Abstract
Although task-switching has been investigated extensively, its interaction with emotionally salient task content remains unclear. Prioritized processing of affective stimulus content may enhance accessibility of affective task-sets and generate increased interference when switching between affective and non-affective task-sets. Previous research has demonstrated that more dominant task-sets experience greater switch costs, as they necessitate active inhibition during performance of less entrenched tasks. Extending this logic to the affective domain, the present experiment examined (a) whether affective task-sets are more dominant than non-affective ones, and (b) what neural mechanisms regulate affective task-sets, so that weaker, non-affective task-sets can be executed. While undergoing functional magnetic resonance imaging, participants categorized face stimuli according to either their gender (non-affective task) or their emotional expression (affective task). Behavioral results were consistent with the affective task dominance hypothesis: participants were slower to switch to the affective task, and cross-task interference was strongest when participants tried to switch from the affective to the non-affective task. These behavioral costs of controlling the affective task-set were mirrored in the activation of a right-lateralized frontostriatal network previously implicated in task-set updating and response inhibition. Connectivity between amygdala and right ventrolateral prefrontal cortex was especially pronounced during cross-task interference from affective features.
Keywords: emotion, task-switching, interference, affect, control, prefrontal cortex
INTRODUCTION
The relationship between neurocognitive systems involved in processing affective representations and those involved in executive control is essential to promoting adaptive functioning. Indeed, disruption of the balance between affect and control is considered the hallmark of many clinical disorders, such as depression and anxiety (Bishop, 2007; Banich, 2009). However, while some aspects of affect-control interactions, for example attentional capture by affectively salient stimuli (Vuilleumier, 2005; Pessoa, 2008; Reeck et al., 2012), have been extensively investigated, it remains unclear how affective and executive control processes interact with one another in adapting to changing task goals that may or may not involve goal-relevant emotional information. Given that task-switching represents a core control function (Monsell, 2003; Miyake and Friedman, 2012), understanding its regulation in the context of affectively salient target or distracter stimuli has great relevance for a better understanding of potential cognitive control deficits in clinical populations. As an initial step in this endeavor, the present experiment sought to characterize how affective and control systems interact to manage affective and non-affective information during task-switching in a healthy subject population.
Cognitive control enables individuals to maintain alignment between internal goals and their thoughts and behavior while navigating a complex and dynamic world (Miller and Cohen, 2001). Affective stimuli in the environment disrupt ongoing mental processing, gaining privileged access to cognitive resources. Representations of affectively salient stimuli in sensory cortical regions are rapidly enhanced compared with neutral stimuli (Pizzagalli et al., 1999; Halgren et al., 2000), and these heightened representations often gain privileged access to attention (Hansen and Hansen, 1994; Ohman, Flykt, and Esteves, 2001; Ohman, Lundqvist, and Esteves, 2001; Fox, 2002; Lim et al., 2008). The amygdala plays a central role in orienting to affective stimuli (LeDoux, 2000; Phelps, 2006; Pessoa, 2008; Vuilleumier and Huang, 2009), and affective attentional capture may occur in a somewhat automatic fashion (Vuilleumier, 2005) or may be contingent on available cognitive resources and current attentional settings (Pessoa, 2008; Reeck et al., 2012). Based on their privileged processing status, affective stimuli often enjoy advantages in cognitive representation, including greater capacity to draw attention, enhanced ability to disrupt ongoing behavior (Reeck and Egner, 2011), and greater likelihood of being encoded in memory (Phelps, 2006; Mather and Sutherland, 2011).
Recognition that affective stimuli gain enhanced access to perceptual and attentional resources has spurred recent theorizing regarding the implications for downstream processing. For instance, the dual-competition model (Pessoa, 2009) is predicated on the notion that emotion–cognition interactions occur not only at the perceptual level, but also at the level of executive control resources. Other theories emphasize the role affective stimuli play in moderating the contents of working memory (Braver and Cohen, 2000). Previous work demonstrates that prefrontal regions play an essential role in resolving interference generated by affective representations (Etkin et al., 2006; Bishop, 2007; Egner, 2008), but it remains unclear how toggling between affective and non-affective task-sets modulates activation in these regions. The relationship between emotion and cognitive control may be especially critical when multiple actions or interpretations are available in response to a given stimulus, suggesting that when an ambivalent stimulus affords both an affective task-set and a non-affective task-set, the affective set should be more potent in driving behavior than the non-affective set. This task-set competition may be disrupted in certain clinical disorders, such that the affective task-set is particularly dominant. Previous work has demonstrated that anxious individuals are more likely to arrive at negative interpretations of ambiguous stimuli (Eysenck et al., 1991; Grupe and Nitschke, 2013), and that amending this bias promotes lasting changes in affective responding (Wilson et al., 2006; Tran et al., 2011; MacLeod and Mathews, 2012). Heightened accessibility of affective task-sets imposes increased demands on systems that toggle between task-sets, yet the regulation of competition between affective and non-affective task-sets has received only limited attention in the literature.
The present experiment sought to clarify (i) whether affective task-sets are more dominant than non-affective ones when switching, and (ii) what neural mechanisms control the putatively more dominant affective task-set, so that the weaker, non-affective task can be executed. Previous research has demonstrated that affectively salient aspects of stimuli are more readily processed (LeDoux, 2000) and more likely to disrupt non-affective task-sets than vice versa (Reeck and Egner, 2011). Research from behavioral task-switching paradigms employing non-affective task-sets reveals that the more dominant task-set is often more challenging to switch to and easier to switch from, with switches to the dominant task-set resulting in more errors and slower response times (Monsell et al., 2000; Wylie and Allport, 2000). This ‘counterintuitive asymmetric switch cost’ is thought to occur because the dominant task-set necessitates greater inhibition to facilitate the performance of the less-dominant task-set. When switching back to the dominant task-set, because it has been more heavily inhibited it takes longer to release from inhibition and is more likely to suffer interference from the now accessible less-dominant task-set (Allport et al., 1994). Assuming that affective task-sets are dominant leads to the hypothesis that switches to an affective task-set will be slower than switches to a non-affective task-set. By combining this basic task design with the simultaneous assessment of neuroimaging data, we can delineate the neural systems that respond to this increased burden on control mechanisms. This approach can provide novel insights into the neural mechanisms underlying affect–control interactions at the basic science level, and it furthermore can identify potential neural targets of therapeutic intervention in future clinical research.
To date, two behavioral investigations have examined shifting between affective and non-affective task-sets (Paulitzki et al., 2008; Johnson, 2009), both reporting larger switch costs for non-affective task-sets. While these findings diverge from the hypothesis outlined above, both studies arguably suffer from several shortcomings. First, these studies did not utilize bivalent stimuli that can be subject to multiple task rules, and thus switching tasks also involved shifting one’s attention to different stimuli presented simultaneously. Second, affective and non-affective stimuli that were presented varied dramatically on basic perceptual features, most notably size, so that the affectively salient stimulus was also vastly more perceptually salient. Finally, transitions between tasks were either predictable (Paulitzki et al., 2008) or were biased such that one task was much less likely to be repeated than the other (Johnson, 2009). Given these concerns, the present experiment sought to examine switching between affective and non-affective task-sets using a paradigm that avoided these limitations.
We developed a novel task-switching paradigm that employed frequency-balanced affective and non-affective task-sets operating over identical, bivalent stimuli that contained both affective and non-affective features. Specifically, affectively expressive faces were presented, and participants were cued to judge either the emotional expression (affective task) or the gender of the face (non-affective task). Both tasks employed overlapping response sets so that we could assess the degree to which task-sets interfered with one another, particularly when switching from one task to the other. Participants performed this task while undergoing functional magnetic resonance imaging (fMRI), enabling the characterization of regions that facilitated shifts between affective and non-affective task-sets and tracked interference generated by switching to or from an affective task-set. We hypothesized that the affective task-set would be dominant, thus predicting an asymmetric switch cost with higher switch costs for the affective than the non-affective task. We anticipated that the enhanced burden on task-set regulation imposed by the affective set would modulate activation in frontal and striatal regions previously implicated in controlling task-sets (e.g., Sohn et al., 2000; Miller and Cohen, 2001; Braver et al., 2003; Brass and von Cramon, 2004; D’Ardenne et al., 2012) and that these regions might exhibit enhanced interaction with the amygdala when confronted with interference from the affective task-set.
METHODS
Participants
Twenty right-handed participants (10 women) were recruited to participate in this experiment. Participants were between the ages of 18 and 35 years old (M = 23.9, s.d. = 3.7) and reported no prior neurological or psychological disorders and were not taking any psychoactive medications. One additional participant completed the experiment but their data were excluded from all analyses due to falling asleep during the neuroimaging portion of the experiment. All procedures were approved by the Duke University Medical Center Institutional Review Board.
Materials
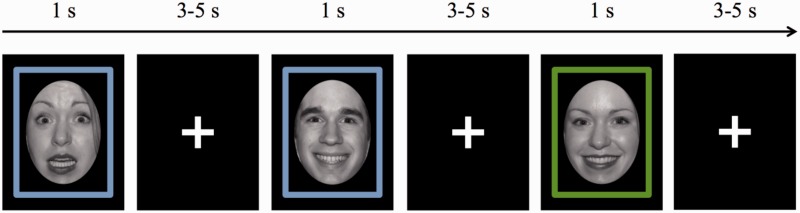
Black and white face images from the NimStim Set of Facial Expressions1 (Tottenham et al., 2009) were employed as stimuli. Specifically, images from four females and four males modeling both fearful and happy expressions were oval-cropped to standardize image size and shape, and to remove non-face information, such as hair, that could facilitate task performance during gender discrimination. On each trial either a blue- or a green-colored frame was presented in conjunction with a face image (Figure 1). Psychophysics Toolbox Version 3 (Brainard, 1997; Pelli, 1997) was employed to control stimulus presentation and data collection.
Fig. 1.
Example trial sequence from experimental paradigm. The frame around the stimulus indicates which task the participant should perform, with a blue frame indicating the emotion expression of the face should be registered and a green frame indicating the gender of the face should be registered. Shown here is one task-stay trial and one task-switch trial, including the jittered intertrial fixation interval.
Procedure
After providing informed consent, participants first completed each of the two tasks employed in the present experiment, gender and emotion expression discrimination, separately, so as to gauge baseline difficulty levels outside the context of a task-switching protocol. The order in which the tasks were performed was counterbalanced across participants. Each trial began with the 1-s presentation of a face stimulus. In the gender discrimination task, participants indicated the gender of the face (male or female). In the emotion expression discrimination task, participants indicated the emotion expression of the face (fearful or happy). Responses were registered via button presses using the index and middle fingers of the right hand. Each task consisted of one run of 48 trials, and the trial sequence in each run was controlled so that each response would occur an equal number of times, there would be an equal number of response repeats and response switches, and no response would need to be repeated more than three times in a row. In addition, each of the 16 unique stimuli employed in the present experiment appeared three times during each run and the trial sequence was controlled so that the same identity never appeared twice in a row to mitigate the influence of perceptual priming results. Trials were separated by a jittered fixation inter-trial interval varying from 2 to 3 s in half-second steps. Data from two participants were excluded, one due to experimenter error and the other due to the participant employing improper response keys.
After performing each task separately, participants then practiced the task-switching paradigm they would perform in the scanner. On each trial, a face stimulus would appear surrounded by a colored frame (Figure 1). The color of the frame indicated which task the participant should perform, with a green frame indicating the participant should perform the gender discrimination and a blue frame indicating the participant should perform the emotion expression discrimination. The frame and face stimulus were presented simultaneously. In this design, each trial could be categorized as a function both of the current task participants were performing (gender or emotion expression) as well as by the transition from the task participants completed on the previous trial (staying with the same task or switching to the alternate task). In addition, as the response mappings for the two tasks overlapped, the stimulus presented on each task could also be classified as either congruent (i.e., the same button press would provide an accurate response on both tasks) or incongruent (i.e., the accurate responses to each task were associated with different button press). Participants were instructed to respond as quickly and accurately as possible, and response mappings were counterbalanced across participants.
After practicing the task outside the scanner, participants then performed four functional runs of the task while undergoing fMRI. Each run consisted of 113 trials, composed of an equal number of trials in each condition (disregarding the first trial). The trial sequence of each run was constrained so that there were an equal number of transitions between each task (gender or emotion expression), response (left or right) and stimulus congruency (congruent or incongruent) and no more than four trials sharing any one of these features ever appeared in a row. The specific face stimuli presented were controlled so that each stimulus appeared an equal number of times in each run and the same identity never appeared on consecutive trials to minimize the influence of perceptual priming effects. A jittered fixation inter-trial interval followed each trial, varying from 3 to 5 s in half-second steps and following a pseudo-exponential distribution.
At the end of the experiment, participants completed a separate localizer task to functionally delineate face responsive regions. Participants performed a 1-back task during block-wise presentation of face and house stimuli, responding whenever identical stimuli appeared consecutively. Each block consisted of 15 stimuli presented for 750 ms followed by 250 ms of fixation, and the localizer task comprised 12 blocks presented in ABAB order and separated by 10 s of fixation.
The present experimental design considered the history of each trial (trial transition factor: task switch versus stay), the task performed on the current trial (task factor: gender vs emotion expression task), and the congruency of the current stimulus (congruency factor: congruent vs incongruent). Accuracy rates were computed for each condition, after omitting the first trial from each run as it had no previous trial history. Mean response times for each condition were calculated, excluding error trials, post-error trials and trials in which response time deviated by more than two standard deviations from the participant’s overall mean response time.
Image acquisition
A General Electric MR750 3.0 Tesla MRI scanner with a multi-channel parallel imaging system (8-channel headcoil) was employed to acquire images. A three-dimensional fast inverse-recovery-prepared SPGR sequence acquired whole-brain high-resolution T1-weighted structural images consisting of 176 axial slices containing 1.0 mm isometric voxels. Whole-brain T2*-weighted images were acquired during functional runs using a single-shot gradient EPI sequence with an echo time of 28 ms, a flip angle of 90°, and a repetition time of 2.0 s. Each volume consisted of 36 contiguous slices containing 3.0 mm isometric voxels acquired in an interleaved sequence.
Imaging analyses
Imaging analyses were conducted using SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). A unified segmentation approach (Ashburner and Friston, 2005) normalized each participant’s bias-corrected structural image to the Montreal Neurological Institute template and co-registered it to the participant’s mean functional image. Functional images were acquisition-time corrected, realigned to the participant’s mean functional image, normalized employing the transformation parameters implemented for the structural images and spatially smoothed utilizing a Gaussian kernel of full-width half-maximum 8 mm3.
The data were modeled at the single-subject level by including one regressor for each of the eight experimental conditions formed by the factorial combination of the factors of trial transition, task and congruency. Onset vectors modeling each 1-s trial in each of these conditions were entered, along with a separate regressor modeling both error trials and the first trial in each run (which could not be classified as task switch or stay as it was not directly preceded by another trial). These vectors were convolved with the canonical hemodynamic response function and analyzed according to the assumptions of the general linear model, with scan run treated as a covariate. Linear contrasts between conditions of interest were estimated individually for each participant and entered into second-level random effects analyses. AlphaSim software (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) was employed to compute a combined cluster-extent and voxel-height threshold to correct for multiple comparisons. A voxel-height threshold of P < 0.005 was employed to generate family-wise error corrected combined thresholds of P < 0.05 in the analyses reported below. For critical analyses, parameter estimates were extracted from 6-mm spheres centered on activation peaks identified in the whole-brain, group-level results and submitted to subsequent analyses.
Psychophyisiological interaction (PPI) analyses assessed whether any regions exhibited differential connectivity with the amygdala when resolving interference from the affective task-set during task transitions. To delineate the amygdala, data from the localizer task were modeled employing two regressors corresponding to the onset and duration of the face and house stimulus presentation blocks. Activation on face stimulus blocks was contrasted with activation on house stimulus blocks. Participant-specific estimates of these effects were entered into a second-level analysis to identify a group-level region of interest, employing a false-discovery rate corrected voxel-height threshold of P < 0.05 and a cluster-extent threshold of 10 voxels. This analysis revealed peak activation in the right amygdala (MNI coordinates: 21, -3, -18), and signal was extracted from a 6-mm sphere centered on this peak. To examine differential connectivity during the resolution of interference from the affective task-set, this analysis focused on the trials involving switches between tasks that also featured incongruent stimuli (whose features primed competing responses), under the assumption that interference between task-sets would be maximal on these trials. Thus, the PPI was conducted examining the contrast of switches between tasks featuring incongruent stimuli comparing transitions to the gender task with transitions to the emotion expression task. PPI results were whole-brain corrected using AlphaSim as described above.
RESULTS
Behavioral data
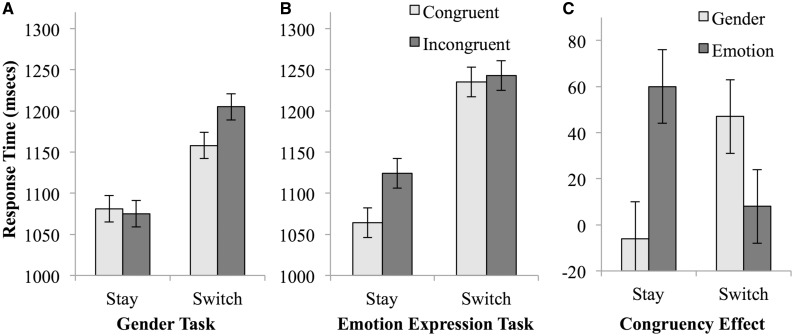
Mean correct response times and accuracy rates were submitted to repeated-measures ANOVAs with the within-subjects factors of trial transition (stay vs switch), task (gender vs emotion expression) and stimulus congruency (congruent vs incongruent). Descriptive statistics are listed in Table 1 and depicted in Figure 2. There was a main effect of trial transition, F(1, 19) = 30.992, P < 0.001, and of stimulus congruency, F(1, 19) = 6.530, P = 0.019. As anticipated, there was also a significant interaction between switch and task, F(1, 19) = 5.867, P = 0.026, which reflected an asymmetric switch cost due to larger costs when switching to the emotion expression task (mean switch cost = 147 ms) than to the gender discrimination task (mean switch cost = 104 ms). Finally, these findings were qualified by a significant three-way interaction between switch, task and congruency, F(1, 19) = 11.615, P = 0.003, as congruency affected stay trial performance in the emotion task and switch trial performance in the gender task. Specifically, in the gender task (Figure 2A), a trial transition × congruency interaction effect, F(1, 19) = 8.314, P = 0.010, arose from the presence of a congruency effect on switch trials, t(19) = 2.102, P = 0.049, but not on stay trials t(19) = 0.360, P = 0.723. In contrast, for the emotion expression task (Figure 2B), a trial transition × congruency interaction effect, F(1, 19) = 6.928, P = 0.016, was attributable to the presence of a congruency effect on stay trials, t(19) = 5.225, P < 0.001, but not on switch trials, t(19) = 0.461, P = 0.650. As can be seen in Figure 2C, the three-way interaction was essentially driven by particularly fast responses during congruent stay trials in the emotional task (which would be expected to be particularly effortless if the emotional task-set were dominant), and by relatively slowed responses in incongruent gender switch trials, that is, when participants had to move to the less dominant task-set while regulating interference from incongruent stimulus information associated with the more dominant set.2
Table 1.
Reaction times from the task-switching paradigm performed during functional neuroimaging
| Gender task | Emotion expression task | |
|---|---|---|
| Congruent stay trial | 1081 (286) | 1064 (316) |
| Incongruent stay trial | 1075 (255) | 1124 (331) |
| Congruent switch trial | 1158 (349) | 1235 (416) |
| Incongruent switch trial | 1205 (340) | 1243 (397) |
Means (in ms) are presented with standard deviations in parentheses
Fig. 2.
Reaction times from the task-switching paradigm. (A) Response times from the gender task. (B) Response times from the emotion expression task. (C) The congruency effect for each condition was computed by subtracting the congruent response time from the incongruent response time. Error bars represent one standard error of the mean.
Accuracy rates displayed a main effect of trial transition, as accuracy was higher on stay trials (mean = 91.2%) than on switch trials (mean = 88.9%), F(1, 19) = 12.889, P = 0.002. There was also a significant main effect of congruency, as accuracy was higher on congruent (mean = 92.2%) than incongruent trials (mean = 87.8%), F(1, 19) = 11.239, P = 0.003. No other effects reached significance, P’s > 0.1.
Overall, these behavioral findings accord well with the hypothesis that when stimuli afford both emotional and non-emotional responses, the former appear to be privileged, thus engendering a dominant emotional response or task-set that leads to an asymmetric switch cost effect. These results set the stage for assessing the neural mechanisms recruited for overcoming these apparently prepotent emotional affordances. However, prior to interrogating the fMRI data, we sought to rule out an alternative explanation, namely that the asymmetric switch effects between gender and emotion tasks were simply due to overall differences in baseline task difficulty rather than a privileged status of the emotional task-set in the context of the task-switching protocol. To gauge baseline difficulty level, participants performed each task separately before being introduced to the task-switching paradigm. Performance on these tasks did not differ significantly with respect to accuracy, t(17) = 1.056, P = 0.306, or response time, t(17) = 1.448, P = 0.166. Thus, it does not appear that either task was inherently more difficult than the other.
Neuroimaging results
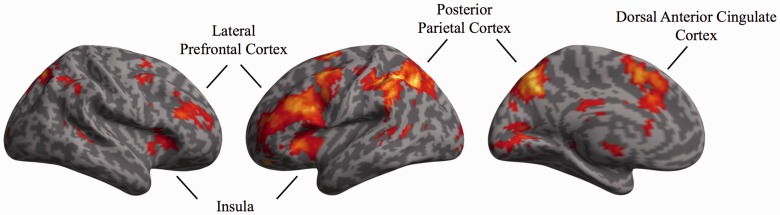
Replicating typical findings in the task-switching literature, enhanced activation was observed in a broad network of regions on trials that involved switching tasks as opposed to continuing to perform the same task as the previous trial (Table 2 and Figure 3). In particular, lateral prefrontal cortex, dorsal anterior cingulate cortex, lateral parietal cortex and caudate all exhibited greater activation on switch trials than on stay trials. Assessing the main effect of task, right ventrolateral prefrontal cortex exhibited greater activation on trials in which the gender task was performed compared to the emotion expression task (Table 2); no regions exhibited the reverse pattern. Finally, no regions exhibited activation that differentiated between congruent and incongruent trials.
Table 2.
Peak activation foci for neuroimaging analyses examining main effects
| Regions | Brodmann area | MNI coordinates (x, y, z) | Cluster extent (voxels) | Z | ||
|---|---|---|---|---|---|---|
| Gender > emotion expression task | ||||||
| Right ventrolateral prefrontal cortex | 44, 45 | 48, | 21, | 21 | 205 | 3.70 |
| Switch > stay trial transition | ||||||
| Left parietal cortex, temporoparietal junction | 7, 40, 39 | −33, | −45, | 39 | 2,574 | 5.61 |
| Lateral prefrontal cortex, dorsal anterior cingulate cortex, insula, caudate | 44, 45, 47, 46, 9, 6, 8, 32, 24 | −30, | 0, | 63 | 4,851 | 5.18 |
| Medial occipital cortex | 18, 17 | 3, | −66, | −12 | 1,078 | 3.99 |
| Posterior hippocampus | 30, | −30, | 0 | 86 | 3.32 | |
| Right dorsolateral prefrontal cortex | 6, 8 | 30, | 9, | 54 | 157 | 3.32 |
| Posterior cingulate cortex | 23 | −6, | −30, | 21 | 86 | 3.20 |
Whole-brain corrected, P < 0.05
Fig. 3.
Neural regions exhibiting greater activation on trials that involved switching tasks as opposed to continuing to perform the same task as the previous trial. Whole-brain corrected, P < 0.05.
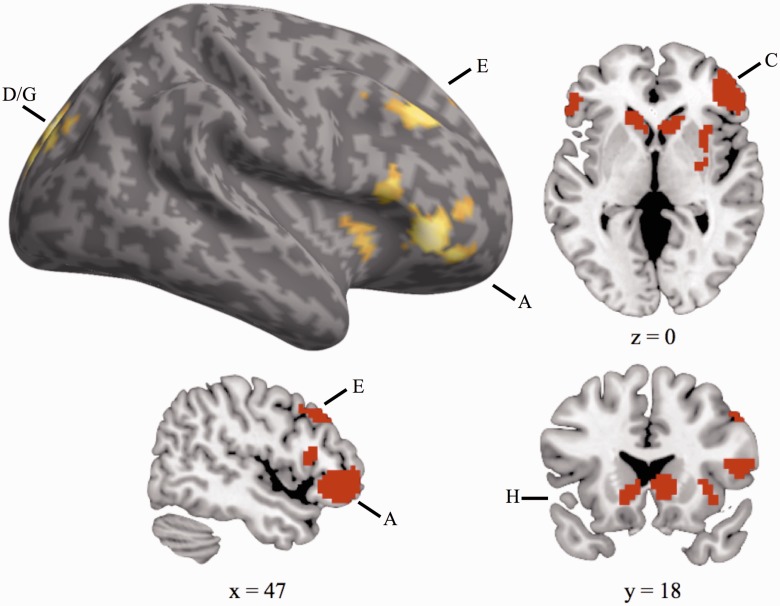
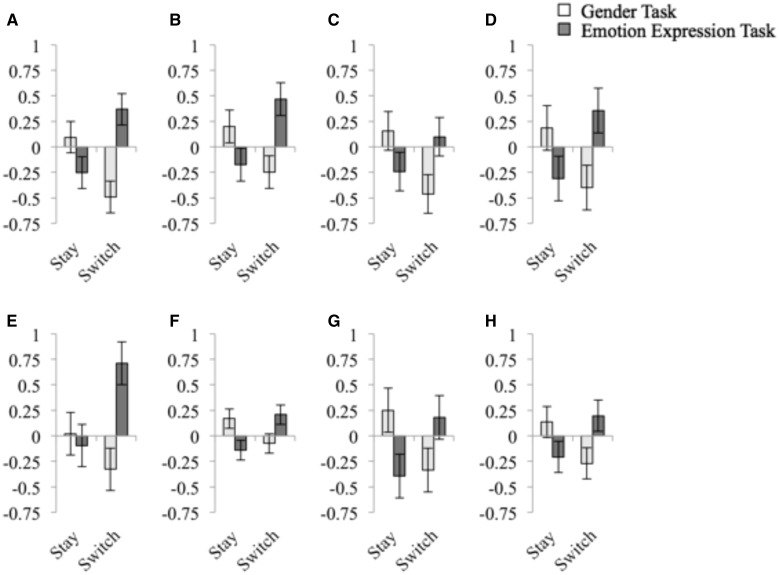
The behavioral data were characterized by a three-way interaction between trial transition (task switch vs stay), task (gender vs emotion expression) and the stimulus congruence (congruent vs incongruent). To determine the neural substrates underlying this pattern of behavioral switch and cross-task interference costs, our neuroimaging analyses therefore focused on identifying regions whose activation also expressed a three-way interaction between these factors. This three-way interaction was observed in a network of right-lateralized frontrostriatal regions, including ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, insula, bilateral caudate and cingulate cortex (Figure 4 and Table 3). To investigate the nature of this interaction, parameter estimates were extracted from peak activation foci in these regions and submitted to additional analyses. For ease of presentation, Figure 5 displays these three-way interactions by means of plotting the neural cross-task interference effects (incongruent minus congruent trial activation) as a function of trial transition and task. As illustrated in Figure 5A–H and Table 4, all regions identified displayed the same basic response pattern. Specifically, on gender task trials, the neural congruency effect was larger on Stay trials than Switch trials. Conversely, on emotion expression task trials the congruency effect was larger on Switch trials than on Stay trials. In other words, on trials that involved a switch to the emotion expression task, these regions exhibited greater activation on incongruent than on congruent trials, whereas this pattern was reversed on trials that involved repeating the emotion expression task. Conversely, on trials that involved a switch to the gender task, these regions exhibited greater activation on congruent than incongruent trials, but this pattern was reversed on trials that involved repeating the gender task. The condition means in the behavioral and neural data display an inverse pattern, in that, on average, a condition that was characterized by larger differences between incongruent and congruent trials at the neural level was associated with a smaller interference effect at the behavioral level. This inverse relationship between behavioral cost and neural activation suggests that activation in this network of regions may track the successful implementation of control (e.g. Kerns et al., 2004; Egner and Hirsch, 2005), with greater differential activation between incongruent and congruent responses potentially reflecting diminished interference from the alternate task-set. In the case of switches to the emotion expression task, activation in these regions may track the successful release of the affective task-set from inhibition and its insulation from interference generated by the non-affective task-set. However, as these analyses did not examine direct relationships between neural activation and behavioral variability across individuals, interpretations regarding the specific relationship between activation in these regions and interference resolution are somewhat speculative.
Fig. 4.
Neural regions whose activation displays a three-way interaction between trial transition (task switch vs stay), task (gender vs emotion expression) and the congruence of the stimulus presented (congruent vs incongruent). Whole-brain corrected, P < 0.05. Letter labels correspond to regions in Figure 5.
Table 3.
Peak activation foci for the three-way interaction between trial transition (task switch vs stay), task (gender vs emotion expression) and the congruence of the stimulus presented (congruent vs incongruent)
| Regions | Brodmann area | MNI coordinates (x, y, z) | Cluster extent (voxels) | Z |
|---|---|---|---|---|
| Right ventrolateral prefrontal cortex, insula | 47, 45, 10 | 57, 36, −3 | 426 | 4.41 |
| Midcingulate cortex | 23, 24, 31 | 3, −24, 33 | 448 | 4.15 |
| Left ventrolateral prefrontal cortex | 47, 45, 10 | −54, 36, −6 | 69 | 3.95 |
| Medial occipital cortex | 19, 18, 7 | −9, −87, 36 | 118 | 3.69 |
| Right dorsolateral prefrontal cortex | 9, 8, 46 | 42, 33, 36 | 127 | 3.63 |
| Caudate, thalamus | 18, −3, 27 | 74 | 3.51 | |
| Right occipital cortex | 19, 18, 7 | 18, −90, 30 | 68 | 3.46 |
| Bilateral caudate | 12, 21, 3 | 154 | 3.40 |
Whole-brain corrected, P < 0.05
Fig. 5.
Neural congruency effects computed by subtracting parameter estimates on congruent trials from activation on incongruent trials. Peak activation foci from the three-way interaction were employed as the basis for parameter estimate extraction. Peaks correspond to clusters listed in Table 3 and congruency effects listed in Table 4: (A) Right ventrolateral prefrontal cortex. (B) Midcingulate cortex. (C) Left ventrolateral prefrontal cortex. (D) Medial Occipital Cortex. (E) Right dorsolateral prefrontal cortex. (F) Posterior Caudate. (G) Right Occipital Cortex. (H) Anterior Caudate. Parameter estimates (arbitrary units) from the Gender task are presented in white and from the Emotion Expression task are presented in dark grey.
Table 4.
Neural congruency effects computed by subtracting parameter estimates on congruent trials from activation on incongruent trials (arbitrary units)
| Regions | Stay gender | Switch gender | Stay emotion | Switch emotion | Gender task-switch comparison | Emotion expression task-switch comparison |
|---|---|---|---|---|---|---|
| R VLPFC | 0.095 | −0.492 | −0.253 | 0.368 | 3.382* | 3.447* |
| MCC | 0.199 | −0.246 | −0.177 | 0.468 | 2.221* | 3.468* |
| L VLPFC | 0.154 | −0.461 | −0.241 | 0.100 | 2.325* | 1.938 |
| MOC | 0.184 | −0.400 | −0.310 | 0.355 | 2.013 | 2.540* |
| R DLPFC | 0.021 | −0.328 | −0.095 | 0.711 | 1.239 | 2.484* |
| P. Caud. | 0.169 | −0.074 | −0.141 | 0.208 | 2.138* | 2.459* |
| R OC | 0.251 | −0.337 | −0.395 | 0.181 | 2.352* | 2.832* |
| A. Caud. | 0.135 | −0.270 | −0.207 | 0.198 | 2.034 | 2.520* |
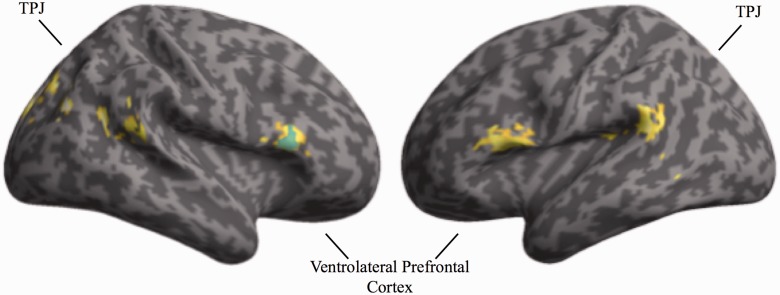
To investigate further how the frontostriatal regions identified above might implement control over affective task content, we conducted functional connectivity analyses. Based on the assumption that the amygdala would mediate affective processing (LeDoux, 2000; Phelps, 2006; Pessoa, 2008; Vuilleumier and Huang, 2009), analyses were conducted to assess whether any regions exhibited differential connectivity with the amygdala when resolving interference from the affective task-set during task transitions. A region of interest in the right amygdala was identified employing an independent localizer and employed as the source region in a PPI analysis contrasting task switches to the gender vs emotion expression task during trials featuring incongruent stimuli, where cross-task interference control would be maximally taxed. This analysis revealed enhanced coupling between the amygdala and bilateral ventrolateral prefrontal cortex, bilateral temporal cortex and several occipital regions (Figure 6 and Table 5). Importantly, the condition-specific amygdala connectivity with right ventrolateral prefrontal cortex overlapped with the region identified in the three-way interaction between trial transition, task and congruency (Figure 6). Interference from the affective task-set should be maximal on switches to the gender task involving incongruent stimuli (as observed in the behavioral results), and the connectivity results suggest functional interplay between the amygdala and the right ventrolateral prefrontal cortex may be involved in detecting and resolving such interference.
Fig. 6.
Neural regions exhibiting greater functional connectivity with the amygdala during switches on incongruent trials to the gender task compared with the emotion expression task as revealed by a PPI analysis. The region depicted in blue overlaps with the right ventrolateral prefrontal cortex cluster identified in the three-way interaction of trial transition, task, and the congruence of the stimulus presented. P < 0.05, corrected.
Table 5.
Peak activation foci for the PPI analysis employing activation from the right amygdala seed and the contrast of switches on incongruent trials to the gender task compared with the emotion expression task
| Regions | Brodmann area | MNI coordinates (x, y, z) | Cluster extent (voxels) | Z |
|---|---|---|---|---|
| Left occipital cortex | 31, 18, 19 | −9, −81, 24 | 385 | 3.76 |
| Left parietal cortex | 7 | −24, −33, 45 | 78 | 3.70 |
| Right occipital cortex | 19, 7 | 39, −69, 27 | 112 | 3.61 |
| Right precuneus | 31 | 21, −51, 27 | 136 | 3.60 |
| Right ventrolateral prefrontal cortex | 44, 45, 46 | 57, 24, 18 | 135 | 3.55 |
| Right occipital cortex | 18 | 12, −69, −6 | 61 | 3.38 |
| Left ventrolateral prefrontal cortex | 44, 45 | −51, 21, 15 | 145 | 3.35 |
| Left temporoparietal junction | 40 | −57, −45, 18 | 122 | 3.24 |
| Right temporoparietal junction | 40, 22 | 63, −45, 15 | 59 | 3.10 |
| Left posterior insula | 13 | −36, −42, 18 | 69 | 3.08 |
Whole-brain corrected, P < 0.05
DISCUSSION
The present experiment investigated how task-switching processes interact with affective task content and sought to identify the neural mechanisms associated with the management of affective tasks. Consistent with the hypothesized dominance of affective task-sets, transitions to the emotion expression task were associated with larger switch costs than transitions to the gender task. Activation in a right-lateralized, frontostriatal network exhibited a pattern similar to the behavioral data, with greater activation in these regions observed in conditions associated with lower mean response times. In addition, differential functional connectivity was observed between the right amygdala and right ventrolateral prefrontal cortex during switches to the gender task on trials that featured competing affective features. These behavioral and neural findings provide novel insights into the mechanisms underlying the management of affective task-sets.
Greater behavioral switch costs were observed during transitions to the affective task-set from the non-affective task-set than vice versa. Critically, these asymmetric switch costs emerged despite the fact that there were no overall differences in performance when these tasks were performed independently, indicating this pattern did not emerge due to basic differences in task difficulty or engagement and instead reflected processes recruited when switching between active task-sets. These observed asymmetric switch costs are consistent with the hypothesized dominance of affective task-sets. Importantly, this general pattern interacted with stimulus congruency. During performance of the affective task-set, responses to congruent stimuli were particularly speeded compared with incongruent stimuli when participants repeated the affective task-set; this congruency effect was not observed during switches to the affective set. Thus, when the affective set was inhibited on the previous trial to enable performance of the non-affective set, responses are equally slow regardless of stimulus congruency while the set was re-instantiated. However, when the affective task-set was activated from the previous trial, the congruent stimuli facilitated responding. Conversely, during performance of the non-affective task-set, responses were particularly slowed to incongruent stimuli following transitions from the affective set, while a significant congruency effect was not observed during repetitions of the non-affective task-set. These findings suggest that when confronted with ambiguous stimuli that contain both affective and non-affective features, the affective features may prime the affective task-set, leading to difficulties performing the weaker, non-affective task. Note that the asymmetric switch costs observed here, while consistent with other task-switching work (Monsell et al., 2000; Wylie and Allport, 2000), revealed a behavioral pattern contrary to those reported in previous manipulations of affective task content (Paulitzki et al., 2008; Johnson, 2009). The present experimental paradigm improves on this previous work by employing bivalent stimuli containing both affective and non-affective features and balancing transitions between the two tasks. Our conclusions regarding the dominance of affective task-sets were also supported by the present neuroimaging findings.
Behavioral performance patterns were reflected in activation in a network of regions previously implicated in the switching or updating of task-sets (e.g. Sohn et al., 2000; Miller and Cohen, 2001; Braver et al., 2003; Brass and von Cramon, 2004; D’Ardenne et al., 2012), including right dorsolateral prefrontal cortex, bilateral ventrolateral prefrontal cortex and caudate. The observed inverse relationship between behavioral costs and neural activation suggests these regions may be involved in the successful implementation of control (Kerns et al., 2004; Egner and Hirsch, 2005) and reduction of cross-task interference. However, the present experiment was not designed as an individual differences investigation and did not specifically evaluate activation in these regions with respect to behavioral variability, and future work should examine this prediction. Focusing on the role of lateral prefrontal regions in managing task-sets, previous work has implicated these regions in task-set reconfiguration (Sohn et al., 2000) and research involving patients with lesions to right lateral prefrontal cortex reveals deficits in inhibitory processes associated with shifting task-sets (Aron et al., 2004). Right ventrolateral prefrontal cortex has been previously hypothesized to play a key role in reinstating previously inhibited task-sets (Dreher and Berman, 2002), and right dorsolateral prefrontal cortex is highly responsive to contextual drivers of task-set configuration (D’Ardenne et al., 2012). While initial activation-based analyses did not identify differential activation in the amygdala in response to particular task conditions, this null finding may reflect the fact that affective stimulus content was presented on each trial. Critically, the amygdala exhibited differential connectivity with the right ventrolateral prefrontal cortex during transitions away from the affective task when stimuli included affective features that primed competing responses. The amygdala is sensitive to interference from affective representations (Etkin et al., 2006; Egner et al., 2008) and the observed connectivity may reflect the detection of conflict between affective stimulus features and the current, non-affective task-set. Enhanced connectivity with right ventrolateral prefrontal cortex may reflect stimulus-driven attentional orienting (Corbetta and Shulman, 2002; Corbetta et al., 2008) or the recruitment of goal-driven interference resolution processes (e.g. Egner, 2011). Although the present experiment does not fractionate the underlying computations in these regions that support successful implementation of control, future work should seek to inform the dynamics of these control processes.
Overall, the present findings support the hypothesized dominance of affective sets, revealing greater switch costs to an affective task-set. Activation in frontostriatal regions was enhanced in conditions involving the implementation of control when shifting between affective and non-affective sets, suggesting activation in this network may enable the reinstatement of a previously inhibited task and offering insight into the neural mechanisms that enable affective task management. The present research elucidates the processes that govern the relationship between task rules and affective content in order to enable adaptive behavior and shifting priorities. Characterizing these neurocognitive mechanisms will inform understanding of how these processes may be disrupted in clinical disorders such as depression, anxiety and post-traumatic stress disorder and, hopefully, point to potential interventions to align priorities and internal processing and promote adaptive behavior.
Acknowledgements
The current research was funded by an NSF Graduate Research Fellowship (C.R.) and an APA Dissertation Award (C.R.). The authors would like to thank Amelia Abbot-Frey for assistance with data collection.
Footnotes
1 Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at nlt7@columbia.edu for more information concerning the stimulus set.
2Given the importance of characterizing the behavioral effects of switching between affective and non-affective task-sets, we sought to replicate this pattern of results in an independent sample of participants. Twenty-two participants were recruited for a behavioral version of the switching task. As in the neuroimaging experiment, there was a significant three-way interaction between switch, task and congruence, F(1,21) = 8.475, P = 0.008. In addition, switches to the emotion expression task were slower than switches to the gender task, t(21) = 2.201, P = 0.039, and no significant response time differences were observed on stay trials, P > 0.4. Thus, the same counterintuitive asymmetric switch costs were observed in this replication sample.
REFERENCES
- Allport A, Styles EA, Hsieh S. Shifting intentional set: exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and Performance XV. Cambridge, MA: MIT Press; 1994. pp. 421–52. [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–73. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: the search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11(7):307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–6. [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. Journal of Neuroscience. 2004;24(40):8847–52. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: the role of dopamine in regulating prefrontal function. In: Monsell S, Driver J, editors. Attention and Performance. Vol. 18. Cambridge, MA: MIT Press; 2000. pp. 713–37. [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39(4):713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(49):19900–9. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14595–600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in Cognitive Sciences. 2008;12(10):374–80. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Egner T. Right ventrolateral prefrontal cortex mediates individual differences in conflict-driven cognitive control. Journal of Cognitive Neuroscience. 2011;23(12):3903–13. doi: 10.1162/jocn_a_00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18(6):1475–84. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8(12):1784–90. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Mogg K, May J, Richards A, Mathews A. Bias in interpretation of ambiguous sentences related to threat in anxiety. Journal of Abnormal Psychology. 1991;100(2):144–50. doi: 10.1037//0021-843x.100.2.144. [DOI] [PubMed] [Google Scholar]
- Fox E. Processing emotional facial expressions: the role of anxiety and awareness. Cognitive Affective and Behavioral Neuroscience. 2002;2(1):52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10(1):69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Hansen RD. Automatic emotion: attention and facial efference. In: Niedenthal PM, Kitayama S, editors. The Heart's Eye: Emotional Influences in Perception and Attention. New York: Academic Press; 1994. pp. 217–43. [Google Scholar]
- Johnson DR. Emotional attention set-shifting and its relationship to anxiety and emotion regulation. Emotion. 2009;9(5):681–90. doi: 10.1037/a0017095. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lim SL, Padmala S, Pessoa L. Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia. 2008;46(5):1267–78. doi: 10.1016/j.neuropsychologia.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Annual Review of Clinical Psychology. 2012;8(8):189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6(2):114–33. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: four general conclusions. Current Directions in Psychological Science. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7(3):134–40. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Monsell S, Yeung N, Azuma R. Reconfiguration of task-set: is it easier to switch to the weaker task? Psychological Research. 2000;63:250–64. doi: 10.1007/s004269900005. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology-General. 2001;130(3):466–78. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Ohman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80(3):381–96. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Paulitzki JR, Risko EF, Oakman JM, Stolz JA. Doing the unpleasant: how the emotional nature of a threat-relevant task affects task-switching. Personality and Individual Differences. 2008;45(5):350–5. [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10(4):437–42. [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–6. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: an ERP study. Neuroreport. 1999;10(13):2691–8. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Reeck C, Egner T. Affective privilege: asymmetric interference by emotional distracters. Frontiers in Psychology. 2011;2(232):1–7. doi: 10.3389/fpsyg.2011.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck C, LaBar KS, Egner T. Neural mechanisms mediating contingent capture of attention by affective stimuli. Journal of Cognitive Neuroscience. 2012;24(5):1113–26. doi: 10.1162/jocn_a_00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal carter in task switching. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13448–53. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TB, Hertel PT, Joormann J. Cognitive bias modification: induced interpretive biases affect memory. Emotion. 2011;11(1):145–52. doi: 10.1037/a0021754. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Huang YM. Emotional attention: uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science. 2009;18(3):148–2. [Google Scholar]
- Wilson EJ, Macleod C, Mathews A, Rutherford EM. The causal role of interpretive bias in anxiety reactivity. Journal of Abnormal Psychology. 2006;115:103–11. doi: 10.1037/0021-843X.115.1.103. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of ‘switch costs’. Psychological Research. 2000;63:212–33. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]