Abstract
Objectives
Autoantibodies reactive with Ro52 are often found in sera of Sjögren’s syndrome (SS) patients. This study was undertaken to investigate the role of Ro52-induced immune responses in pathogenesis of SS.
Methods
New Zealand Mixed (NZM) 2758 mice were immunized with Ro52 in alum adjuvant. Control mice were immunized either with Maltose binding protein (MBP) or injected with alum alone. Mice were monitored for anti-Ro52 antibody, sialoadenitis and pilocarpine induced salivation. Antibody binding to salivary gland (SG) cells was analyzed in vivo and in vitro by immunofluorescence. Sera from immunized mice were passively transferred into untreated or alum injected NZM2758 mice.
Results
By day 30 post-immunization, Ro52 immunized mice generated immunoprecipitating anti-Ro52 antibodies and they had the maximum drop in saliva production. Both Ro52 immunized and control mice showed evidence of mild sialoadenitis. However, only Ro52 immunized mice had antibody deposition in their SG. Passive transfer of Ro52-immune sera induced SG dysfunction in recipient mice, only if the recipients were primed with alum. In vitro, antibodies from Ro52-immune sera were internalized by a SG cell line and this uptake was inhibited by Cytochalasin D treatment.
Conclusion
Our data shows for the first time that antibodies induced by Ro52 are capable of inducing SG dysfunction, and that this phenomenon is dependent on the activation of innate immunity. The mouse model described in this study implies that autoantibody deposition in the SG might be an important step in the induction of xerostomia and pathogenesis of SS.
Keywords: Autoantibodies, Mouse, Salivary gland, Sjögren’s syndrome, Ro52
INTRODUCTION
Autoantibodies reactive with Ro52 are found in almost 70% of patients with Sjögren’s syndrome (SS), a chronic autoimmune disorder mainly affecting the salivary and lacrimal glands.[1, 2] Ro52, also known as TRIM21, is an E3 ubiquitin ligase that either positively or negatively regulates type I interferon (IFN) responses.[3–7] Presence of antibodies against Ro52 and Ro60 are used in the classification criteria of SS.[8] Earlier reports have collectively termed these antibodies as anti-SSA (Sjögren’s syndrome antigen A), without clearly distinguishing their specificity. However, recent analysis of sera from SS patients clearly shows that the frequency of anti-Ro52 autoantibodies is higher than that of anti-Ro60 autoantibodies.[2, 9,10] The presence of anti-Ro52 autoantibodies is linked to higher lymphocytic focus scores within the SG.[11] Furthermore, B cells reactive with Ro52 have been detected within the lymphocytic foci in SG of SS patients.[12] Despite this extensive knowledge and epidemiological data on anti-Ro52 antibody, it is still not clear whether this autoantibody specificity exerts pathogenic effects in SS.
To specifically address the role of Ro52 induced immune responses in SS, we developed an immunization model using the New Zealand Mixed (NZM) 2758 mouse strain. The NZM mouse strains have been derived by breeding and backcrossing the NZB and NZW strains of mice.[13, 14] The NZM2758 mice do not spontaneously develop anti-Ro52 antibodies and SS. However, this mouse strain has susceptibility genes for development of SS. We have recently reported that treatment of NZM2758 mice with the aluminum containing adjuvant alum induces a SS-like disorder.[15] In this study we investigated the effects of Ro52 induced immune responses on the development of SS-like disorder in NZM2758 mice.
METHODS
Proteins
The full length mRo52 cDNA (NM_009277.2) was cloned into the pMAL-c5E vector (New England BioLabs, Ipswich, MA) to generate a maltose-binding protein (MBP)-Ro52 fusion protein. Empty vector was used for the production of MBP. Both proteins were purified under native conditions following manufacturer’s instructions.
Mice
All experiments were approved by the Institutional Animal Care and Use Committees. The NZM2758 strain of mice were bred and maintained under specific pathogen-free conditions in the University of Virginia, and The Oklahoma Medical Research Foundation vivarium. Outbred CD1 mice were purchased from National Cancer Institute, Frederick MD and used for generation of hyper-immune sera. Female mice were immunized either with 50μg of MBP-mRo52 or MBP adsorbed on to adjuvant alum (Thermo Scientific, Rockford, IL). An additional group of mice were injected with only alum as described previously.[15] Mice were bled at different time points to obtain serum for antibody analyses. Pilocarpine induced saliva was measured as described previously.[16]
Quantitative immunoprecipitation assay (IP)
Reactivity to native Ro52 was analyzed by a quantitative immunoprecipitation assay as described previously.[17]. In vitro transcribed and translated and 35S-Met labeled mRo52 was used as substrate.
Histology
Submandibular SG (SMG) harvested from mice were fixed in 10% buffered formalin and sections were stained with hematoxylin and eosin (H&E). The slides were evaluated for sialoadenitis as reported previously.[18]
Direct immunofluorescence staining
SG were fixed in paraformaldehyde-lysine-periodate, transferred to 30% sucrose in PBS and were embedded in OCT for cryostat sectioning. Five micron sections were used for detection of IgG antibody deposits as described previously.[19]
Internalization of antibodies
SCA9-15 cells were seeded on to glass cover-slips and grown over-night at 37°C in 5% CO2. Cells were incubated with different mouse sera for 1h and then fixed with Cytofix/CytoPerm Kit (BD Biosciences, San Jose, CA) for 30 minutes at room temperature. Bound antibody was detected by incubation with FITC conjugated goat anti-mouse IgG (SouthernBiotech, Birmingham, AL) and mounted with Prolong Gold with DAPI (Life technologies, Grand Island, NY). In some experiments, cells were pre-treated with 1.5 μg/ml Cytochalasin D (Enzo Life Sciences, Farmingdale, NY) for 15 minutes at 37°C followed by incubation with different mouse sera. To visualize actin filaments, cells were co-stained with Phalloidin Alexa Fluor 568 conjugate (Life technologies). Images were captured by optical sectioning on a Zeiss LSM-510 META laser scanning confocal microscope using LSM software.
Passive transfer of Serum
Recipient female NZM2758 mice were either untreated or injected with alum as described previously.[15] Pooled sera from either Ro52 or MBP or alum immunized mice were transferred into recipient mice (100μl/mouse) on day 30 by intraperitoneal route. Saliva production was measured after 24 hours.
Patients
The primary SS (pSS) classification was based on the American European Consensus Group (AECG) criteria.[8] Clinical data from the Oklahoma Sjögren’s Syndrome Center of Research Translation (OSSCORT) pSS cohort was analyzed for autoantibody reactivity, biopsy scores, and whole unstimulated salivary flow (WUSF).[20] The patient cohort and data collection has been described in detail previously.[20] All procedures were approved by the Oklahoma Medical Research Foundation IRB.
Statistical Methods
To compare differences in saliva production between different groups of mice, non-parametric Kruskal-Wallis test was used. Mann-Whitney test was used to compare anti-Ro52 antibody levels. Spearman’s test was used to determine correlation between anti-Ro52 antibody levels and saliva volume. Graph Pad Prism software was used to perform all statistical tests.
RESULTS
Immunization of NZM2758 mice with Ro52 induces anti-Ro52 antibodies and SG dysfunction
Day 30 post-immunization sera were analyzed for reactivity to native mRo52. Figure 1A shows that seven out of eight mice generated IgG antibodies capable of immunoprecipitating Ro52. None of the 5 MBP immunized mice had these antibodies. Similar results were obtained in an additional cohort of mice (5 per group).
Figure 1. SG dysfunction is most prominent in Ro52 immunized mice and it correlates with levels of anti-Ro52 antibody.
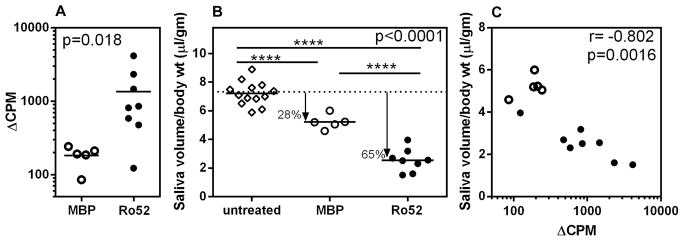
A. Immunoprecipitating anti-mRo52 antibodies are generated in 7 out of 8 NZM2758 mice immunized with Ro52. Data is represented as ΔCPM, which is CPM with serum sample minus CPM without serum. None of the MBP immunized mice have antibodies over the cutoff, which is mean ΔCPM+2SD for MBP immunized mice. Mann-Whitney test was used to determine the statistical significance. B. In comparison with untreated mice, Ro52 immunized mice show a 65% drop and MBP immunized mice show a 28% drop in mean saliva volumes. Kruskal–Wallis test was used to determine the statistical significance and ****indicates p<0.0001. The number of mice used provides the experiment >99% power at a significance level (alpha) of 0.05 (two-tailed) C. Inverse correlation is seen between saliva volume and level of anti-Ro52 antibody (p=0.0016; r = −0.802) as determined by Spearman correlation test.
To determine the effects of Ro52 immunization on SG function, pilocarpine induced saliva was measured on day 35 (figure 1B). In comparison with untreated mice, both MBP and Ro52 immunized mice, showed a significant drop in the mean saliva volume. However, maximum drop (65%, p<0.001) was observed in the Ro52 immunized group. The 28% drop in mean saliva volume in MBP immunized mice is similar to that observed in alum injected NZM2758 mice, previously [15]. Thus, the loss of function in MBP immunized mice can be attributed to effects of innate immune activation by alum. The anti-Ro52 antibody levels showed an inverse correlation (r=0.802, p=0.0016), with the amount of saliva produced (figure 1C). Collectively, the results demonstrate that SG dysfunction could be exacerbated by Ro52 immunization.
Mild sialoadenitis and immunoglobulin deposition in SMG of immunized mice
Our previous studies have demonstrated that 4 months after alum treatment, NZM2758 mice show evidence for sialoadenitis.[15] In the Ro52 immunized mice, since significant drop in saliva production occurred just 1 month post-immunization, SMG were obtained at this time point and H&E sections analyzed for sialoadenitis. Only 1 out of 9 Ro52 immunized mice, and 4 out of 8 MBP immunized mice had small inflammatory foci in their SMG (figure 2A and 2B). CD4+ T cells were readily detected in these foci (Supp figure S1). However, there was no statistically significant difference in the incidence (Fisher’s exact test, p=0.132) and severity of sialoadenitis between the MBP immunized and Ro52 immunized groups.
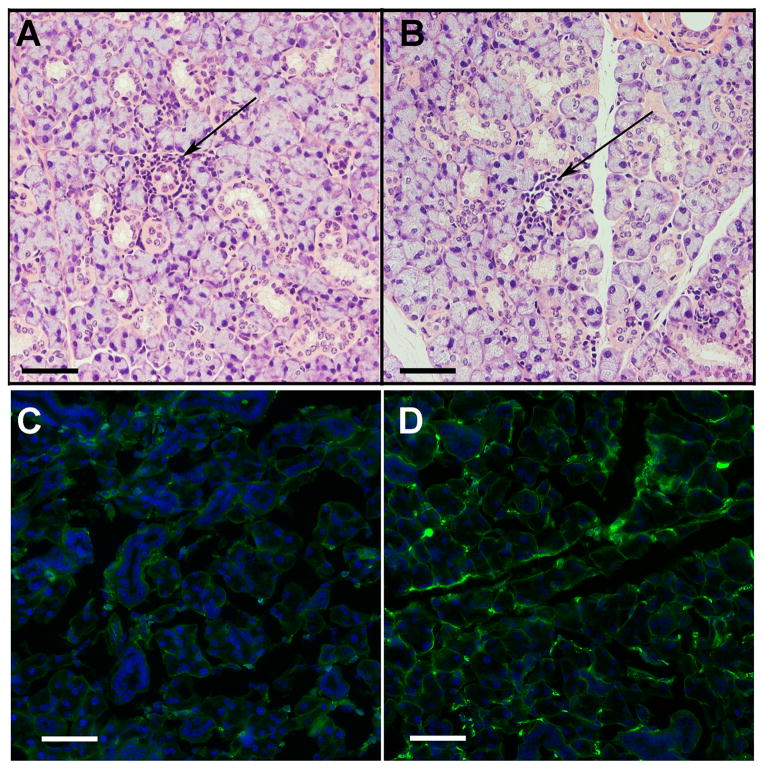
Figure 2. Mild sialoadenitis was seen in NZM2758 mice injected either with Ro52 or MBP, but IgG deposition was only seen in Ro52 immunized mice.
A, B. H&E stained sections of SMG obtained on day 35 from MBP immunized (A) and Ro52 immunized mouse (B) showing mild sialoadenitis. C, D. Representative pictures of direct immunofluorescence staining of SMG from mice injected either with MBP (C) or with Ro52 (D). IgG deposition was detected by direct immunofluorescence using FITC conjugated goat anti-mouse IgG. Scale bar = 50μm.
To determine the possibility that direct deposition of antibodies within the SG might influence glandular dysfunction, presence of immunoglobulin within the SMG was analyzed by direct immunofluorescence. IgG deposits were readily detected along the base of acinar and ductal cells, and the interacinar regions in SG from Ro52 immunized mice, but not in the MBP immunized mice (figure 2C, 2D, Supp figure S2).
Antibody mediated SG dysfunction
To further investigate the role of antibodies in glandular dysfunction, we performed passive transfer of sera from immunized mice into either naïve untreated mice or alum injected mice. As shown in Figure 3 (left panel), passive transfer of sera from Ro52 immunized mice did not affect saliva production in untreated NZM2758 recipients. However, injection of alum one month prior to Ro52-immune serum transfer induced a significant drop in saliva production compared to control mice injected with alum alone (figure 3, right panel). Transfer of sera from alum or MBP immunized mice did not significantly affect saliva production in alum injected recipient mice. Immunoglobulins eluted from SMG of Ro52-immune sera recipient mice reacted with Ro52 (Supp figure S3), suggesting that anti-Ro52 antibodies are deposited in SMG.
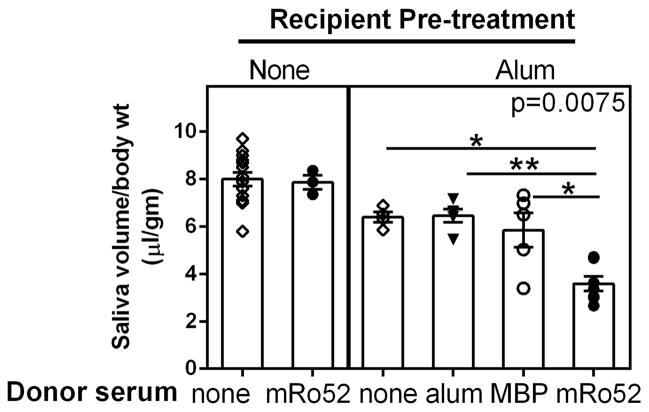
Figure 3. Passive transfer of immune sera from Ro52 immunized mice rapidly induces SG dysfunction only in recipients primed with alum.
NZM2758 mice were either untreated (left panel) or injected with alum (right panel). On day 30, sera obtained from mice immunized either with alum alone, or MBP or Ro52 were used for passive transfer and saliva production was measured after 24h. There was no drop in saliva production in untreated mice receiving Ro52-immune sera. Compared to untreated mice, all groups of alum injected mice showed a drop in mean saliva volume. Of the mice pre-injected with alum, only transfer of Ro52-immune sera led to a significant reduction in saliva production. (p = 0.0075, Kruskal–Wallis test).
Internalization of antibodies by SG cell line SCA9-15
Since antibodies from Ro52-immune sera deposited in the SG, we analyzed the reactivity of these sera with a mouse SG ductal cell line, SCA9-15. Antibodies from Ro52-immune sera entered live cells and mainly stained the cytoplasm as dots (figure 4). This staining pattern was not seen in sera obtained from mice immunized with MBP. Cytochalasin D treatment of cells prevented the entry of antibodies inside the cell and antibodies were retained on the surface, indicating that the internalization was an active process of uptake (figure 5). In addition, pre-incubation of immune sera with Ro52 significantly reduced the internalization and intracellular staining (Supp figure S4).
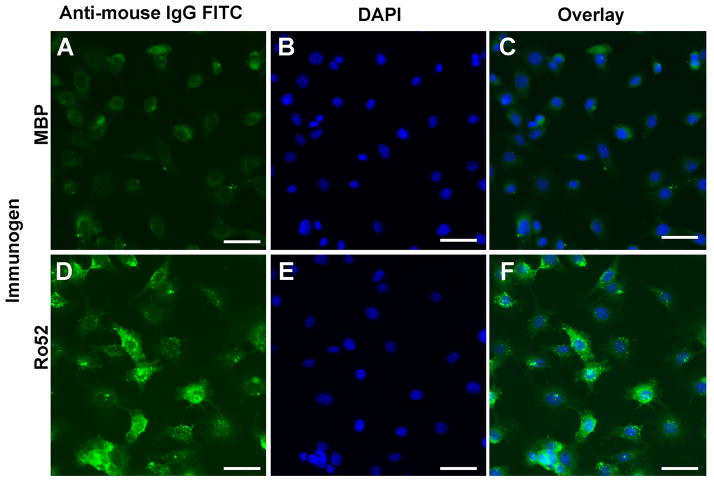
Figure 4. Antibodies from Ro52-immune sera are actively internalized by SG cell line.
SCA9-15 cells were grown on coverslips and incubated with immune sera at 1:250 dilution. Bound antibodies were detected with FITC conjugated goat anti-mouse IgG (A & D, green) and nuclei were stained with DAPI (B & E, blue). Antibodies from Ro52-immune sera show strong cytoplasmic staining in comparison with MBP-immune sera (C & F, overlay). Scale bar = 20μm.
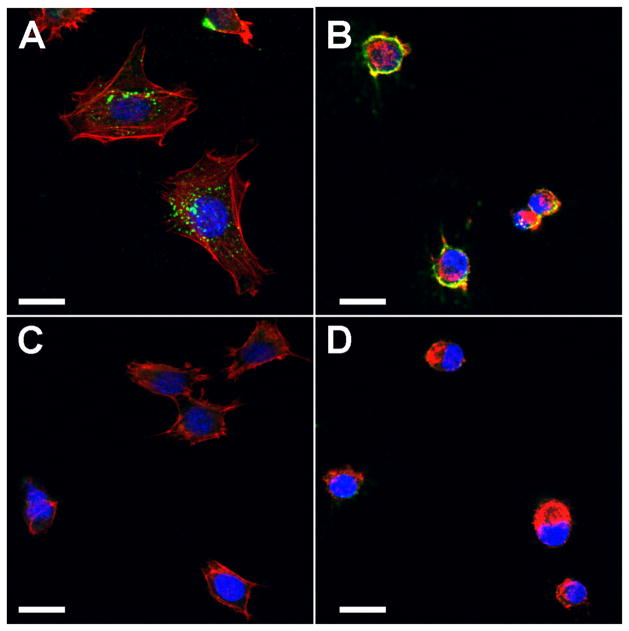
Figure 5. Cytochalasin D treatment prevents internalization of antibodies by SCA9-15 cells.
Confocal images show that cells incubated with Ro52-immune sera have cytoplasmic IgG (green) staining (A), which is not seen in cells incubated with control sera (C). Cytochalasin D treatment localizes antibody reactivity to the surface (B). Cells treated with Cytochalasin D, become rounded due to collapse in actin filaments (red) stained with Phalloidin Alexa Fluor 568. Similar results were obtained in 3 additional experiments. Scale bar = 10μm.
Ro52 immunized NZM2758 mice mimic a subset of SS patients
At day 30 post-immunization time point, Ro52 immunized mice did not show evidence for sialoadenitis. However, they had circulating anti-Ro52 antibodies and SG dysfunction. Interestingly, a small subset of SS patients, in the Oklahoma SS cohort show similar features (Table 1). Of the 321 patients, 73 patients (~23%) were biopsy negative by AECG criteria (focus score <1). Amongst these, 49 were anti-Ro positive and 24 were anti-Ro negative. Amongst the 49 anti-Ro positive patients, 10 patients also had anti-La antibodies.
Table 1.
Summary of anti-Ro reactivity and objective dry mouth in pSS patients in OK cohort with negative histopathology of minor salivary gland biopsy.
| 1Biopsy Negative (Focus Score <1.0) | |||
| 73/321 (22.74%)2 | |||
| Anti-Ro+ | 3Anti-Ro− | ||
| 49 (15.26%) | 24 (7.47%) | ||
| 4WUSF+ | WUSF− | WUSF+ | WUSF− |
| 33 (10.28%) | 16 (4.98%) | 14 (4.36%) | 10 (3.11%) |
As per AECG criteria.[8] Focus Score is defined as number of lymphocytic foci (which are adjacent to normal-appearing mucous acini and contain more than 50 lymphocytes) per 4 mm2 of glandular tissue.[21]
% values represent proportion of the total number of pSS patients (n=321).
All anti-Ro negative patients were anti-La positive and thus met AECG criteria for pSS.[8] The methods employed for autoantibody analysis in OK are provided in supplemental methods.
WUSF: Whole unstimulated salivary flow. A value of ≤1.5ml per 15 min is considered WUSF+.[20]
DISCUSSION
In this study we demonstrate for the first time that antibodies induced by Ro52 immunization can cause SG dysfunction. Apart from our study, the direct pathogenic role of anti-Ro52 antibodies has only been demonstrated in the experimental rat models of congenital heart block.[22, 23] Considered together, these studies clearly establish the pathogenic potential of anti-Ro52 antibodies.
Although multiple mouse models have been described for SS, each model recapitulates only certain features of this complex disorder.[24] The experimental model system described in this study resembles clinical observations reported in a subset of pSS patients who meet the AECG classification criteria (Table 1). These patients do not have sialoadenitis, are anti-Ro or/and La positive and have xerostomia. However, only longitudinal studies in both patients and our model system would clarify, whether this represents an early stage of the disease, which is followed by development of severe sialoadenitis. In this regard, it should be noted that in the Ro60-peptide immunization model, only 45% of mice developed sialoadenitis by 8 months post-immunization.[25] However, the severity of sialoadenitis did not correlate with the extent of salivary gland dysfunction. The Ro52-model system clearly demonstrates the role of autoantibodies in SG dysfunction and suggests that this might represent an early feature of SS in some patients.
Passive transfer experiments show that ability of antibodies to induce SG dysfunction was dependent on prior activation of innate immunity through alum. Alum used in this study has been reported to activate innate immune responses through the inflammasome pathway, leading to the production of pro-inflammatory cytokines.[26, 27] Inflammatory conditions in SG of pSS patients have been shown to induce ductal expression of Ro52.[28] In addition, expression and location of Ro52 in different cellular compartments has been modulated by activation of innate immunity through TLR3, IFN-α and nitric oxide.[29–31] In NZM2758 mice, we observed that alum treatment caused significant upregulation of circulating KC, IL-1α, MIG, MIP2 and PDGF-β (Supp fig S4). At present it is unclear as to how these cytokines influence antibody mediated SG dysfunction. It is possible that systemic up regulation of pro-inflammatory cytokines can make the SG conducive for antibody mediated injury, such as, changes in the expression and localization of proteins targeted by the Ro52-immune sera. Anti-Ro/La antibody responses in SS patients have been associated with a positive type I IFN signature.[32] Thus, whether an elevated type I IFN response occurs in Ro52 immunized mice and contributes towards glandular dysfunction needs to be evaluated.
In both active immunization and passive transfer studies, immunoglobulin deposition was detected in the mouse SG. These data suggest that antibody deposition within the SG might be an important factor for the induction of glandular dysfunction, particularly in cases lacking lymphocytic foci. IgG antibody deposition in the SG ducts has been previously demonstrated in a limited study of SS patients.[33]. In addition, circulating antibodies reacting with SG ductal antigens have been previously reported.[34] Clearly additional studies with patient samples are warranted to further confirm the association between ductal antigen recognition, immunoglobulin deposition, and xerostomia.
Since, immunoglobulin deposition was observed in SG of Ro52 immunized mice, we analyzed the reactivity of immune sera with a mouse SG cell line SCA9-15. Live SCA9-15 cells internalized antibodies, specifically from the Ro52-immune sera. The antibody uptake was prevented by Cytochalasin D treatment, and the antibodies showed cell surface binding. Although pre-incubation of immune sera with Ro52 considerably inhibited antibody binding to SCA9-15 cells, the precise target of these antibodies on cell surface is not known. Previous studies have demonstrated that apoptotic human ductal cells cause subcellular redistribution and surface exposure of Ro52, Ro60 and La.[35] However, in Ro52 immunized NZM2758 mice we did not observe epitope spreading and generation of anti-Ro60 and anti-La antibodies (Supp fig S6). Thus, it is possible that antibodies in Ro52-immune sera bind either Ro52 or a cross-reactive protein(s) expressed on the surface of SG cells. Indeed, anti-Ro52 antibodies have been shown to cross-react with serotoninergic 5-HT4 receptor on cell surface.[36] Whether such cross-reactive antibodies are generated in Ro52 immunized NZM2758 mice will be investigated in future. Alternatively, the antibody uptake might be occurring through the Fcγ receptors. A previous report has demonstrated that human SG cell line A-253 internalizes anti-Ro and anti-La antibodies from SS patients through Fcγ receptor binding.[37] Although plausible, this mechanism cannot explain the lack of uptake of antibodies from MBP immunized mice.
The internalized antibodies in SCA9-15 cells were localized in the cytoplasm as dots (figure 4). Previous studies have demonstrated that Ro52 is present in cytoplasmic bodies that cannot be classified as endosomes, lysosomes, mitochondria, caveolae or proteasome-enriched structures. [38] A characteristic feature of Ro52 cytoplasmic bodies is their transport along the microtubule network. It would be of interest to determine, whether such structures are seen in salivary gland epithelial cells and whether anti-Ro52 antibodies localize to these structures, in vivo.
In summary, this study demonstrates the critical role played by Ro52 induced autoantibodies in pathogenesis of SS, particularly in the induction of SG dysfunction. Considering that this phenomenon was also dependent on the activation of innate immunity, novel immunotherapies targeting both innate and adaptive immunity might prove to be highly beneficial for treating SS.
Supplementary Material
Acknowledgments
This study has been funded by research grants from the National Institute of Dental and Craniofacial Research, DE019883 (USD), DE022977 (USD), National Institute of Allergy and Infectious Diseases, AI079621 (USD) and National Institute of Arthritis and Musculoskeletal and Skin Diseases, P50 AR060804 (KLS). Financial support was also provided by the Oklahoma Medical Research Foundation (USD and HB). Technical assistance by the OMRF imaging core facility is acknowledged.
Footnotes
The authors have no financial disclosures.
References
- 1.Reksten TR, Jonsson MV. Sjögren’s syndrome: an update on epidemiology and current insights on pathophysiology. Oral Maxillofac Surg Clin North Am. 2014;26:1–12. doi: 10.1016/j.coms.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J Autoimmunity. 2014;51:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Ozato K, Shin DM, Chang TH, et al. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–60. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong HJ, Anderson DE, Lee CH, Jang, et al. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF-8 and enhances cytokine expression in macrophages. J Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Higgs R, NíGabhann J, Ben Larbi N, et al. The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J Immunol. 2008;181:1780–6. doi: 10.4049/jimmunol.181.3.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Bao M, Lu N, et al. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat Immunol. 2013;14:172–8. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manocha GD, Mishra R, Sharma N, et al. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J Neuroinflammation. 2014;11:24. doi: 10.1186/1742-2094-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghillani P, André C, Toly C, et al. Clinical significance of anti-Ro52 (TRIM21) antibodies non-associated with anti-SSA 60kDa antibodies: results of a multicentric study. Autoimmun Rev. 2011;10:509–13. doi: 10.1016/j.autrev.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Menéndez A, Gómez J, Escanlar E, et al. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity. 2013;46:32–9. doi: 10.3109/08916934.2012.732131. [DOI] [PubMed] [Google Scholar]
- 11.Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmunity. 2012;39:77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Aqrawi LA, Skarstein K, ØijordsbakkenK, et al. Ro52- and Ro60-specific B cell pattern in the salivary glands of patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2013;172:228–37. doi: 10.1111/cei.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudofsky UH, Evans BD, Balaban SL, et al. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68:419–26. [PubMed] [Google Scholar]
- 14.Hudson CA, Cao L, Kasten-Jolly J, et al. Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J Toxicol Environ Health A. 2003;66:895–918. doi: 10.1080/15287390306456. [DOI] [PubMed] [Google Scholar]
- 15.Bagavant H, Nandula SR, Kaplonek P, et al. Alum, an aluminum-based adjuvant, induces Sjögren’s syndrome-like disorder in mice. Clin Exp Rheumatol. 2014;32:251–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Deshmukh US, Nandula SR, Thimmalapura PR, et al. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009;38:42–7. doi: 10.1111/j.1600-0714.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshmukh US, Sim DL, Dai C, et al. HLA-DR3 restricted T cell epitope mimicry in induction of autoimmune response to lupus-associated antigen SmD. J Autoimmunity. 2011;37:254–62. doi: 10.1016/j.jaut.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandula SR, Scindia YM, Dey P, et al. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjögren’s syndrome-like disease. Oral Dis. 2011;17:801–7. doi: 10.1111/j.1601-0825.2011.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagavant H, Tung KS. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175:944–50. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen A, Ice JA, Li H, et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterized sicca cohort. Ann Rheum Dis. 2014;73:31–8. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels TE, Cox D, Shiboski CH, et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63:2021–30. doi: 10.1002/art.30381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosi A, Wahren-Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res Ther. 2012;14:208. doi: 10.1186/ar3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosi A, Dzikaite V, Park J, et al. Anti-Ro52 monoclonal antibodies specific for amino acid 200-239, but not other Ro52 epitopes, induce congenital heart block in a rat model. Ann Rheum Dis. 2012;71:448–54. doi: 10.1136/annrheumdis-2011-200414. [DOI] [PubMed] [Google Scholar]
- 24.Delaleu N, Nguyen CQ, Peck AB, et al. Sjögren’s syndrome: studying the disease in mice. Arthritis Res Ther. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scofield RH, Asfa S, Obeso D, et al. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren’s syndrome. J Immunol. 2005;175:8409–14. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Willingham SB, Ting JP, et al. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kool M, Pétrilli V, De Smedt T, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–9. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 28.Aqrawi LA, Kvarnström M, Brokstad KA, et al. Ductal epithelial expression of Ro52 correlates with inflammation in salivary glands of patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2014;177:244–52. doi: 10.1111/cei.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyriakidis NC, Kapsogeorgou EK, Gourzi VC, et al. TLR3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway. Clin Exp Immunol. 2014 doi: 10.1111/cei.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strandberg L, Ambrosi A, Espinosa A, et al. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J Clin Immunol. 2008;28:220–31. doi: 10.1007/s10875-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa A, Oke V, Elfving A, et al. The autoantigen Ro52 is an E3 ligase resident in the cytoplasm but enters the nucleus upon cellular exposure to nitric oxide. Exp Cell Res. 2008;314:3605–13. doi: 10.1016/j.yexcr.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Brkic Z, Maria NI, van Helden-Meeuwsen CG, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis. 2013;72:728–35. doi: 10.1136/annrheumdis-2012-201381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes JD, Nico MM, Aoki V, et al. Xerostomia in Sjögren’s syndrome and lupus erythematosus: a comparative histological and immunofluorescence study of minor salivary glands alterations. J Cutan Pathol. 2010;37:432–8. doi: 10.1111/j.1600-0560.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- 34.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren’s disease. J Clin Pathol. 1968;21:656–60. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohlsson M, Jonsson R, Brokstad KA. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: a possible mechanism in the pathogenesis of Sjögren’s syndrome. Scand J Immunol. 2002;56:456–69. doi: 10.1046/j.1365-3083.2002.01072_79.x. [DOI] [PubMed] [Google Scholar]
- 36.Eftekhari P, Sallé L, Lezoualc’h F, et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000;30:2782–90. doi: 10.1002/1521-4141(200010)30:10<2782::AID-IMMU2782>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Lisi S, Sisto M, Soleti R, et al. Fc gamma receptors mediate internalization of anti-Ro and anti-La autoantibodies from Sjögren’s syndrome and apoptosis in human salivary gland cell line A-253. J Oral Pathol Med. 2007;36:511–23. doi: 10.1111/j.1600-0714.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Tanji K, Niida M, et al. Dynamic movements of Ro52 cytoplasmic bodies along microtubules. Histochem Cell Biol. 2010;133:273–84. doi: 10.1007/s00418-009-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.