n = 125 | GM by school
|
Pairwise comparisona
|
|||||
|---|---|---|---|---|---|---|---|
| Primary (P) n = 38 | Middle (M) n = 40 | High (H) n = 47 | P-M | P-H | M-H | ||
| Culturable microbe (CFU/g) | |||||||
| Total fungi | 86 000 (3.7) | 150 000 | 61 000 | 73 000 | *** | ** | |
| Total bacteria | 1 212 000 (4.2) | 1 819 000 | 997 000 | 1 029 000 | |||
| GPB | 563 000 (5.7) | 967 000 | 401 000 | 485 000 | |||
| GNB | 54 000 (7.1) | 73 000 | 74 000 | 32 000 | |||
| Fungal biomass (ng/g) | |||||||
| Ergosterol | 1300 (2.1) | 2200 | 900 | 1100 | *** | *** | |
| (1 → 3)-β-D-Glucan | 57 000 (2.0) | 90 500 | 75 200 | 39 000 | *** | ** | *** |
| Bacterial biomass | |||||||
| Endotoxin (EU/g) | 69 000 (1.9) | 82 100 | 66 700 | 61 800 | |||
| Muramic acid (ng/g) | 7500 (1.4) | 9200 | 6900 | 7000 | *** | *** | |
| Relative moisture contentb | |||||||
| Maximum | 105 (2.1) | 77 | 117 | 143 | *** | *** | |
GM, geometric mean; GSD, geometric standard deviation; CPU, colony forming unit; GPB, Gram-positive bacteria; GNB, Gram-negative bacteria; ng, nanogram; EU, endotoxin unit.
Pairwise multiple comparisons with Scheffé’s correction.
The total number of rooms with measured relative moisture content were 212 (77, 75, and 60 rooms from the primary, middle, and high schools, respectively). For statistical analysis, we used the maximum value among all values measured from room components within a room.
P ≤ 0.01;
0.01 < P ≤ 0.05.
Fig. 1.
Differences in the mixed microbial exposure index (I) between schools. Boxplot: box = 25th, 50th (median), and 75th percentiles of mixed microbial exposure indices; lower and upper whiskers = 10th and 90th percentiles; closed circles = outliers; and an open circle inside the box = an arithmetic mean. The total number of samples was 125. ***P ≤ 0.01 using pairwise multiple comparisons with Scheffé’s correction
Associations between observational dampness scores and levels of microbial agents
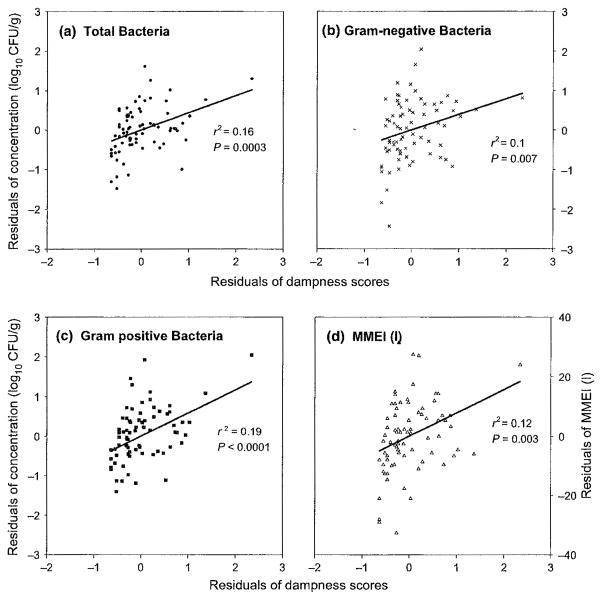
We found significant (all P-values ≤0.01) linear associations for the continuous observational dampness score with culturable bacteria (total, Gram-positive, and Gram-negative), and all three types of MMEIs but not with culturable fungi, biomass (fungi and bacteria), and relative moisture content. The significant associations remained regardless of whether zero scores were excluded from or included in the models. However, in the models, dampness scores only explained 10 to 19% of total variance in culturable bacteria and the MMEIs (Figure 2). All associations remained significant even after an outlier was excluded from each of the analyses although r-squared values slightly decreased.
Fig. 2.
Partial regression plots between total, Gram-negative, or Gram-positive bacteria, or mixed microbial exposure index I [MMEI (I)] and nonzero continuous observational dampness scores after adjusting for the school effect: x-axis = residual of nonzero observational dampness scores (N = 76) regressed against school; y-axis: residual of levels of bacteria or mixed microbial exposure index regressed against school
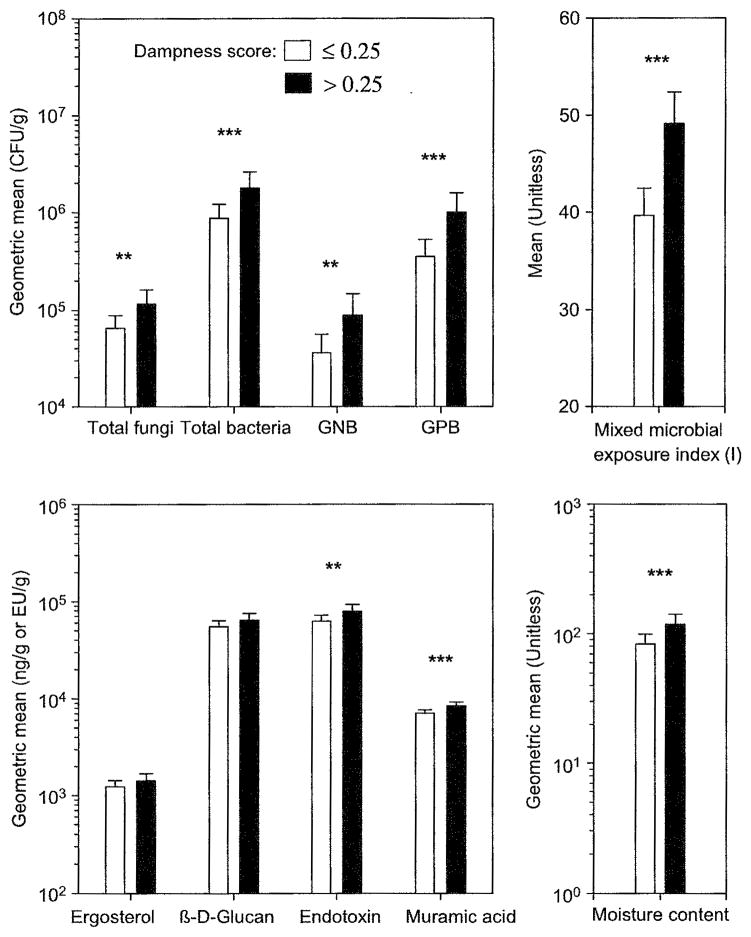
Figure 3 shows adjusted least squares means of microbial agents and moisture content for rooms with higher dampness scores (>0.25) and lower scores (≤0.25). Rooms with the higher scores had significantly (P < 0.05) higher GMs of culturable microbes (total fungi and culturable bacteria) and bacterial biomass (endotoxin and muramic acid) than those with the lower scores. Rooms with the higher dampness scores also had significantly (P < 0.001) higher MMEIs (I, II, and III) than those with the lower scores. We observed similar trends when we used microbial load per m2 floor area instead of concentration (per g of dust) in all the models (data not shown). These associations of observational dampness categorized as high or low with measurements of various microbial agents or MMEIs generally remained when we ran these models stratified by building engineers. In the models with two-three-level variables for gypsum board on walls and carpet on floor and dampness (as high or low) as independent variables, rooms with the higher dampness scores had significantly (P = 0.04) or marginally (P = 0.06) higher MMEIs (I and III, respectively) than those with the lower scores.
Fig. 3.
Adjusted means of microbial agents and moisture content in two groups of rooms categorized by the median observational dampness score: ≤0.25 and >0.25. Adjusted means were estimated using general linear models, after adjusting for a school effect. Each error bar shows the upper 95% confidence limit of the adjusted mean. ***P ≤ 0.01; **0.01 < P ≤ 0.05; GNB, Gram-negative bacteria; GPB, Gram-positive bacteria; Mixed microbial exposure index (I) = sum of deciles over 8 fungal and bacterial measurements
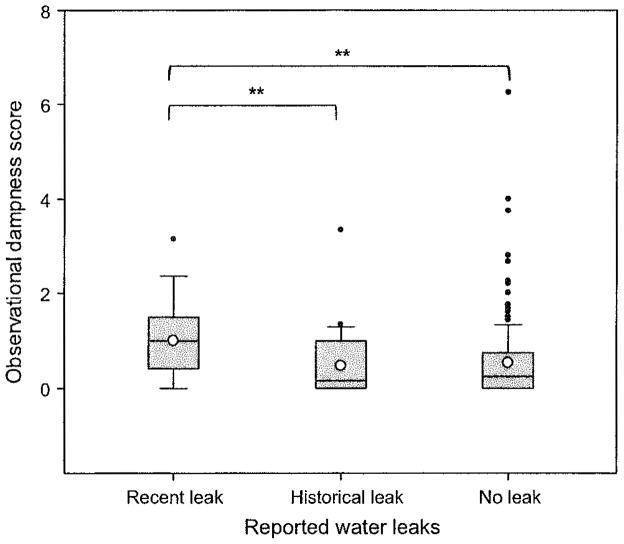
Figure 4 shows associations between water leaks reported by building maintenance employees or long-tenured workers and dampness scores. Rooms with recent water leaks had significantly (P < 0.05) higher adjusted mean dampness scores (n = 17, median=1.00) than rooms with historical (n = 29, median=0.17) or no water leaks (n = 173, median = 0.25) documented. Compared to rooms with historical or no water leaks, rooms with recent leaks had significantly higher levels of MMEIs (adjusted means: 53 vs. 43 for type I MMEI; 25 vs. 21 for type II; and 25 vs. 21 for type III; P-values <0.05) and relative moisture content (175 vs. 93, P < 0.01) as well as total bacteria (4.3 vs. 1.1 million CFU/g, P < 0.01), and Gram-negative (411 000 vs. 46 000, P < 0.01) and Gram-positive bacteria (2.8 vs. 0.5 million, P < 0.01) in floor dust. We found that measurements of moisture content of building materials were weakly negatively correlated (correlation coefficients: −0.22 to −0.30; P-values <0.05) with levels of total fungi, glucan, and ergosterol (thus, higher moisture content was associated with lower fungal, glucan, and ergosterol levels), but were not correlated with other agents.
Fig. 4.
Associations between water leaks reported by building maintenance workers or long-tenured workers and observational dampness scores. Boxplot: box = 25th, 50th (median), and 75th percentiles of observational dampness scores; lower and upper whiskers = 10th and 90th percentiles; closed circles = outliers; and an open circle inside the box = arithmetic mean. **0.01 < P ≤ 0.05 using the Kruskal–Wallis nonparametric ANOVA with a multiple comparison test (Dunn’s method)
Discussion
Signs of dampness and visible mold have frequently been used as a dampness-related exposure surrogate in indoor epidemiologic studies (Bornehag et al., 2001, 2004; Borràs-Santos et al., 2013; Jaakkola et al., 2002). There is also ample evidence of their associations with a wide range of respiratory morbidities (IOM, 2004b; Norbäck et al., 2013; WHO, 2009b; Zhang et al., 2012). However, there is little information available on whether observational assessment of dampness and mold is associated with objective measurements of microbial agents in floor dust which may be more representative of occupants’ longer-term exposure as compared to airborne levels (Park et al., 2006). Our study of three schools shows that observational assessment of dampness and mold was associated with the level of fungal and bacterial contamination of floor dust. We also found that rooms with recent water leaks had significantly higher observational dampness scores, levels of culturable bacteria, and relative moisture content than those with no recent water leaks (rooms with historical or no leaks documented). Our findings suggest that observational assessment of dampness/mold using a standardized evaluation form is a useful tool for monitoring and identifying water damage-related microbial contamination in buildings. Thus, considering our previous findings of associations between dampness/mold indices and occupants’ health in water-damaged buildings (Cox-Ganser et al., 2009; Park et al., 2004), this dampness/mold assessment tool could be used for justifying proactive remediation action to enhance building sustainability as well as protect occupants’ health. This simple observational tool may also help building management save substantial resources by minimizing the need for expensive indoor air sampling and analyses for microbial agents, which may produce false negative results.
Our study showed significant linear associations between continuous dampness scores and measurements of culturable bacteria (log-transformed) in dust and mixed microbial exposure indices. These linear associations remained significant regardless whether an extreme value was excluded from regression models. However, only a small portion of the total variance in those quantitative measurements was explained by dampness scores in the regression models. This implies that other building-related factors as well as measurement error may have contributed to the large variance of the measurements. It is known that the culture method captures only a small proportion of viable microbes in the samples on selected media (Torvinen et al., 2010). Nonetheless, the significant and positive linear association implies that microbial contamination in rooms generally increases as the observational dampness score increases. This finding suggests that repeated application of the observational tool over time may provide important information on the changes of the status in microbial contamination related to water damage.
Among our observational factors, visible mold, mold odor, and wetness are likely to indicate ongoing or recent water damage because mold cannot grow without moisture, and mold odor indicates the presence of microbial volatile organic compounds as a result of active metabolism (Borràs-Santos et al., 2013; Jones et al., 2011). However, we found from our study that approximately 70% (29 of 42) of rooms with the signs of these factors did not have reported water leaks or water incursions. This finding implies that documentation of water damage can be improved using an observational dampness and mold assessment and that the tool may also help management detect early water incursions before they become severe.
As damp indoor environments promote the growth of various dampness-related biological agents, including fungi and bacteria, we created mixed microbial exposure indices using decile ranks and summation of the ranks over all different agents investigated. Indeed, we found strong and consistent associations between the dampness scores and the mixed microbial exposure indices. Considering the low to moderate correlations (range: 0.09–0.66) among microbial agents and the significant but weaker associations between the dampness scores and individual microbial agents, this finding indicates that the observational dampness scores may better represent overall microbial contamination than concentrations of individual microbial agents.
In our study, three experienced and trained building engineers carried out the observational assessments using a standardized assessment form. Our finding that each engineer’s dampness and mold scores were associated with quantitative measurements of microbial agents reflects the robustness of the observational tool in the hands of trained observers, despite the lower statistical power reflected in a subset of the measurements. A prior study of ours found that the concordance rates between two teams of industrial hygienists were 88% for water stains, 63% for visible mold, 75% for mold odor, and 100% for moisture (Park et al, 2004). However, several other studies that examined agreement of dampness/mold evaluation between and within observers showed large inter- and intra-observer variability (Aamodt et al., 1999; Engman et al., 2007; Haverinen-Shaughnessy et al., 2005; Naydenov et al., 2008; Sun et al., 2007). Haverinen-Shaughnessy et al. (2005) discussed that an engineering background combined with extensive experience in building investigation and use of a standardized assessment form may lessen interobserver variability.
Observational assessment in our study could have been affected by the degree of maintenance and repair before the time of the assessment. If superficial maintenance such as painting over water stains and replacing ceiling tiles was performed without repairing the sources of water intrusions, the observational scores would be falsely low. In contrast, if sources of water incursion were fixed but signs of water stains or water damage were allowed to remain, the observational scores would be high, but not be an indication of active water incursions. Furthermore, the observational assessment does not involve intrusive methods and can miss hidden damage or contamination behind walls or above ceiling tiles during evaluation. To partly address this issue, mold odor was included in the assessment tool to help indicate the presence of hidden mold. Even with these limitations, our findings showed that the observational assessment of dampness and mold is a useful screening or surveillance tool.
We found no association between continuous variables of relative moisture content and the dampness score. We also found no associations between the continuous dampness score and continuous variables of total fungi or microbial biomass measurements. Therefore, the observation of weak negative correlations between relative moisture content and culturable fungi, ergosterol, or glucan might be type I error resulting from potentially large spatial variability in moisture measurements in building materials within a room or instrument variability of moisture readings among users.
In conclusion, we found that observational dampness scores obtained using a standardized evaluation form were positively and consistently associated with various objective measurements of environmental parameters such as culturable microbes, their biomasses, mixed microbial exposure indices, and relative moisture content in three schools. Our study suggests that the observational assessment of dampness/mold using a standardized form may be a valuable screening tool: (i) to identify and document water damage-related issues, (ii) to prioritize and guide timely remediation in buildings, and (iii) to effectively utilize resources by minimizing the cost for indoor air sampling and analysis of microbes in water-damaged buildings that may result in false negative findings.
Practical Implications.
Our study shows that higher observational dampness scores were positively associated with higher levels of fungal and bacterial contamination in floor dust of three school buildings. This indicates that observational assessment of dampness/mold using a standardized form may be the most practical screening tool to identify and document water damage-related issues and guide timely remediation by minimizing the cost for indoor air sampling and analysis of microbes in buildings.
Acknowledgments
We thank Dr. Lennart Larsson for analyzing dust aliquots for ergosterol and muramic acid. We are also grateful to Turner Building Science, LLC. for conducting observational assessment of dampness and visible mold, our colleagues for collecting environmental samples, and management of the school district and facilities for allowing our research team to conduct the study.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Aamodt AH, Bakke P, Gulsvik A. Reproducibility of indoor environment characteristics obtained in a walk through questionnaire, A pilot study. Indoor Air. 1999;9:26–32. doi: 10.1111/j.1600-0668.1999.t01-3-00005.x. [DOI] [PubMed] [Google Scholar]
- American Industrial Hygiene Association (AIHA) Ecology of fungi and bacteria found in uilding environments. In: Hung L-L, Miller JD, Dillon HK, editors. Field Guide for the Determination in Environmental Samples. Fairfax: American Industrial Hygiene Association; 2005. pp. 29–38. [Google Scholar]
- Bornehag CG, Blomquist G, Gyntelberg F, Jarvholm B, Malmberg P, Nordvall L, Nielsen A, Pershagen G, Sundell J. Dampness in buildings and health. Nordic interdisciplinary review of the scientific evidence on associations between exposure to “dampness” in buildings and health effects (NORDDAMP) Indoor Air. 2001;11:72–86. doi: 10.1034/j.1600-0668.2001.110202.x. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Sundell J, Bonini S, Custovic A, Malmberg P, Skerfving S, Sigsgaard T, Verhoeff A. Dampness in buildings as a risk factor for health effects, EUROEXPO: a multidisciplinary review of the literature (1998–2000) on dampness and mite exposure in buildings and health effects. Indoor Air. 2004;14:243–257. doi: 10.1111/j.1600-0668.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- Borràs-Santos A, Jacobs JH, Täubel M, Haverinen-Shaughnessy U, Krop EJ, Huttunen K, Hirvonen MR, Pekkanen J, Heederik DJ, Zock JP, Hyvärinen A. Dampness and mould in schools and respiratory symptoms in children: the HITEA study. Occup Environ Med. 2013;70:681–687. doi: 10.1136/oemed-2012-101286. [DOI] [PubMed] [Google Scholar]
- Choi H, Bryne S, Larson LS, Sigsgaard T, Thorne PS, Larson L, Sebastian A, Bornehag CG. Residential culturable fungi, (1–3, 1–6)-β-D-glucan, and ergosterol concentrations in dust are not associated with asthma, rhinitis, or ezema diagnoses in children. Indoor Air. 2014;24:158–170. doi: 10.1111/ina.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Ganser JM, Rao CY, Park J-H, Schumpert JC, Kreiss K. Asthma and respiratory symptoms in hospital workers related to dampness and biological contaminants. Indoor Air. 2009;19:280–290. doi: 10.1111/j.1600-0668.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- Elliott AC, Hynan LS. A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Programs Biomed. 2011;102:75–80. doi: 10.1016/j.cmpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Engman LH, Bornehag CG, Sundell J. How valid are parents’ questionnaire responses regarding building characteristics, mouldy odour, and signs of moisture problems in Swedish homes? Scand J Public Health. 2007;35:125–132. doi: 10.1080/14034940600975658. [DOI] [PubMed] [Google Scholar]
- Haverinen U, Husman T, Vahteristo M, Koskinen O, Moschandreas D, Nevalainen A, Pekkanen J. Comparison of two-level and three-level classifications of moisture-damaged dwellings in relation to health effects. Indoor Air. 2001;11:192–199. doi: 10.1034/j.1600-0668.2001.011003192.x. [DOI] [PubMed] [Google Scholar]
- Haverinen U, Vahteristo M, Moschandreas D, Nevalainen A, Husman T, Pekkanen J. Knowledge-based and statistically modeled relationships between residential moisture damage and occupant reported health symptoms. Atmos Environ. 2003;37:577–585. [Google Scholar]
- Haverinen-Shaughnessy U, Hyvärinen A, Pekkanen J, Nevalainen A, Husman T, Korppi M, Halla-aho J, Koivisto J, Moschandreas D. Occurrence and characteristics of moisture damage in residential buildings as a function of occupant and engineer observations. Indoor Built Environ. 2005;14:133–140. [Google Scholar]
- Institute of Medicine of the National Academies (IOM); Committee on Damp Indoor Spaces and Health, editor. Damp Indoor Spaces and Health. Washington, DC: The National Academies Press; 2004a. Damp buildings; pp. 29–89. [Google Scholar]
- Institute of Medicine of the National Academies (IOM); Committee on Damp Indoor Spaces and Health, editor. Damp Indoor Spaces and Health. Washington, DC: The National Academies Press; 2004b. Human health effects associated with damp indoor environments; pp. 183–269. [Google Scholar]
- Jaakkola MS, Nordman H, Piipari R, Uitti J, Laitinen J, Karjalainen A, Hahtola P, Jaakkola JJK. Indoor dampness and molds and development of adult-onset asthma: a population-based incident case-control study. Environ Health Perspect. 2002;110:543–547. doi: 10.1289/ehp.02110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Recer GM, Hwang SA, Lin S. Association between indoor mold and asthma among children in Buffalo, New York. Indoor Air. 2011;21:156–164. doi: 10.1111/j.1600-0668.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect. 2011;119:748–756. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton DK, Feldman HA, Neuberg DS, Bruckner RJ, Greaves IA. Environmental endotoxin measurement: the kinetic Limulus assay with resistant-parallel-line estimation. Environ Res. 1992;57:212–230. doi: 10.1016/s0013-9351(05)80081-7. [DOI] [PubMed] [Google Scholar]
- Naydenov K, Melikov A, Markov D, Stankov P, Bornehag CG, Sundell J. A comparison between occupants’ and inspectors’ reports on home dampness and their association with the health of children: the ALLHOME study. Build Environ. 2008;43:1840–1849. [Google Scholar]
- Norbäck D, Zock JP, Plana E, Heinrich J, Svanes C, Sunyer J, Künzli N, Villani S, Olivieri M, Soon A, Jarvis D. Mould and dampness in dewelling places, and onset of asthma: the population-based cohort ECRHS. Occup Environ Med. 2013;70:325–331. doi: 10.1136/oemed-2012-100963. [DOI] [PubMed] [Google Scholar]
- Park J-H, Schleiff PL, Attfield MD, Cox-Ganser JM, Kreiss K. Building-related respiratory symptoms can be predicted with semi-quantitative indices of exposure to dampness and mold. Indoor Air. 2004;14:425–433. doi: 10.1111/j.1600-0668.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- Park J-H, Cox-Ganser JM, Rao C, Kreiss K. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 2006;16:192–203. doi: 10.1111/j.1600-0668.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19:690–693. [Google Scholar]
- Sebastian A, Larsson L. Characterization of the microbial community in indoor environments: a chemical-analytical approach. Appl Environ Microbiol. 2003;69:3103–3109. doi: 10.1128/AEM.69.6.3103-3109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren ES, Park JH. Pre-sampling contamination of filters used in measurements of airborne (1 –> 3)-beta-D-glucan based on glucan-specific Limulus amebocyte lysate assay. J Environ Monit. 2011;13:1082–1087. doi: 10.1039/c0em00495b. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sundell J, Zhang Y. Validity of building characteristics and dorm dampness obtained in a self-administrated questionnaire. Sci Total Environ. 2007;387:276–282. doi: 10.1016/j.scitotenv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Torvinen E, Torkko P, Rintala AN. Real-time PCR detection of environmental mycobacteria in house dust. J Microbiol Methods. 2010;82:78–84. doi: 10.1016/j.mimet.2010.04.007. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Building dampness and its effect on indoor exposure to biological and non-biological pollutants. In: Heseltine E, Rosen J, editors. WHO Guidelines for Indoor Air Quality: Dampness and Mould. Copenhagen: WHO Regional Office for Europe; 2009a. pp. 7–29. [Google Scholar]
- World Health Organization (WHO) Health effects associated with dampness and mould. In: Heseltine E, Rosen J, editors. WHO Guidelines for Indoor Air Quality: Dampness and Mould. Copenhagen: WHO Regional Office for Europe; 2009b. pp. 63–96. [Google Scholar]
- Zhang X, Sahlberg B, Wieslander G, Janson C, Gislason T, Norbäck D. Dampness and moulds in workplace buildings: associations with incidence and remission of sick building syndrome (SBS) and biomarkers of inflammation in a 10 year follow-up study. Sci Total Environ. 2012;430:75–81. doi: 10.1016/j.scitotenv.2012.04.040. [DOI] [PubMed] [Google Scholar]