Abstract
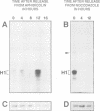
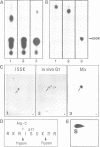
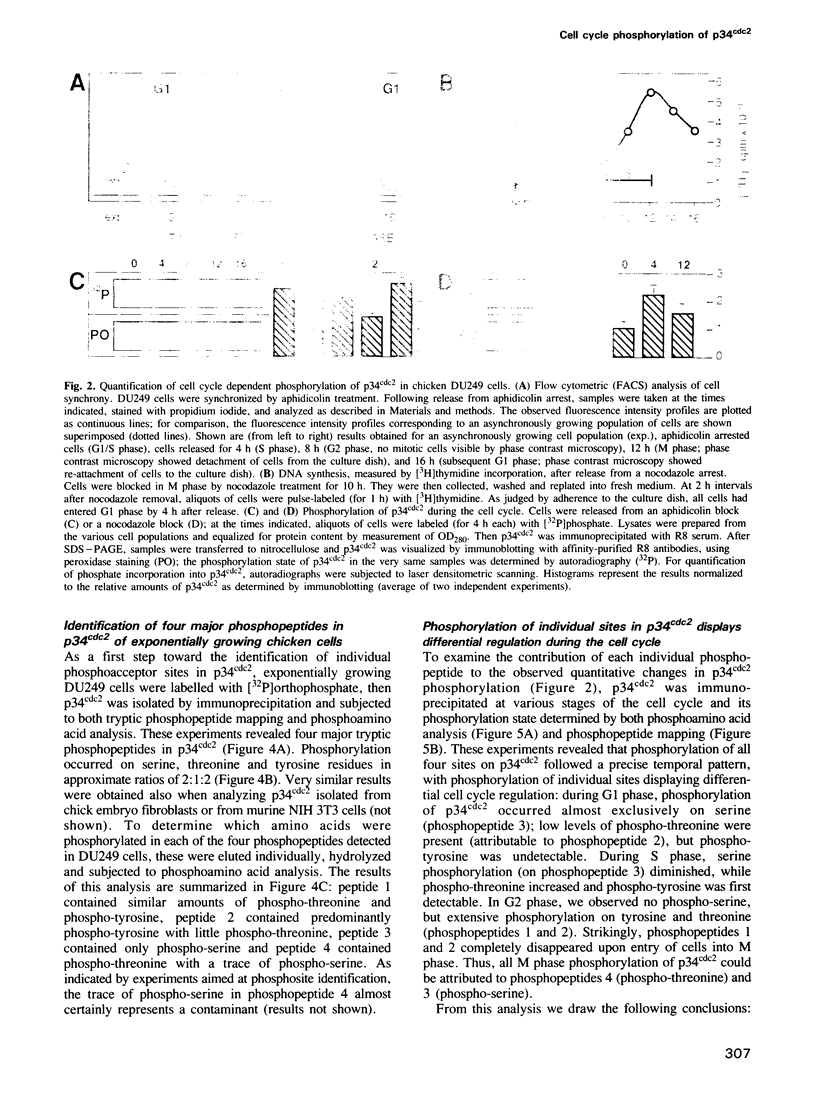
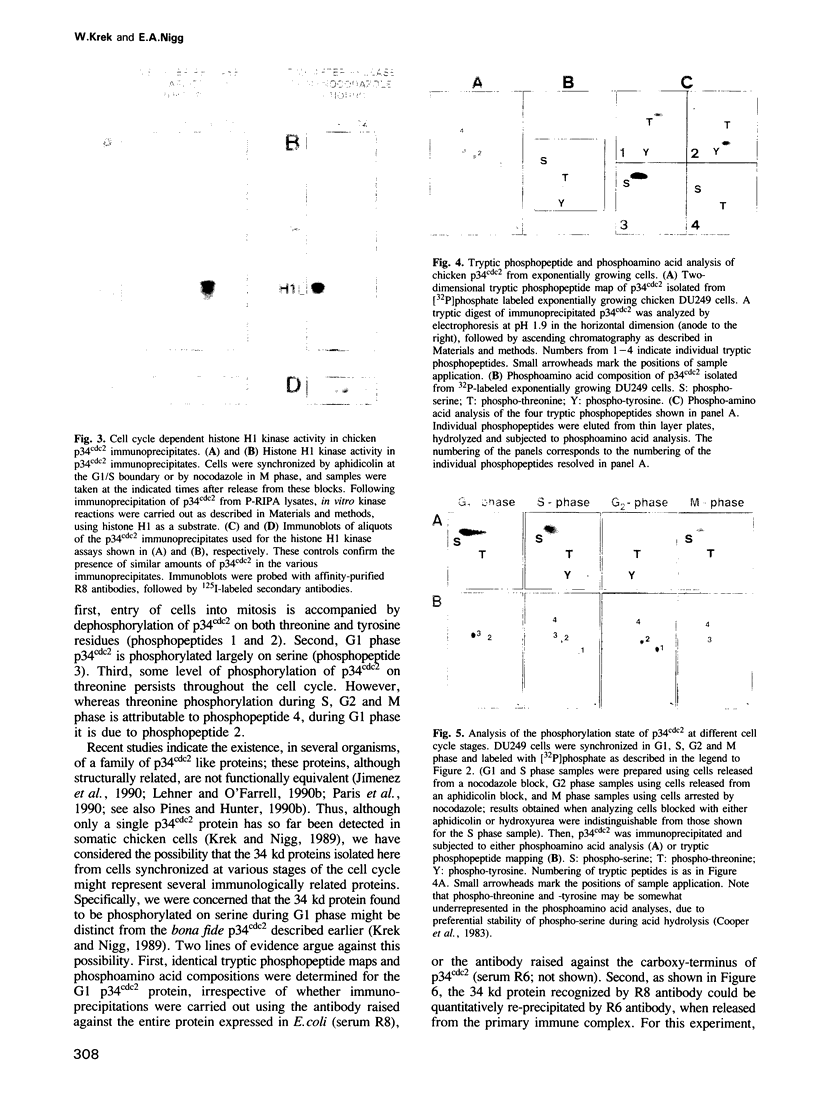
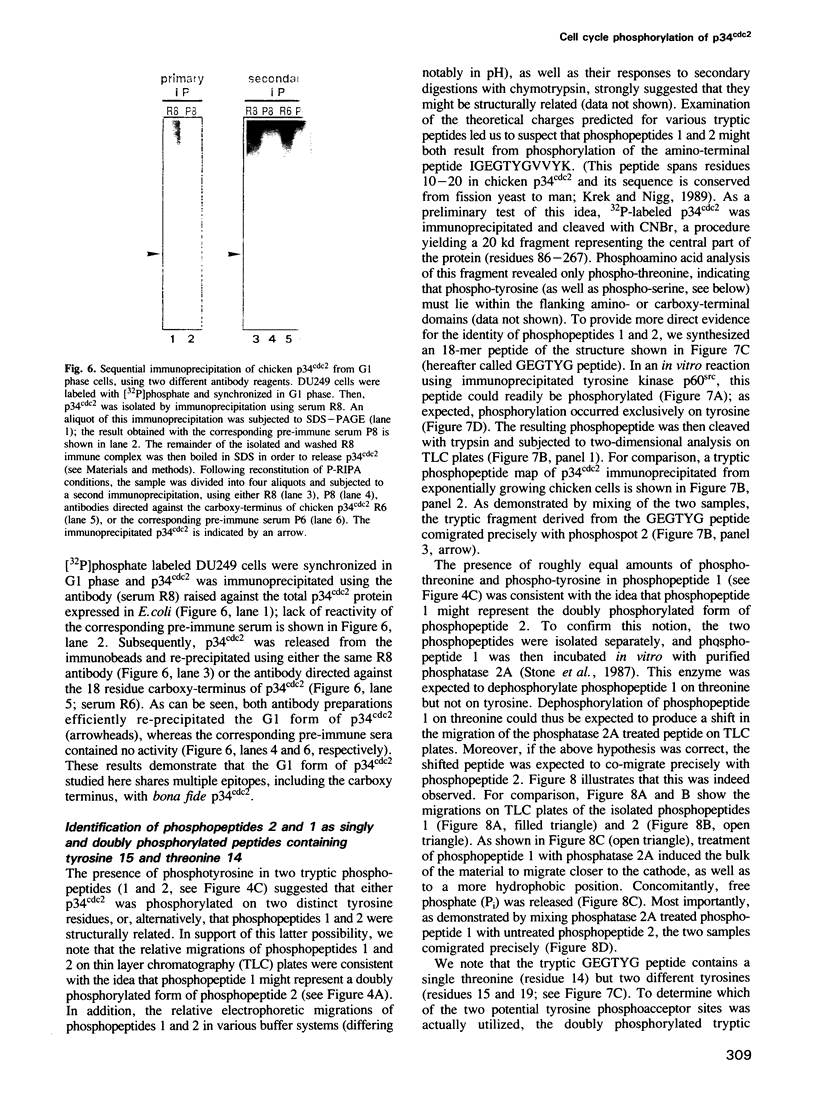
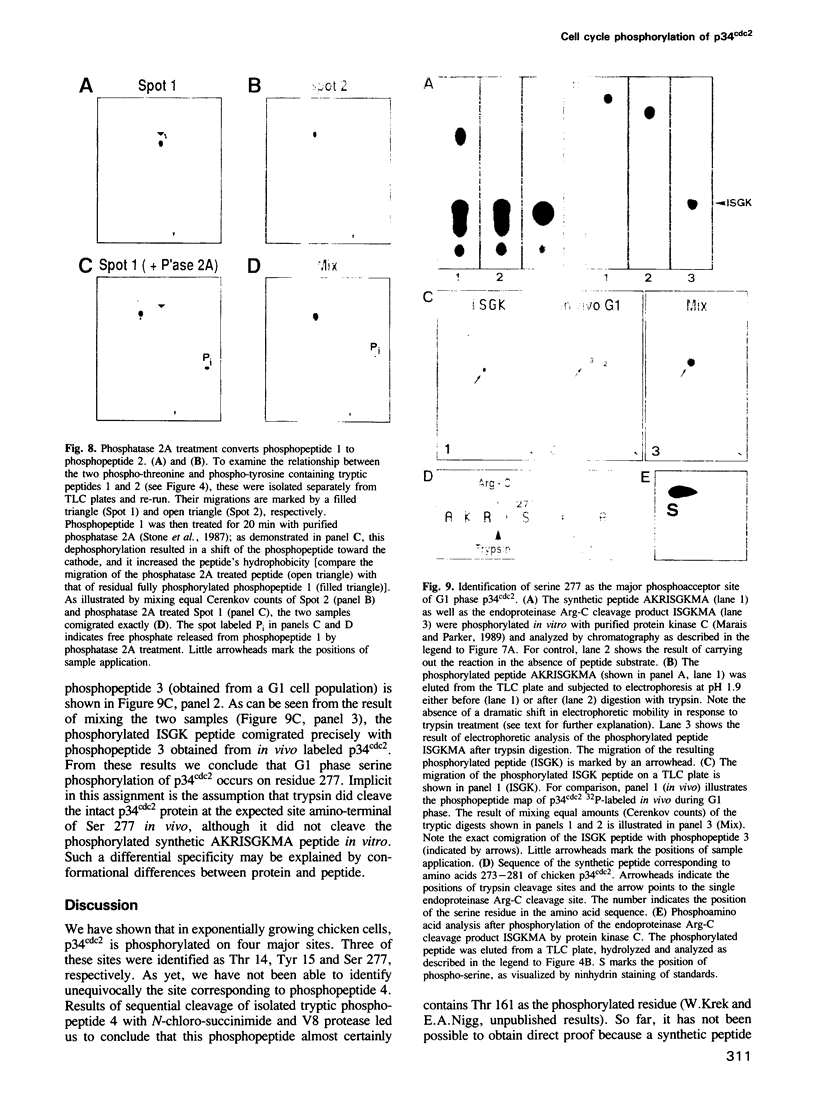
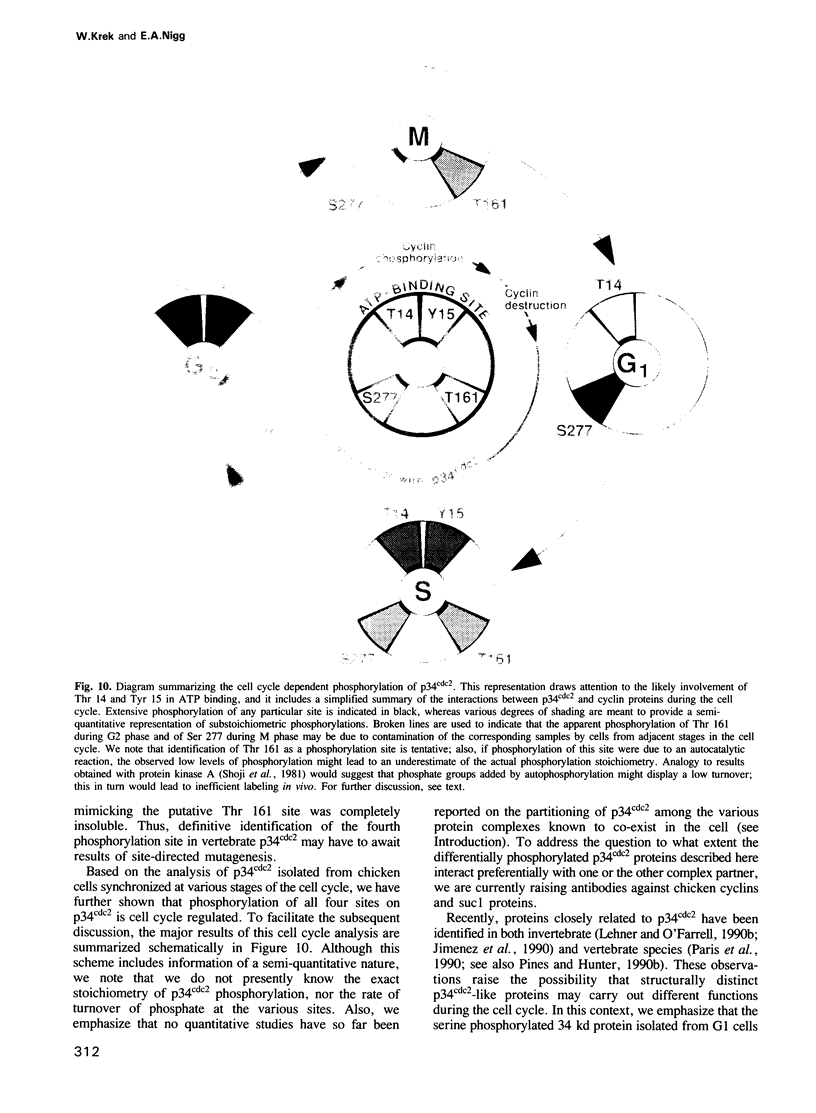
The cdc2 kinase is a key regulator of the eukaryotic cell cycle. The activity of its catalytic subunit, p34cdc2, is controlled by cell cycle dependent interactions with other proteins as well as by phosphorylation--dephosphorylation reactions. In this paper, we examine the phosphorylation state of chicken p34cdc2 at various stages of the cell cycle. By peptide mapping, we detect four major phosphopeptides in chicken p34cdc2; three phosphorylation sites are identified as threonine (Thr) 14, tyrosine (Tyr) 15 and serine (Ser) 277. Analysis of synchronized cells demonstrates that phosphorylation of all four sites is cell cycle regulated. Thr 14 and Tyr 15 are phosphorylated maximally during G2 phase but dephosphorylated abruptly at the G2/M transition, concomitant with activation of p34cdc2 kinase. This result suggests that phosphorylation of Thr 14 and/or Tyr 15 inhibits p34cdc2 kinase activity, in line with the location of these residues within the putative ATP binding site of the kinase. During M phase, p34cdc2 is also phosphorylated, but phosphorylation occurs on a threonine residue distinct from Thr 14. Finally, phosphorylation of Ser 277 peaks during G1 phase and drops markedly as cells progress through S phase, raising the possibility that this modification may contribute to control the proposed G1/S function of the vertebrate p34cdc2 kinase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990 Sep 7;62(5):855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- Booher R. N., Alfa C. E., Hyams J. S., Beach D. H. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989 Aug 11;58(3):485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Site-specific mutagenesis of cdc2+, a cell cycle control gene of the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1986 Oct;6(10):3523–3530. doi: 10.1128/mcb.6.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cross F., Roberts J., Weintraub H. Simple and complex cell cycles. Annu Rev Cell Biol. 1989;5:341–396. doi: 10.1146/annurev.cb.05.110189.002013. [DOI] [PubMed] [Google Scholar]
- D'Urso G., Marraccino R. L., Marshak D. R., Roberts J. M. Cell cycle control of DNA replication by a homologue from human cells of the p34cdc2 protein kinase. Science. 1990 Nov 9;250(4982):786–791. doi: 10.1126/science.2173140. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990 Oct;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Mendenhall M. D., Reed S. I. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol Cell Biol. 1989 May;9(5):2034–2041. doi: 10.1128/mcb.9.5.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hayles J., Beach D., Durkacz B., Nurse P. The fission yeast cell cycle control gene cdc2: isolation of a sequence suc1 that suppresses cdc2 mutant function. Mol Gen Genet. 1986 Feb;202(2):291–293. doi: 10.1007/BF00331653. [DOI] [PubMed] [Google Scholar]
- Hayles J., Nurse P. Cell cycle regulation in yeast. J Cell Sci Suppl. 1986;4:155–170. doi: 10.1242/jcs.1986.supplement_4.10. [DOI] [PubMed] [Google Scholar]
- Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989 Apr;1(2):268–274. doi: 10.1016/0955-0674(89)90099-9. [DOI] [PubMed] [Google Scholar]
- Jessus C., Ducommun B., Beach D. Direct activation of cdc2 with phosphatase: identification of p13suc1-sensitive and insensitive steps. FEBS Lett. 1990 Jun 18;266(1-2):4–8. doi: 10.1016/0014-5793(90)90002-c. [DOI] [PubMed] [Google Scholar]
- Jimenez J., Alphey L., Nurse P., Glover D. M. Complementation of fission yeast cdc2ts and cdc25ts mutants identifies two cell cycle genes from Drosophila: a cdc2 homologue and string. EMBO J. 1990 Nov;9(11):3565–3571. doi: 10.1002/j.1460-2075.1990.tb07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Structure and developmental expression of the chicken CDC2 kinase. EMBO J. 1989 Oct;8(10):3071–3078. doi: 10.1002/j.1460-2075.1989.tb08458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A. J., Lapis K., Ishizaki R., Beard J. W., Bolognesi D. P. Isolation of a transplantable cell line induced by the MC29 avian leukosis virus. Cancer Res. 1974 Jun;34(6):1457–1464. [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Drosophila cdc2 homologs: a functional homolog is coexpressed with a cognate variant. EMBO J. 1990 Nov;9(11):3573–3581. doi: 10.1002/j.1460-2075.1990.tb07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989 Mar 24;56(6):957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka M. J. Mitotic control by metaphase-promoting factor and cdc proteins. J Cell Sci. 1989 Feb;92(Pt 2):131–135. doi: 10.1242/jcs.92.2.131. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Parker P. J. Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem. 1989 Jun 1;182(1):129–137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Pines J., Golsteyn R., Standart N., Mackie S., Colman A., Blow J., Ruderman J. V., Wu M., Hunt T. The role of cyclin synthesis, modification and destruction in the control of cell division. J Cell Sci Suppl. 1989;12:77–97. doi: 10.1242/jcs.1989.supplement_12.8. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles J., Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989 Jul 28;58(2):361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Kitten G. T., Nigg E. A. A somatic cell-derived system for studying both early and late mitotic events in vitro. J Cell Sci. 1989 Nov;94(Pt 3):449–462. doi: 10.1242/jcs.94.3.449. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Controls of cell proliferation in yeast and animals. Ciba Found Symp. 1990;150:168–183. doi: 10.1002/9780470513927.ch11. [DOI] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Picard A., Capony J. P., Brautigan D. L., Dorée M. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J Cell Biol. 1989 Dec;109(6 Pt 2):3347–3354. doi: 10.1083/jcb.109.6.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott J. R., Rai R., Carter B. L. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982 Jul 22;298(5872):391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunt T. Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 1987 Oct;6(10):2987–2995. doi: 10.1002/j.1460-2075.1987.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Pondaven P., Meijer L., Beach D. Activation of M-phase-specific histone H1 kinase by modification of the phosphorylation of its p34cdc2 and cyclin components. Genes Dev. 1990 Jan;4(1):9–17. doi: 10.1101/gad.4.1.9. [DOI] [PubMed] [Google Scholar]
- Potashkin J. A., Beach D. H. Multiple phosphorylated forms of the product of the fission yeast cell division cycle gene cdc2+. Curr Genet. 1988 Sep;14(3):235–240. doi: 10.1007/BF00376743. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Wittenberg C. Mitotic role for the Cdc28 protein kinase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5697–5701. doi: 10.1073/pnas.87.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H. E., Stueland C. S., Thomas J., Russell P., Reed S. I. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990 Aug;4(8):1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., Wittenberg C., Cross F., Reed S. I. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989 Dec 22;59(6):1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Titani K., Demaille J. G., Fischer E. H. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1979 Jul 25;254(14):6211–6214. [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Standart N., Minshull J., Pines J., Hunt T. Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev Biol. 1987 Nov;124(1):248–258. doi: 10.1016/0012-1606(87)90476-3. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J., Hemmings B. A. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987 Nov 17;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., González C., Sánchez-Herrero E., Glover D. M. Transcripts of one of two Drosophila cyclin genes become localized in pole cells during embryogenesis. Nature. 1989 Mar 23;338(6213):337–340. doi: 10.1038/338337a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg C., Sugimoto K., Reed S. I. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell. 1990 Jul 27;62(2):225–237. doi: 10.1016/0092-8674(90)90361-h. [DOI] [PubMed] [Google Scholar]