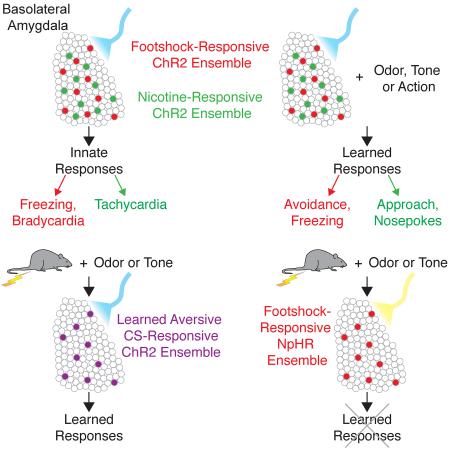
Summary
Stimuli that possess inherently rewarding or aversive qualities elicit emotional responses and also induce learning by imparting valence upon neutral sensory cues. Evidence has accumulated implicating the amygdala as a critical structure in mediating these processes. We have developed a genetic strategy to identify the representations of rewarding and aversive unconditioned stimuli (USs) in the basolateral amygdala (BLA) and have examined their role in innate and learned responses. Activation of an ensemble of US-responsive cells in the BLA elicits innate physiological and behavioral responses of different valence. Activation of this US ensemble can also reinforce appetitive and aversive learning when paired with differing neutral stimuli. Moreover, we establish that the activation of US-responsive cells in the BLA is necessary for the expression of a conditioned response. Neural representations of conditioned and unconditioned stimuli must therefore ultimately connect to US-responsive cells in the BLA to elicit both innate and learned responses.
Introduction
Emotions may arise in response to unconditioned and conditioned stimuli from each of the sensory modalities (Cardinal et al., 2002, Davis, 1998, LeDoux, 2000, Rosen, 2004 and Schultz, 2001). Unconditioned stimuli (USs) possess inherently rewarding or aversive qualities and elicit innate emotional responses. However, the responses to most stimuli are not innate but learned, allowing an organism to respond appropriately to a variable and often unpredictable world. Stimuli that drive innate responses also contribute to learning by imparting meaning on neutral sensory cues. An animal can therefore predict the consequence of a conditioned stimulus (CS) after learning and respond with appropriate behavioral output (Lang and Davis, 2006, LeDoux, 2000, Pavlov, 1927 and Schultz, 2006). Thus an unconditioned stimulus may participate in both innate and learned responses.
Representations of unconditioned stimuli are generated at the earliest stages of sensory processing. These representations must connect with neural circuits that elicit both innate and learned emotional responses. Anatomical, electrophysiological and behavioral experiments provide evidence that the basolateral amygdala (BLA) may connect sensory representations and behavioral output (Amaral et al., 1992, Everitt et al., 2003, Fendt and Fanselow, 1999, Gallagher and Holland, 1994, Janak and Tye, 2015, Lang and Davis, 2006, McDonald, 1998, Russchen et al., 1985, Sah et al., 2003, Salzman and Fusi, 2010 and Sarter and Markowitsch, 1985). Neural representations of appetitive and aversive USs have been identified in the BLA (Belova et al., 2007, Bermudez and Schultz, 2010, Knapska et al., 2007, Livneh and Paz, 2012, Muramoto et al., 1993, Paton et al., 2006, Romanski et al., 1993, Uwano et al., 1995 and Wolff et al., 2014).
Pharmacologic silencing and lesions of the BLA impair aversive conditioning and some forms of appetitive conditioning (Amano et al., 2011, Ambroggi et al., 2008, Anglada and Quirk, 2005, Balleine and Killcross, 2006, Hatfield et al., 1996 and Maren et al., 2001). Optogenetic activation of random populations of neurons in the lateral amygdala can entrain a neutral tone to elicit freezing behavior (Johansen et al., 2010, Yiu et al., 2014), and activation of different populations of BLA neurons or their distinct projections can elicit either anxiety-related or self-stimulation behaviors (Felix-Ortiz et al., 2013, Kim et al., 2013, Namburi et al., 2015, Stuber et al., 2011 and Tye et al., 2011). Finally, photoactivation of BLA cells activated by an appetitive or aversive conditioning paradigm can generate valence-specific responses (Redondo et al., 2014). These studies indicate that the BLA is involved in linking sensory representations to behavioral output, but the nature of the neural representations of different USs in the BLA and their causal role in the generation of innate responses and emotional learning has remained elusive.
We have developed a genetic strategy to examine the functional role of US representations in the BLA. This approach permits the identification and optogenetic manipulation of the activity of BLA neurons responsive to an appetitive or aversive US. We demonstrate that photoactivation of an ensemble of US-responsive cells in the BLA elicits valence-specific innate responses. These US ensembles can also drive appetitive and aversive learning. Moreover, activation of US-responsive cells in the BLA is necessary for the expression of a conditioned response. Thus, representations of sensory stimuli ultimately connect to an US representation in the BLA to elicit both innate and learned responses.
Results
An appetitive and an aversive unconditioned stimulus are represented by distinct but intermingled subpopulations in the BLA
In initial experiments, we examined the neural representations of two opposing USs in the BLA. Footshock, which elicits defensive behaviors, was used as an aversive US (LeDoux, 2000). Intraperitoneal injection (i.p.) of nicotine at a dose that elicits a conditioned place preference (Supplemental Figure 1) was used as an appetitive US (Merritt et al., 2008). Footshock or i.p. injection of nicotine resulted in the expression of c-fos, an activity-dependent gene, in ~6% of neurons in the BLA (shock treated 5.94±0.43%, n=6; nicotine treated 6.13±0.46%, n=6). Spatial segregation of neurons responsive to footshock or nicotine was not observed. Control experiments with untreated animals or animals treated with an i.p. injection of saline revealed that less than 1% of neurons stained for c-fos (untreated 0.88±0.14%, n=6; i.p. saline 0.83±0.13% n=8). The observation that appetitive and aversive USs result in significant increases in c-fos-expressing cells with little background c-fos staining suggested the use of the c-fos promoter to drive the expression of the photoactivatable cation channel channelrhodopsin after US exposure. This genetic strategy allowed us to mark and manipulate the activity of neurons that respond to either an appetitive or aversive US.
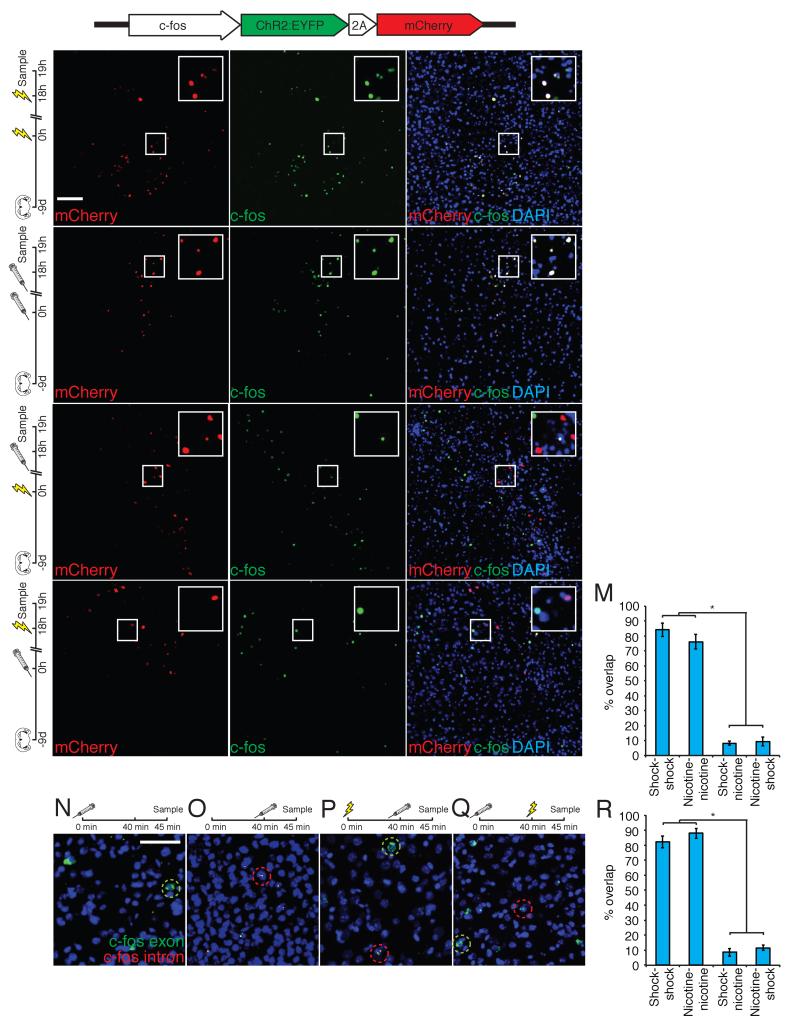
We next asked whether footshock and nicotine administration activated different neurons in the BLA. We injected a bicistronic lentiviral vector encoding channelrhodopsin2 fused to enhanced yellow fluorescent protein (ChR2-EYFP) and nuclear-targeted mCherry under the control of the c-fos promoter into the BLA (c-fos:ChR2-EYFP-2A-mCherry). Nine days later animals were exposed to footshock or nicotine. Endogenous c-fos expression was observed 1 hour after footshock or nicotine exposure, whereas mCherry expression was not detected at this time. Conversely, animals sampled 19 hours after footshock or nicotine exhibit mCherry expression in the BLA but endogenous c-fos expression is not observed (Supplemental Figure 2).
This temporal separation in the expression of lentiviral mCherry and endogenous c-fos allowed us to identify the populations of neurons activated by sequential stimuli in the same animal and to ask whether the expression of mCherry is a faithful reporter of endogenous c-fos activity. Animals were injected with virus, treated with 2 sessions of 20 footshocks separated by 18 hours, and sacrificed 1 hour later allowing us to determine the overlap of cells expressing mCherry and c-fos. Staining for endogenous c-fos and mCherry revealed that 84.07±4.46% of mCherry+ cells also expressed c-fos (n=6, Figure 1A-C, M). Mice were also injected with virus and treated with 2 sessions of i.p. nicotine administration separated by 18 hours and sacrificed 1 hour later. 76.02±4.90% of the mCherry+ cells also expressed c-fos (n=5, Figure 1D-F, M). Moreover, the expression of mCherry and ChR2 was observed in 47.73±4.48% of the cells expressing endogenous c-fos. Therefore, expression of mCherry faithfully identifies a population of neurons responsive to the different USs: 80% of mCherry expressing cells after US exposure are c-fos+, and 50% of c-fos+ cells also express mCherry.
Figure 1. Anatomically distinct, yet intermingled populations of cells in the BLA respond to appetitive and aversive unconditioned stimuli.
A-C: Animals injected with lentivirus expressing c-fos:ChR2-EYFP-2A-mCherry were exposed to 2 footshock treatments separated by 18 hours and stained for mCherry (A), c-fos (B), and merged (C). D-F: Animals injected with lentivirus expressing c-fos:ChR2-EYFP-2A-mCherry were treated with 2 i.p. nicotine injections separated by 18 hours and stained for mCherry (D), c-fos (E), and merged (F). G-I: Animals injected with lentivirus expressing c-fos:ChR2-EYFP-2A-mCherry were treated with footshock followed by nicotine 18 hours later and stained for mCherry (G), c-fos (H), and merged (I). J-L: Animals injected with lentivirus expressing c-fos:ChR2-EYFP-2A-mCherry were treated with nicotine followed by footshock 18 hours later and stained for mCherry (J), and c-fos (K), and merged (L). M. Percent overlap ((mCherry+ + c-fos+)/mCherry+) of c-fos positive and mCherry positive neurons in the BLA (shock-shock 84.07±4.46, n=6; nicotine-nicotine 76.02±4.90, n=5; shock-nicotine 8.22±1.40, n=6; nicotine-shock 9.28±2.94, n=5. One-way ANOVA, F3,18=130.43, P<0.0001). N-Q: N. Animals exposed to nicotine treatment 45 minutes prior to sacrifice and stained for intronic c-fos RNA (nuclear, red) and exonic c-fos RNA (cytoplasmic, green). O. Animals exposed to nicotine treatment 5 minutes prior to sacrifice and stained for intronic c-fos RNA and exonic c-fos RNA. P. Animals exposed to footshock treatment 45 minutes prior to sacrifice and nicotine treatment 5 minutes prior to sacrifice and stained for intronic c-fos RNA and exonic c-fos RNA. Q. Animals exposed to nicotine treatment 45 minutes prior to sacrifice and footshock treatment 5 minutes prior to sacrifice and stained for intronic c-fos RNA and exonic c-fos RNA. R. Percent overlap (yellow/green) of c-fos intronic RNA positive neurons (nuclear red) with c-fos exonic RNA positive neurons (cytoplasmic green) (shock-shock 82.19±3.86%, n=3; nicotine-nicotine 87.93±3.29%, n=4; shock-nicotine 8.63±2.67%, n=4; nicotine-shock 11.62±1.75%, n=6. One-way ANOVA, F3,13=254.29, P<0.0001). Scale bars, 100μm. All error bars for all figures represent ± s.e.m. See also Figure S2.
We next examined whether USs of distinct valence activated different populations of BLA neurons. Animals were injected with c-fos:ChR2-EYFP-2A-mCherry, and exposed to footshock. Eighteen hours later nicotine was injected and animals were sampled 1 hour later. This protocol results in only 8.22±1.40% overlap of neurons expressing mCherry (presumably representing shock) and c-fos expressing neurons (presumably representing nicotine administration, n= 6, Figure 1G-I, M). Similarly, animals were injected with virus and exposed to nicotine. Eighteen hours later animals were treated with footshock and sampled 1 hour later. In these animals we observed 9.28±2.94% overlap of neurons expressing mCherry and c-fos expressing neurons (n=5, Figure 1J-L, M). Similar levels of overlap were observed in animals where the populations of cells activated by footshock and nicotine were examined using cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH, Guzowski et al., 2001) (shock-nicotine 8.63±2.67%, n=4; nicotine-shock 11.62±1.75%, n=6; Figure 1N-R). These data demonstrate that distinct but intermingled subpopulations of BLA neurons are activated by exposure to an aversive and appetitive US. We cannot distinguish whether these 2 neural representations reflect valence or simply different sensory qualities of the USs independent of valence. Nonetheless, we can define the valence of these representations by virtue of the behaviors they elicit upon activation. We therefore employed this lentiviral strategy to manipulate the activity of neurons responsive to specific unconditioned stimuli.
Photoactivation of US representations in the BLA elicits innate responses
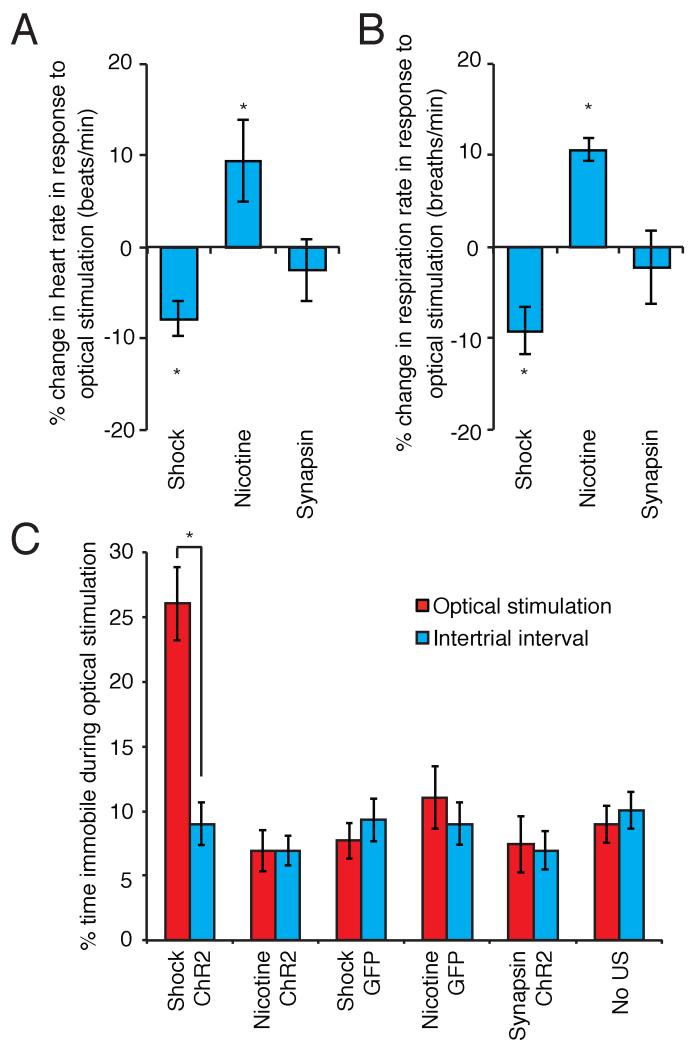
We asked whether BLA neurons that express ChR2 in response to an US could elicit valence-specific physiological and behavioral responses upon stimulation with light. Mice injected with the c-fos:ChR2-EYFP-2A-mCherry lentivirus were implanted with a guide cannula 250μm above the injection site. Nine days following surgery, animals were exposed to either footshock (shock-induced animals), or received an i.p. nicotine injection (nicotine-induced animals) to induce ChR2 and mCherry expression. This resulted in mCherry expression in ~3% neurons (shock-induced animals 3.01±0.44%, n=7; nicotine-induced animals 3.29±0.54%, n=6). This was significantly greater than the number of neurons expressing mCherry in animals not exposed to an US (0.82±0.13%, n=5. One way ANOVA F2,15=8.52, P<0.01). After 18 hours to allow ChR2 expression, a fiber-optic cable connected to a 473nm laser was positioned above the BLA for optical stimulation. Excitation of footshock-responsive neurons decreased both heart and respiration rate (n=5, Figure 2A, B) (Belkin, 1968 and Lang and Davis, 2006). Conversely, excitation of nicotine-responsive neurons increased heart and respiration rate (n=5, Figure 2A, B). In control experiments, we measured the effects on heart and respiratory rate upon photoactivation of a random population of BLA neurons. Mice were injected with a lentivirus expressing ChR2-EYFP-2A-mCherry under the control of the synapsin promoter to achieve a frequency of ChR2 expression similar to that observed in shock or nicotine treated mice infected with the c-fos:ChR2-EYFP-2A-mCherry lentivirus (3.48±0.64% of neurons expressing mCherry, n=7). Photoactivation of this random population of neurons did not elicit a change in heart or respiratory rate (n=6, Figure 2A, B). In vivo electrophysiological experiments as well as recordings in slice demonstrated that photostimulation of cells induced to express ChR2 by footshock or nicotine exposure elicits photocurrents and spiking (Supplemental Figure 3).
Figure 2. The exogenous activation of cells responsive to footshock and nicotine in the BLA is sufficient to elicit valence-specific physiologic and behavioral responses.
A. Percent change in heart rate from baseline in response to optical stimulation of footshock or nicotine responsive cells, or a random ensemble (shock −7.88±1.93% n=5; nicotine 9.49±4.52% n=5; synapsin −2.55±3.40%, n=6. Two-way ANOVA, group × optical stimulation interaction, F2,26=6.24, P<0.01). B. Percent change in respiration rate from baseline in response to optical stimulation of footshock or nicotine responsive cells, or a random ensemble (shock −9.20±2.55%, n=5; nicotine 10.63±1.21%, n=5; synapsin - 2.19±4.05%, n=6. Two-way ANOVA, group × optical stimulation interaction, F2,26=10.14, P<0.001). C. Percent of time spent freezing in response to optical stimulation compared to the intertrial interval (ITI) (shock ChR2 optical stimulation 26.05±2.83%, ITI 8.99±1.66% n=7; nicotine ChR2 optical stimulation 6.93±1.58%, ITI 6.94±1.44 n=6; shock GFP optical stimulation 7.70±1.37%, ITI 9.32±1.65, n=6; nicotine GFP optical stimulation 11.07±2.42%, ITI 9.02±1.62%, n=6; synapsin ChR2 optical stimulation 7.43±2.19%, ITI 6.95±1.46%, n=5; no US optical stimulation 8.96±1.42%, ITI 10.05±1.40, n=5. Two-way ANOVA, group × optical stimulation interaction, F5,58=8.28, P<0.0001). See also Figures S3 and S4.
Shock-induced animals also exhibited significantly elevated levels of freezing upon photostimulation, compared with either nicotine-induced animals, or with animals expressing ChR2 in a random population of neurons (shock 26.05±2.83%, n=7; nicotine 6.93±1.58%, n=6; synapsin 7.43±2.19%, n=5; Figure 2C). Animals that were injected but not exposed to an US failed to show freezing behavior to optical stimulation (No US 8.96±1.42%, n=5, Figure 2C). In addition, mice injected with a lentivirus encoding c-fos:GFP and treated with footshock or nicotine did not freeze in response to optical stimulation (shock GFP 7.70±1.37%, n=6; nicotine GFP 11.07±2.42%, n=6; Figure 2C). Control experiments demonstrate that the freezing behavior we observe results from activation of neurons responsive to footshock rather than activation of neurons that represent a rapidly formed contextual association (Fanselow, 1980, Supplemental Figure 4). Thus, photoactivation of US-responsive cells in the BLA elicits innate behavioral and physiological responses of different valence. We are unable to provide evidence of an innate behavioral response to the photoactivation of an appetitive US representation because it is difficult to conceive of a behavioral assay that reports an innate, as opposed to an instrumental, appetitive response.
Photoactivation of US representations in the BLA drives learning
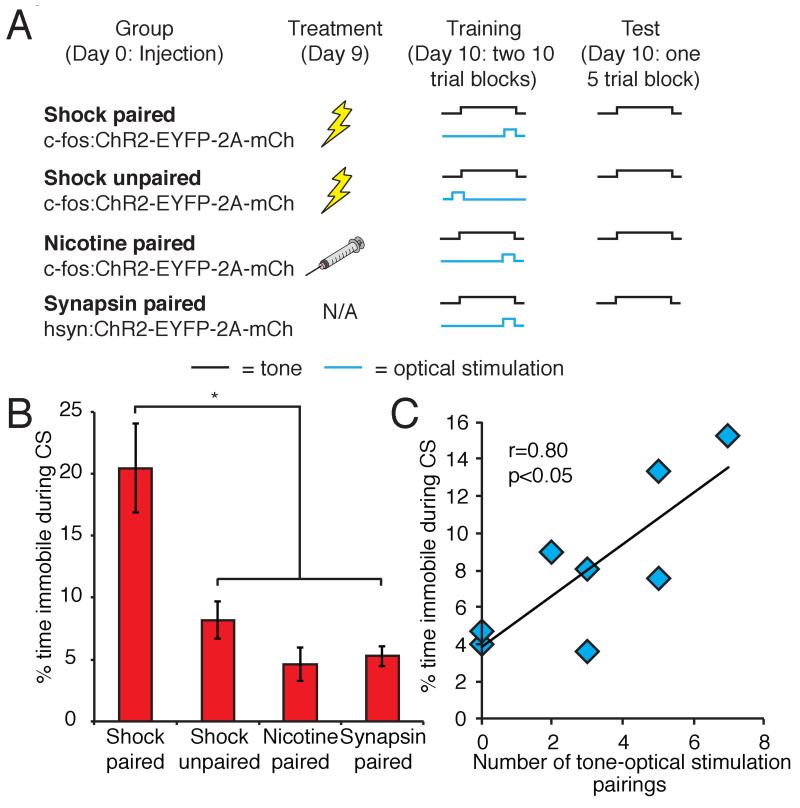
Rewarding and aversive USs can drive associative learning when paired with a CS. We therefore tested whether the activation of an ensemble of cells responsive to an US in the BLA, when temporally paired with a CS, results in valence-specific learning. We subjected mice to a modified fear-conditioning paradigm in which a tone served as the CS and tested whether optical stimulation of neurons responsive to footshock could serve as an US (Figure 3A). Animals were injected with lentivirus and treated with footshock or nicotine to induce ChR2 expression. The following day, shock-induced and nicotine-induced animals were placed into a behavioral testing chamber where they received 20 10-second tone presentations (CS), co-terminating with 2 seconds of optical stimulation of the BLA (US). A second group of shock-induced animals (shock unpaired) received 20 randomly timed presentations of the CS and US. Freezing behavior was then assessed during 5 presentations of the CS. Shock paired animals showed significantly more freezing in response to the CS compared to both shock unpaired and nicotine paired control animals (shock paired 20.46±3.59%, n=10; shock unpaired 8.19±1.51%, n=8; nicotine paired 4.62±1.38%, n=8, Figure 3B). Activation of a random population of BLA neurons did not drive aversive learning in animals trained with this fear conditioning protocol (synapsin 5.26±0.80% freezing, n=6, Figure 3B). Furthermore, shock unpaired animals that received random presentations of tone and optical stimulation showed freezing behavior that was significantly correlated with the number of chance paired presentations of the CS and optical stimulation (r=0.80, P<0.05, n=8, Figure 3C). Thus, selective optical reactivation of footshock-responsive cells in the BLA, when temporally paired with a neutral tone, can induce aversive learning. The levels of freezing observed using optical stimulation are, however, lower than those exhibited by animals exposed to a similar training protocol using footshock as the US (classical shock paired 55.53±6.14%, n=8; classical shock unpaired 19.85±3.11%, n=8).
Figure 3. The exogenous activation of footshock-responsive cells can act as an US in an auditory fear conditioning paradigm.
A. Modified fear conditioning paradigm. B. Percent time spent freezing in response to the auditory CS following fear conditioning using optical stimulation of the BLA as the US (shock paired 20.46±3.59%, n=10; shock unpaired 8.19±1.51%, n=8; nicotine paired 4.62±1.38%, n=8, synapsin paired 5.26±0.80%, n=6. One-way ANOVA, F3,28=9.74, P<0.0005). C. Correlation between number of tone-optical stimulation pairings and percent immobility to CS in shock unpaired animals.
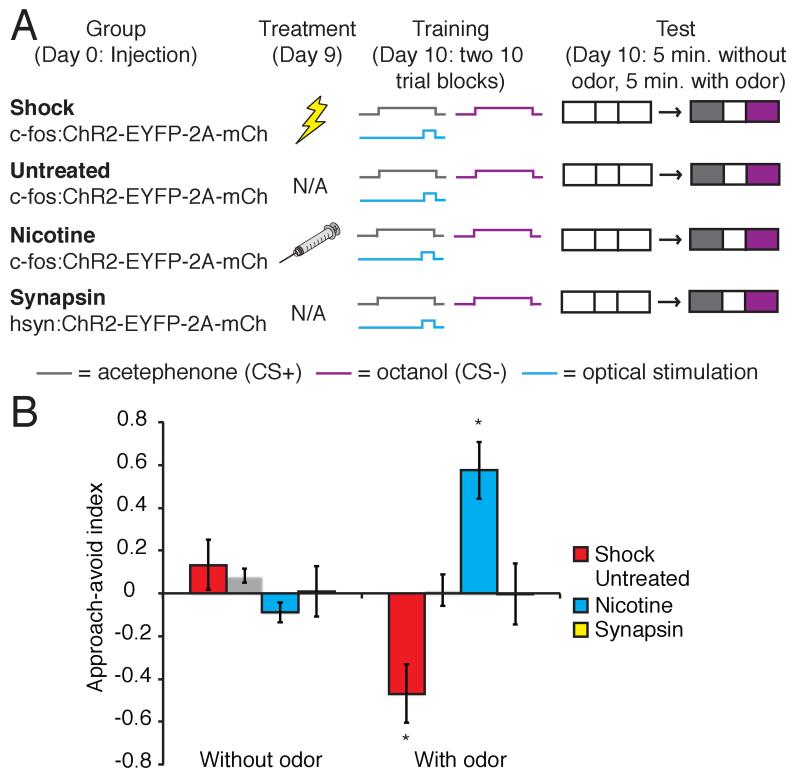
We next employed an odor-learning task to determine whether the photoactivation of an ensemble of nicotine-responsive cells could induce appetitive learning (Figure 4A). This experimental design also tested whether photoactivation of footshock-responsive cells could impart an aversive valence upon a neutral odor. Animals were injected with lentivirus and treated with either nicotine or footshock to induce ChR2 expression. Shock-induced and nicotine-induced animals were placed into a chamber where they received 20 presentations of 1% acetephenone (CS+) that co-terminated with optical stimulation of the BLA. As a control, animals also received 20 randomly interleaved presentations of 2% octanol (CS-). Mice were then placed in the center of a 3-compartment chamber. CS+ and CS-odors were infused from opposite ends of the apparatus. In the absence of odor, nicotine- and shock-induced animals, as well as untreated controls, spent equal amounts of time in the CS+ and CS-compartments (Figure 4B). Following odor delivery, shock-induced animals avoided of the CS+ compartment, while nicotine-induced animals approached the CS+ compartment. Animals not exposed to an US showed no compartment preference (Approach-avoid index: shock without odor 0.13±0.12, with odor −0.47±0.14, n=6; untreated without odor 0.08±0.03, with odor 0.02±0.07, n=6; nicotine without odor −0.09±0.05, with odor 0.57±0.13, n=6, Figure 4B). Animals injected with lentivirus expressing ChR2 under the control of the synapsin promoter to generate a random ensemble of ChR2 expressing neurons showed no compartment preference when trained and tested in the same manner (synapsin without odor 0.01±0.12, with odor 0.00±0.14, n=5, Figure 4B). The selective reactivation of BLA neurons responsive to footshock can therefore induce aversive learning across two sensory modalities, auditory and olfactory. Each modality can evoke behaviorally distinct defensive behaviors, freezing and avoidance. Moreover, reactivation of BLA neurons responsive to nicotine can drive appetitive learning about olfactory stimuli.
Figure 4. The exogenous activation of footshock- and nicotine-responsive cells can reinforce aversive and appetitive olfactory conditioning, respectively.
A. Behavioral training protocol for associative olfactory learning. B. Approach-avoid index (difference in time spent in CS+ and CS-compartments of a 3 compartment chamber, divided by the time spent in both compartments) of shock-induced, untreated and nicotine-induced animals trained to associate odor with optical stimulation of the BLA, as well as animals expressing ChR2 in a random population of BLA neurons (shock without odor 0.13±0.12, with odor −0.47±0.14, n=6; untreated without odor 0.08±0.03, with odor 0.02±0.07, n=6; nicotine without odor −0.09±0.05, with odor 0.57±0.13, n=6; synapsin without odor 0.01±0.12, with odor 0.00±0.14, n=5. Two-way ANOVA, group × conditioning interaction, F3,38=12.65, P<0.0005).
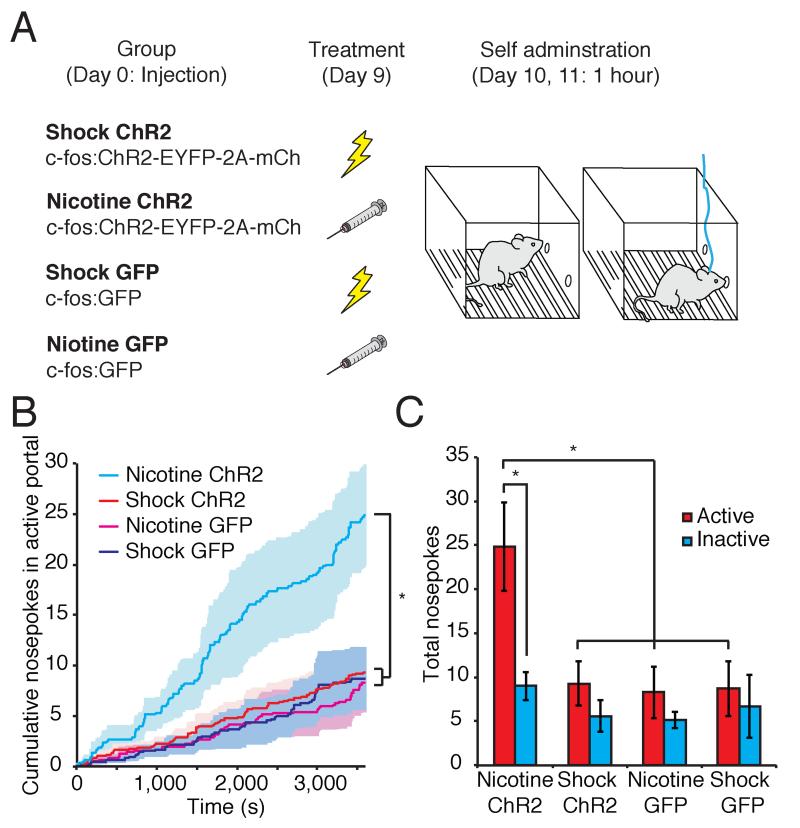
In addition to Pavlovian learning, unconditioned stimuli can reinforce operant behavior. We therefore asked whether US-responsive cells in the BLA could direct instrumental conditioning. Animals injected with lentivirus were exposed to either footshock or nicotine to induce ChR2 expression in BLA neurons. Shock-induced and nicotine-induced animals were placed into a chamber equipped with an active and an inactive portal for 1 hour. Nosepoke entry into the active portal, but not the inactive portal, resulted in 5 seconds of optical stimulation of the BLA (Figure 5A). On the second day of testing, nicotine-induced mice performed significantly more nosepokes into the active portal than the inactive portal (nicotine-ChR2 active 24.83±5.02, inactive 9.00±1.57, n=6). Footshock-induced mice and control animals injected with a virus encoding c-fos:GFP and exposed to footshock or nicotine showed a small, but statistically insignificant, bias towards the active portal (shock-ChR2 active 9.30±2.54, inactive 5.60±1.81, n=10; shock-GFP active 8.71±3.11, inactive 6.71±3.58, n=7; nicotine-GFP active 8.29±2.92, inactive 5.14±0.91, n=7; Figure 5B, C). Overall, the total number of nosepokes was 2.5 times greater in the nicotine-induced mice expressing ChR2 than in all other groups. These observations demonstrate that selective reactivation of neurons responsive to nicotine in the BLA can reinforce instrumental behavior. Thus BLA neurons encode behaviorally relevant information about the valence of unconditioned stimuli and the exogenous activation of these ensembles is sufficient to drive both Pavlovian and instrumental learning.
Figure 5. The exogenous activation of nicotine-responsive cells can reinforce instrumental conditioning.
A. Behavioral protocol for instrumental conditioning B. Average cumulative nosepokes for the active portal on the second day of testing in animals expressing ChR2 or GFP in footshock or nicotine responsive cells. Shading represents ± s.e.m. (One-way ANOVA, F3,26=5.08, P<0.01). C. Total nosepokes in the active and inactive portal on the second day of testing (nicotine-ChR2 active 24.83±5.02, inactive 9.00±1.57, n=6; shock-ChR2 active 9.30±2.54, inactive 5.60±1.81, n=10; nicotine-GFP active 8.29±2.92, inactive 5.14±0.91, n=7; shock-GFP active 8.71±3.11, inactive 6.71±3.58, n=7. Two-way ANOVA, group F3,52=5.08, P<0.01, portal F1,52=8.03, P<0.01).
Learning connects CS and US representations in the BLA
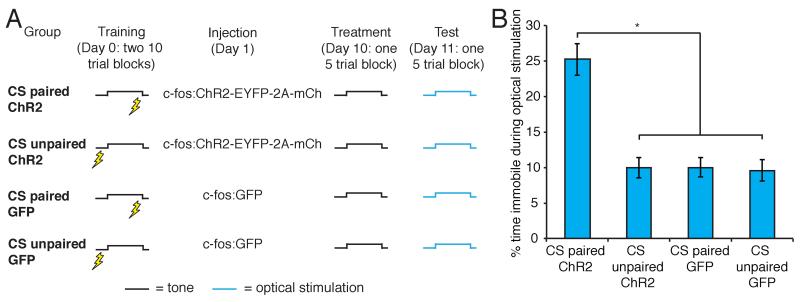
These data suggest a concise circuit in which CS-responsive cells connect with US-responsive neurons in the BLA such that this connection is only capable of driving behavior after learning. We therefore devised a strategy to express ChR2 in CS-responsive neurons in the BLA to test whether these neurons can elicit behavior after conditioning (Figure 6A). One group of animals, CS paired, received 20 presentations of a tone (CS) that co-terminated with footshock. A second group of animals, CS unpaired, received random presentations of the CS and footshock. Both groups were then injected with lentivirus expressing c-fos:ChR2-EYFP-2A-mCherry; 9 days later, animals received 5 presentations of the CS. Expression of ChR2 in the BLA was significantly elevated in CS paired compared to CS unpaired animals (% neurons expressing mCherry: CS paired 4.85±0.52%, n=9; CS unpaired 2.19±0.28%, n=10. Unpaired t-test, P<0.001). As expected, CS paired animals showed significantly elevated freezing in response to the CS compared to CS unpaired animals (CS paired 59.84±8.58%, n=9; CS unpaired 14.92±1.86%, n=10. Unpaired t-test, P<0.001). CS paired animals showed significantly elevated freezing levels in response to optical stimulation compared to CS unpaired controls (CS paired 25.22±2.25%, n=9; CS unpaired 9.93±1.40%, n=10, Figure 6B). Animals injected with a lentivirus expressing c-fos:GFP after training did not freeze upon optical stimulation of the CS representation (CS paired GFP 9.98±1.36%, n=6; CS unpaired GFP 9.59±1.50%, n=6; Figure 6B). Thus, a population of CS-responsive cells exists in the BLA after learning, and activation of this population can elicit learned behavior.
Figure 6. The exogenous activation of a learned aversive CS representation can drive freezing behavior, whereas the exogenous activation of an unlearned CS representation cannot.
A. Behavioral protocol for the reactivation of learned and unlearned CS representations in the BLA. B. Percent of time spent freezing in response to optical stimulation of the CS representation in the BLA (CS paired ChR2 25.22±2.25%, n=9; CS unpaired ChR2 9.93±1.40%, n=10; CS paired GFP 9.98±1.36%, n=6; CS unpaired GFP 9.59±1.50%, n=6. One-way ANOVA, F3,26=20.10, P<0.00001).
We next asked whether CS-responsive cells mediate learned behavior through the activation of US-responsive cells in the BLA. Animals were trained to associate a tone with footshock and the following day mice were injected with c-fos:ChR2-EYFP-2A-mCherry. Nine days later animals were exposed to footshock to induce mCherry expression and the following day they received 5 presentations of the auditory CS to induce endogenous c-fos expression. Animals were sacrificed 1 hour later and the overlap of endogenous c-fos and mCherry was calculated ((mCherry+ + c-fos+)/mCherry+). Mice that received paired training demonstrated higher levels of overlap than unpaired controls (CS paired 34.76±2.75%, n=6; CS unpaired 16.23±3.33%, n=6. Unpaired t-test, P<0.05). Thus, CS-activated neurons (expressing endogenous c-fos) overlap with shock-responsive neurons (expressing mCherry) and this overlap is 2 fold greater after learning.
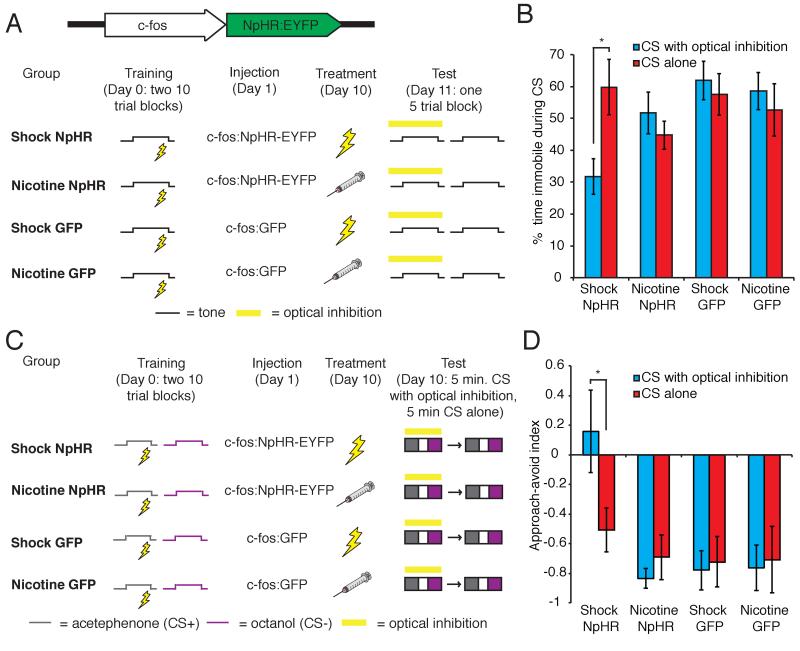
These data suggest that a CS ensemble connects with an US representation in the BLA and this US representation may be necessary for the expression of a conditioned response. Mice were therefore trained with 20 presentations of a 10 second tone that co-terminated with 2 seconds of footshock (Figure 7A). The following day, a lentivirus expressing the neural silencer halorhodopsin (NpHR) fused to EYFP under the control of the c-fos promoter (c-fos:NpHR-EYFP) was injected into the BLA of both brain hemispheres. Nine days later, animals were treated with either footshock or nicotine to induce NpHR in US-responsive cells (Supplemental Figure 5). The next day a fiber optic cable connected to a 593nm laser was positioned above each BLA. Animals then received 5 presentations of the auditory CS with photostimulation of NpHR, followed by 5 presentations of the auditory CS without photostimulation. In the absence of optical silencing, footshock-treated animals demonstrated comparable levels of freezing to classically fear conditioned animals (shock NpHR without light 59.84±8.72%, n=6; CS paired 59.84±8.58%, n=10). However, optical inhibition of footshock-responsive cells attenuated freezing in response to the CS (shock NpHR with light 31.70±5.62%, n=6, Figure 7B). Freezing was not completely abolished, which may be due to incomplete optical silencing or the existence of parallel pathways for fear conditioning.
Figure 7. Learning connects auditory and olfactory CS representations to US-responsive neurons in the BLA.
A. Behavioral protocol for the silencing of US-responsive cells during auditory CS presentation. B. Percent immobility in response to the CS in the presence and absence of optical inhibition of footshock and nicotine-responsive cells (shock NpHR with yellow light 31.75±5.62%, without yellow light 59.84±8.72%, n=6; nicotine NpHR with yellow light 51.70±6.54%, without yellow light 44.73%±4.45%, n=9; shock GFP with yellow light 61.92±6.06%, without yellow light 57.59±6.55%, n=6; nicotine GFP with yellow light 58.59±5.84%, without yellow light 52.64±8.18%, n=6. Two-way ANOVA, group × optical inhibition interaction, F3,46=3.16, P<0.05). C. Behavioral protocol for the silencing of US-responsive cells during olfactory CS presentation. D. Approach-avoid index in the presence and absence of optical inhibition of footshock and nicotine-responsive cells (shock NpHR with yellow light 0.16±0.28, without yellow light −0.51±0.15, n=6; nicotine NpHR with yellow light - 0.83±0.07, without yellow light −0.69±0.15, n=6; shock GFP with yellow light - 0.78±0.13, without yellow light, −0.72±0.17, n=6; nicotine GFP with yellow light - 0.76±0.15, without yellow light −0.71±0.22, n=6. Two-way ANOVA, group F3,40=5.36, P<0.005). See also Figures S5 and S6.
In control experiments, nicotine-treated animals showed equal levels of freezing in response to the CS in the presence or absence of optical inhibition (nicotine NpHR with light 51.70±6.54%, without light 44.73±4.45%, n=9, Figure 7B). Similarly, animals injected with a lentivirus expressing c-fos:GFP and treated with either footshock or nicotine showed equal levels of freezing in response to the CS with or without optical inhibition (shock GFP with light 61.92±6.06%, without light 57.59±6.55%, n=6; nicotine GFP with light 58.59±5.84%, without light 52.64±8.19%, n=6; Figure 7B). Whole cell electrophysiological recordings in slice preparations confirmed that photostimulation of cells induced to express NpHR by footshock or nicotine exposure can inhibit spiking elicited by current injection (Supplemental Figure 6). These experiments demonstrate that an auditory CS activates a US representation in the BLA to generate learned behavior.
We next asked whether cells responsive to an olfactory CS also mediate learned behavior through the activation of US-responsive neurons. Animals were placed into a chamber where they received 20 presentations of 10 seconds of 1% acetephenone (CS+) that coterminated with 2 seconds of footshock. Animals also received 20 randomly interleaved presentations of 2% octanol (CS-). The following day, lentivirus expressing c-fos:NpHR-EYFP was injected into the BLA of both brain hemispheres. Nine days later animals were treated with footshock or nicotine to induce NpHR expression in US-responsive neurons. The next day animals were placed in the center of a 3-compartment chamber. CS+ and CS-odors were infused from opposite ends of the apparatus. Animals explored the chamber for 5 minutes with optical stimulation of NpHR, followed by 5 minutes without optical stimulation (Figure 7C). In the absence of optical silencing, animals expressing NpHR in footshock-responsive neurons avoided the CS+ odor. However, in the presence of optical silencing, avoidance of the CS+ was abolished (approach avoid index: shock NpHR with light 0.16±0.28, without light −0.51±0.15, n=6, Figure 7D). In control experiments, nicotine-treated animals showed no reduction in avoidance of the CS+ upon optical inhibition (nicotine NpHR with light −0.83±0.07, without light −0.69±0.15, n=6, Figure 2D). Similarly, animals injected with c-fos:GFP and treated with either footshock or nicotine showed equal levels of avoidance of the CS+ in the presence or absence of yellow light (shock GFP with light −0.78±0.13, without light −0.72±0.17, n=6; nicotine GFP with light −0.76±0.15, without light −0.71±0.22, n=6, Figure 7D). These data indicate that the activity of cells responsive to the entraining US is necessary for the expression of conditioned responses to CSs of two different modalities. Thus the expression of learned behavior requires the projection of CS inputs onto US representations in the BLA.
Discussion
Stimuli that possess inherently rewarding or aversive qualities elicit emotional responses and also induce learning by imparting valence upon neutral sensory cues (Lang and Davis, 2006, Pavlov, 1924, Rosen, 2004, Schultz, 2001 and Seymour and Dolan, 2008). We used a genetic strategy to identify and manipulate the representations of innately rewarding and aversive stimuli in the BLA. Our experiments demonstrate that the activation of these representations can generate innate physiological and behavioral responses and can also reinforce Pavlovian and operant learning. Furthermore, the convergence of a CS representation onto an US ensemble in the BLA is required for the expression of learned behavior. These data suggest that US representations in the amygdala link representations of sensory stimuli to appropriate behavioral output.
The US Representation
Electrophysiological studies have demonstrated that neurons in the BLA respond to appetitive and aversive USs (Belova et al., 2007, Bermudez and Schultz, 2010, Livneh and Paz, 2012, Muramoto et al., 1993, Paton et al., 2006, Romanski et al., 1993, Uwano et al., 1995 and Wolff et al., 2014). Our data demonstrate that an appetitive and an aversive US activate distinct representations in the BLA. Photoactivation of the aversive US ensemble elicits innate responses and also reinforces aversive learning. Activation of this US representation is also necessary for the expression of a conditioned response. Representations of USs are likely to reside in multiple brain structures. Our data imply that the representation in the BLA participates in the innate responses to an US and the same representation is an essential component of the neural circuit that mediates learned responses. We emphasize, however, that we have not demonstrated the necessity of the US representation in the BLA for the generation of innate behaviors.
The observation that the activation of valence-specific representations elicits different behavioral responses poses the question as to whether different USs of the same valence activate the same or different representations in the BLA. Innate behaviors are complex and consist of multiple components (Lang and Davis, 2006 and Rosen, 2004). The response to different aversive USs may therefore result from the activation of different representations that elicit subtly different behaviors. Consistent with this idea, lesion experiments suggest that the BLA encodes information about the sensory quality of USs (Blundell et al., 2001, Corbit and Balleine, 2005 and Balleine and Killcross, 2006). This would imply that the BLA contains multiple, distinct representations encoding different USs of each valence, and each of these representations connects to US-specific output circuitry.
We have identified distinct representations of unconditioned stimuli in the BLA that can generate behaviors of opposing valence. In one model of BLA circuitry, these distinct subpopulations of neurons are determined to receive inputs of specific valence and project to different downstream targets that elicit appropriate behavioral responses. Consistent with this model, activation of different populations of BLA neurons or their distinct projections can elicit valence-specific responses in the absence of US presentation (Felix-Ortiz et al., 2013, Kim et al., 2013, Namburi et al., 2015, Stuber et al., 2011 and Tye et al., 2011). An alternative model proposes that cells in the BLA do not possess an inherent valence and only acquire valence after experience. In this model, each BLA neuron would be connected to both appetitive and aversive outputs. US exposure activates an arbitrary subset of these neurons and potentiates valence-specific outputs appropriate for a given US. At present, we cannot distinguish among these alternative models for the origin of the valence-specific neuronal populations we have identified.
Extensive evidence has established the importance of the BLA in aversive learning whereas the role of the BLA in appetitive learning and behavior is more nuanced (Balleine and Killcross, 2006, Everitt et al., 2003 and LeDoux, 2000). Our results demonstrate that activation of the representation of an appetitive US in the BLA can elicit innate responses and can drive both instrumental and Pavlovian conditioning. Consistent with this finding, instrumental responses to appetitive conditioned stimuli require the BLA (Ambroggi et al., 2008 and Stuber et al., 2011). However, lesion and pharmacological inactivation studies indicate that the BLA is not necessary for the formation of simple CS-appetitive US associations (Hatfield et al., 1996 and Holland, 1997). These data suggest the presence of multiple representations of an appetitive US in the brain capable of eliciting multiple forms of appetitive behavior.
The CS Representation
We have shown that the activation of US-responsive neurons in the BLA can drive conditioning of auditory and olfactory CSs. Electrophysiological studies have identified neurons in the BLA responsive to CSs of all sensory modalities (Herry et al., 2008, Livneh and Paz, 2012, Paton et al., 2006, Schoenbaum et al., 1998, Shabel and Janak, 2009 and Uwano et al., 1995), and neurons have been observed that respond to both CSs and USs (Barot et al., 2008, Belova et al., 2008, Paton et al., 2006 and Romanski et al., 1993). Moreover, appetitive and aversive learning modulates the activity of CS-responsive cells in the amygdala (Morrison et al., 2011, Paton et al., 2006, Quirk et al., 1995, Rogan et al., 1997, Rosenkranz and Grace, 2002 and Tye et al., 2008) and this activity correlates with behavioral output (Belova et al., 2008, Repa et al., 2001). We have shown that optogenetic activation of an auditory CS representation generated after learning can elicit an appropriate behavioral response. In addition, we demonstrate that the projection of a CS representation onto an US ensemble in the BLA is required for the expression of learned behavior. These findings are in accord with models in which CS-US pairing results in the Hebbian potentiation of CS inputs onto US representations in the BLA (Holland, 1990, Johansen et al., 2011, Maren and Quirk, 2004, Pape and Paré, 2010 Pickens and Holland, 2004, Rescorla, 1988 and Sah et al., 2008).
We have also shown that an olfactory CS connects to US representations in the BLA to generate learned behavior, indicating that olfactory conditioning may utilize the same circuit mechanisms as those proposed for auditory fear conditioning. In olfaction each odor activates a distinct ensemble of neurons in piriform cortex and each unique ensemble is capable of serving as a CS (Choi et al., 2011, Illig and Haberly, 2003, Rennaker et al., 2007 and Stettler and Axel, 2009). Piriform cortex projects directly to the BLA (Luskin and Price, 1983 and Schwabe et al., 2004) and we demonstrate that an US representation in the BLA is essential for the expression of learned olfactory behavior. These experiments suggest that an odor representation in piriform cortex must ultimately connect with US representations in the BLA, extending an olfactory circuit responsible for odor conditioning from the nose to the BLA.
Unconditioned stimuli are likely to elicit innate behavioral and physiological responses through determined neural circuits that have emerged over long periods of evolutionary time. Most sensory stimuli, however, have no inherent meaning and only generate responses upon learning during the life of an organism. An unconditioned stimulus can therefore elicit innate responses and also drive learning about neutral stimuli. In all sensory modalities, brain areas proximal to the amygdala, such as sensory cortices and thalamic nuclei, provide the initial representation of both conditioned and unconditioned stimuli. These sensory representations must ultimately engage neural circuits that produce valence-specific innate and learned responses. We have identified populations in the BLA that are both responsive to conditioned and unconditioned stimuli and are able to elicit valence-specific responses. These populations therefore serve to link earlier stage sensory representations to neural circuits that generate appropriate emotional responses.
Experimental Procedures
Constructs and viruses
Codon-optimized lentiviral vector expressing ChR2-EYFP under the control of the human synapsin promoter was a kind gift from Dr. Karl Deisseroth. To generate the hsyn:ChR2-EYFP-2A-mCherry construct, ChR2-EYFP was replaced by codon optimized ChR2-EYFP-T2A-mCherry (synthesized by Genewiz, Inc.). To generate the c-fos:ChR2-EYFP-2A-mCherry construct, a 720 nucleotide fragment of the c-fos promoter (residues 85493280-85473999 of mus musculus chromosome 12; ascension NC_000078.6) replaced the synapsin promoter. To generate the c-fos:NpHR-EYFP construct, NpHR-EYFP was PCR amplified from pAAV-EF1a-DIO-eNpHR 3.0-EYFP (also a gift from Dr. Karl Deisseroth) and this was used to replace ChR2-EYFP-T2A-mCherry in the c-fos:ChR2-EYFP-2A-mCherry construct. c-fos:GFP was generated by replacing the ChR2-EYFP-2A-mCherry construct with a codon optimized EGFP construct (synthesized by Genewiz, Inc.). Lentiviruses for in vivo injection were produced as previously described (Zhang et al., 2010).
Experimental subjects and stereotactic surgery
Adult (25-30g) male C57BL/6J mice (Jackson laboratory) were group-housed until surgery. Animals were anaesthetized with ketamine/xylazine (100mgkg−1 or 10mgkg−1, respectively, Henry Schein) and placed in a stereotactic frame (Kopf Instruments). Custom-made microinjection needles (Drummond) were then inserted (coordinates from Bregma: −1.55AP, +3.3ML, −4.75 DV) and each BLA was injected with 1μl of lentivirus over 0.5μm (from −4.75DV to −4.25DV). A 6mm guide cannula (Plastics One) was placed 250μm above the virus injection site and fixed in place using a small amount of dental cement (Parkell Inc.). Buprenorphine (0.05mgkg−1, Henry Schein) was administered. As exclusion criteria, we only included mice with viral expression confined to the BLA, and cannula placement above the BLA (Supplemental Figure 7). All experiments were conducted according to approved protocols at Columbia University.
Footshock and nicotine treatment
Footshock-treated animals were removed from their homecage and placed into a standard Med-Associates operant chamber equipped with a grid floor and aversive stimulator. Animals received 20 1.5mA footshocks over 10 minutes before being returned to their homecage. Nicotine treated animals were removed from their homecage and administered an intraperitoneal (i.p.) injection of nicotine hydrogen tartrate (0.7mgkg−1 free base, Sigma-Aldrich), prepared in saline, before being returned to their homecage. Both unconditioned stimuli were used at higher intensities than were used in studies that have previously reported little induction of c-fos expression in the BLA in response to either footshock or nicotine (Knapska et al., 2007).
Further methods, including behavioral assays, electrophysiological protocols and histological procedures, can be found in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
We thank D. Paré and L. Abbott for critical comments of the manuscript; K. Deisseroth for ChR2 and NpHR reagents; Monica Mendelsohn and Nataliya Zabello for help with mice; Phyllis Kisloff for assistance in preparing the manuscript; and Miriam Gutierrez for laboratory support. This work was supported by the Howard Hughes Medical institute and the Marthers Foundation. E.C.S. was supported by a Jane Coffin Childs fellowship. C.D.S. was supported by NIMH (R01-MH082017).
Footnotes
Author Contributions
F.G., E.C.S., C.D.S and R.A. conceived the project, participated in its development, wrote the manuscript and participated in all data analysis. F.G. and E.C.S. performed all experiments. S.A. and B.C.B. performed cloning, lentivirus production, mouse husbandry and histology. J.M.S., E.L. and J.A.G. performed in vivo electrophysiology and analysis. M.J.R. performed ex vivo electrophysiology and analysis.
Conflict of Interest The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Price J, Pitkanen A, Carmichael S. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J, editor. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Barot SK, Yasuhiro K, Clark EW, Bernstein IL. Visualizing stimulus convergence in amygdala neurons during associative learning. Proc Natl Acad Sci USA. 2008;105:20959–20963. doi: 10.1073/pnas.0808996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin DA. Bradycardia in response to threat. American Zoologist. 1968;8:775. [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. Reward magnitude coding in primate amygdala neurons. J Neurophysiol. 2010;104:3424–3432. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforce representation in rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Anatomic and physiologic substrates of emotion in an animal model. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of post-shock freezing. Pav J Bio Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci USA. 1994;91:11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Worley PF. Cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0108s15. Chapter 1: Unit 1.8. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Hans JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of the basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforce devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Swtiching on and off fear by distinct neural circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Holland PC. Brain mechanisms for changes in processing of conditioned stimuli in Pavlovian conditioning: implications for behavior theory. Anim Learn Behav. 1997;25:373–399. [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, et al. Diverging neural pathways assemble a behavioral state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animals and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Livneh U, Paz R. Aversive-bias and stage-selectivity in neurons of the primate amygdala during acquisition, extinction, and overnight retention. J Neurosci. 2012;32:8592–8610. doi: 10.1523/JNEUROSCI.0323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Saez A, Lau B, Salzman Different time course for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71:1127–1140. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Pickens CL, Holland PC. Conditioning and cognition. Neurosci Biobehav Rev. 2004;28:651–661. doi: 10.1016/j.neubiorev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513:426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Luthi A. Fear conditioning and long-term potentiation in the amygdala: what really is the connection? Ann NY Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Involvement of the amygdala in learning and memory: a critical review, with emphasis on anatomical relations. Behav Neurosci. 1985;99:342–380. doi: 10.1037//0735-7044.99.2.342. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Ebert U, Loscher W. The central piriform cortex: anatomical connections and anticonvulsant effect of GABA elevation in the kindling model. Neurosci. 2004;126:727–741. doi: 10.1016/j.neuroscience.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Janak PH. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc Natl Acad Sci USA. 2009;106:15031–15036. doi: 10.1073/pnas.0905580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Panak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Gründermann J, Tovote P, Krabbe S, Jacobson GA, Müller C, Herry C, Ehrlich I, Freidrich RW, Letzkus JJ, Lüthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.